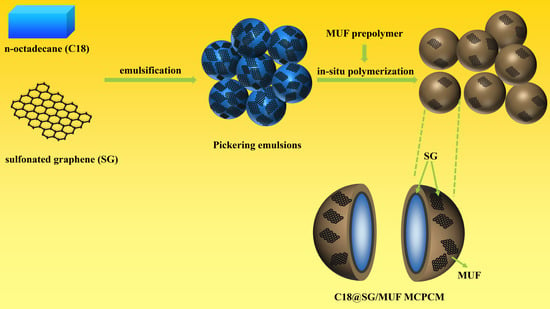
Preparation of Microencapsulated Phase Change Materials from Sulfonated Graphene Stabilized Pickering Emulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pickering Phase Change Material Emulsions
2.3. Preparation of Microencapsulated Phase Change Materials
- (1)
- A predetermined amount of melamine, formaldehyde solution, and 70 mL of deionized water were weighed and combined in a beaker. The pH value of the mixture was adjusted to 8.5 by adding triethanolamine, and the reaction was sustained for 1 h at 70 °C with mechanical stirring. The mixture was cooled to room temperature, and 0.92 g of urea were added to obtain the melamine-urea-formaldehyde (MUF) prepolymer.
- (2)
- The phase change material emulsion was then transferred into a three-neck flask and placed in a constant temperature oil bath at 40 °C with mechanical stirring at 350 rpm. The oil bath temperature was gradually elevated to 75 °C, and the MUF prepolymer solution was slowly added dropwise to the three-necked flask for 20–30 min. Upon reaching the predetermined oil bath temperature, a 10 wt% citric acid solution was gradually added dropwise to regulate the pH of the system to approximately 5, and the reaction was carried out for 3.5 h. The pH of the reaction mixture was then adjusted to 9 with triethanolamine to stop the polymerization reaction.
2.4. Characterization
3. Results and Discussion
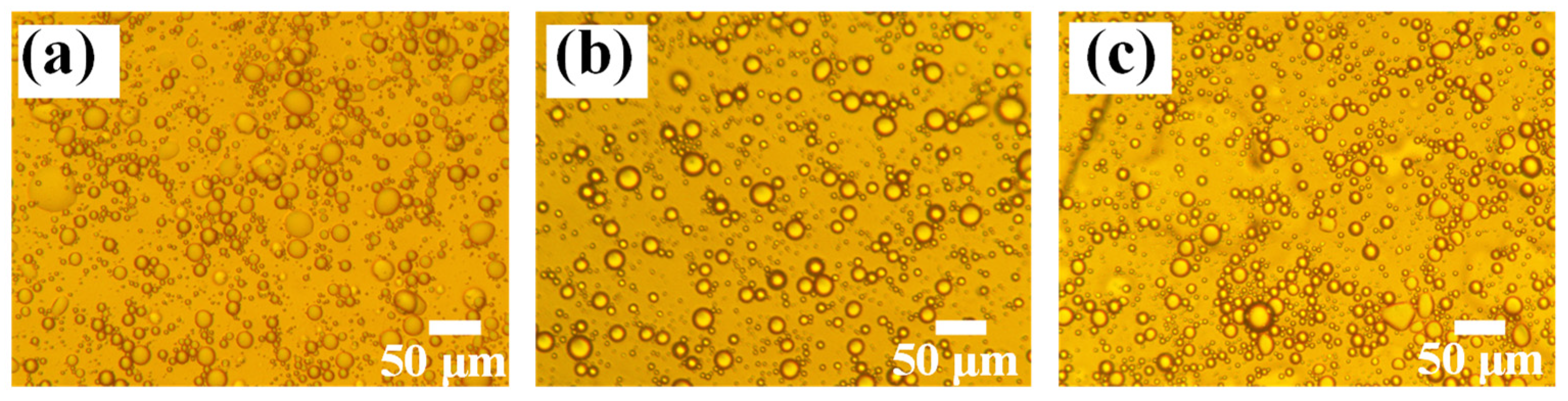
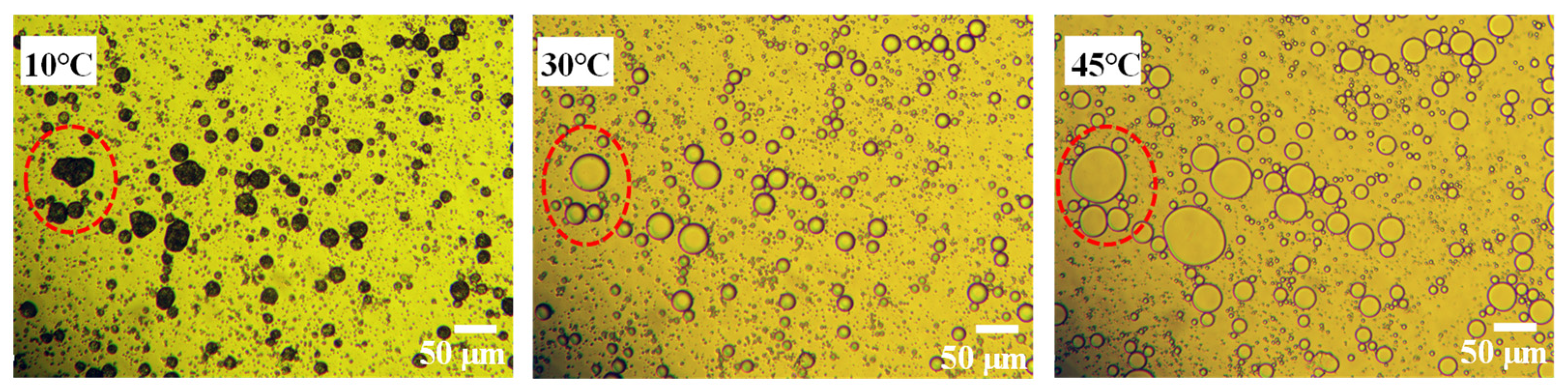
3.1. Pickering Emulsion Stabilized by SG
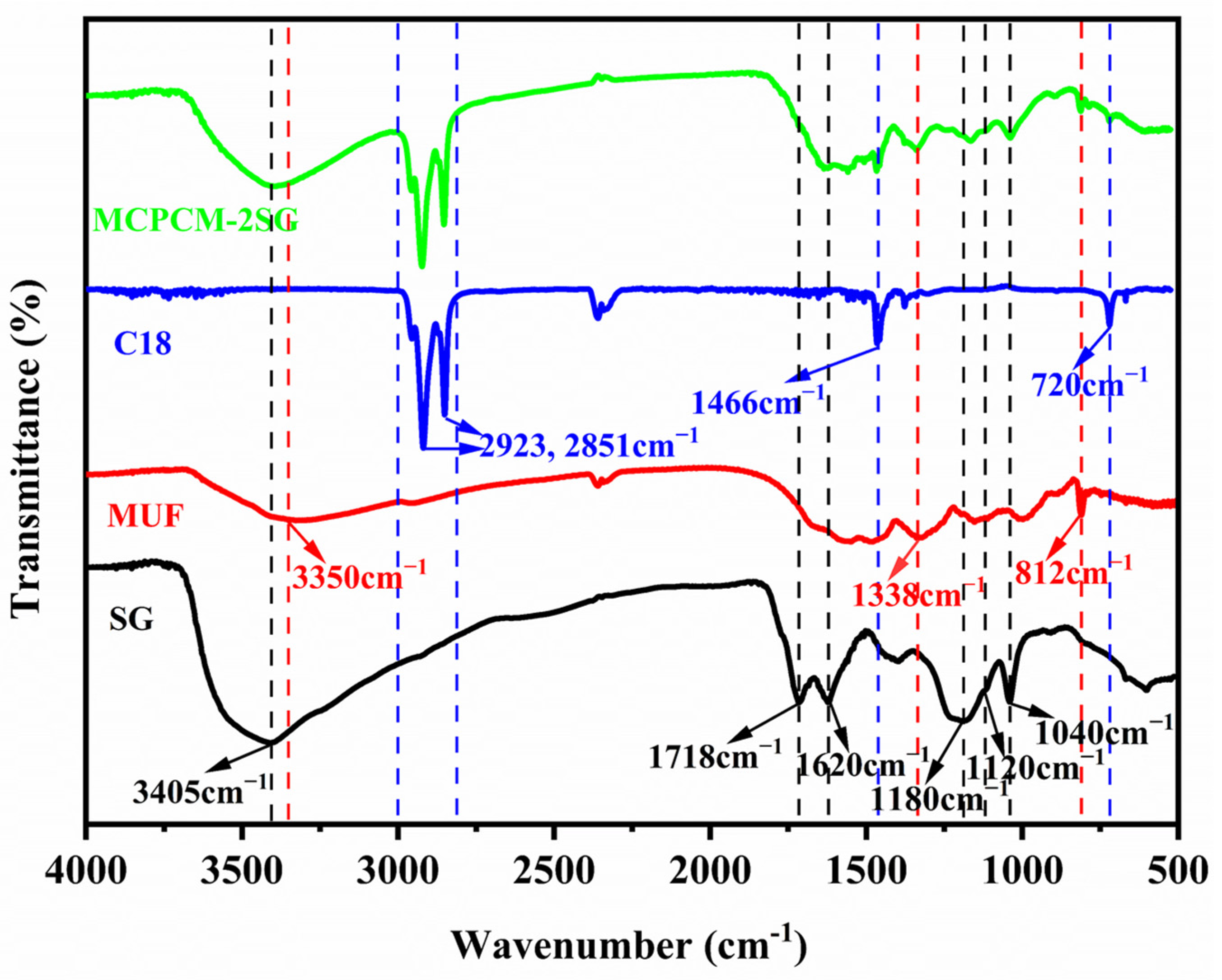
3.2. Chemical Composition of MCPCM
3.3. Morphology of MCPCM
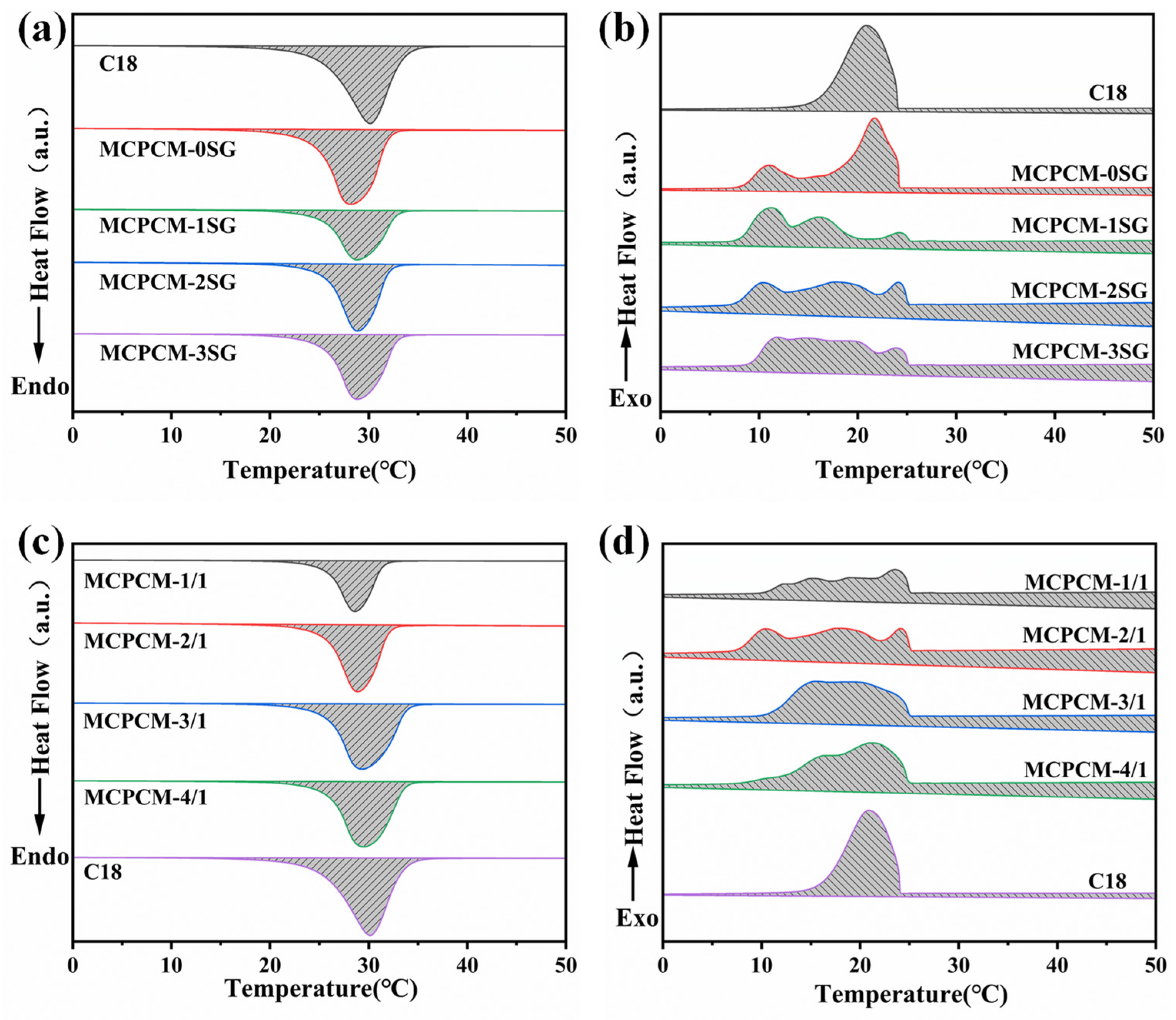
3.4. Thermophysical Properties of MCPCM
3.5. Water Contact Angle of MCPCM
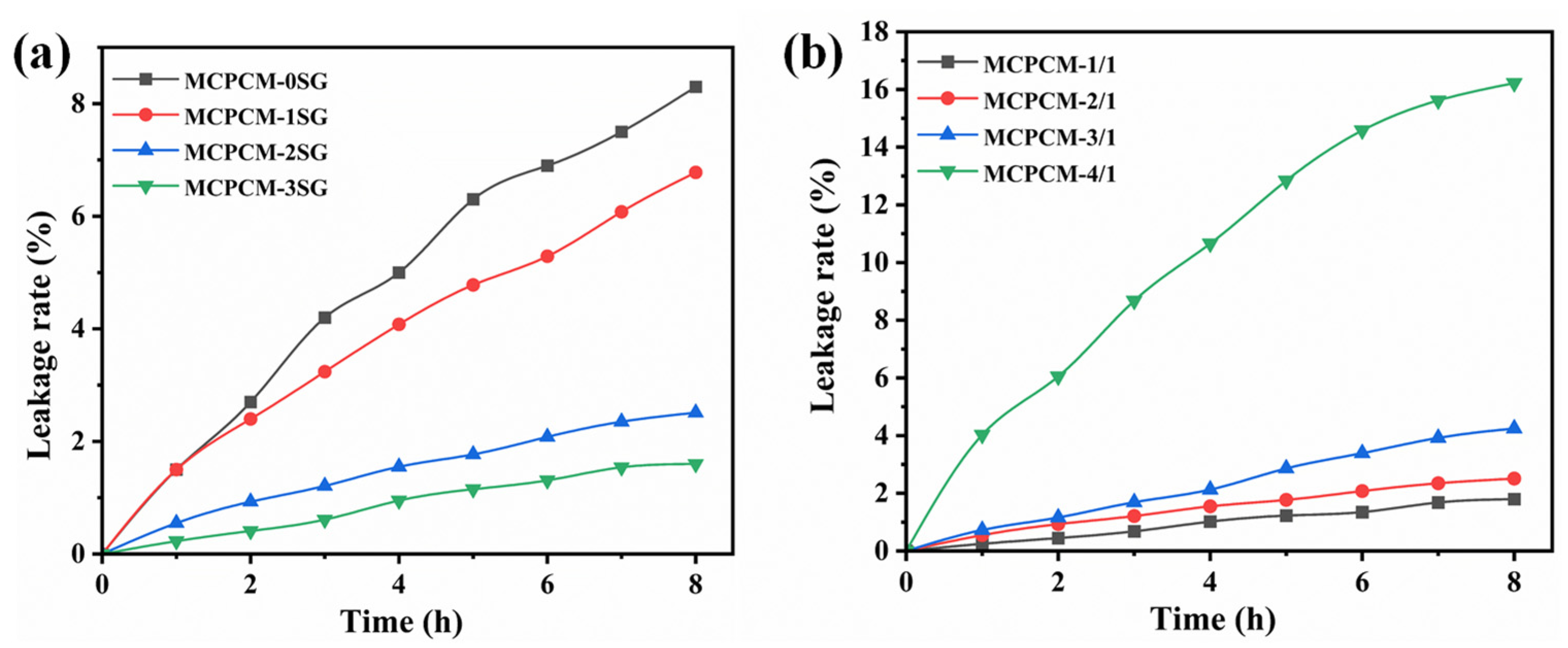
3.6. Leakage Prevention of MCPCM
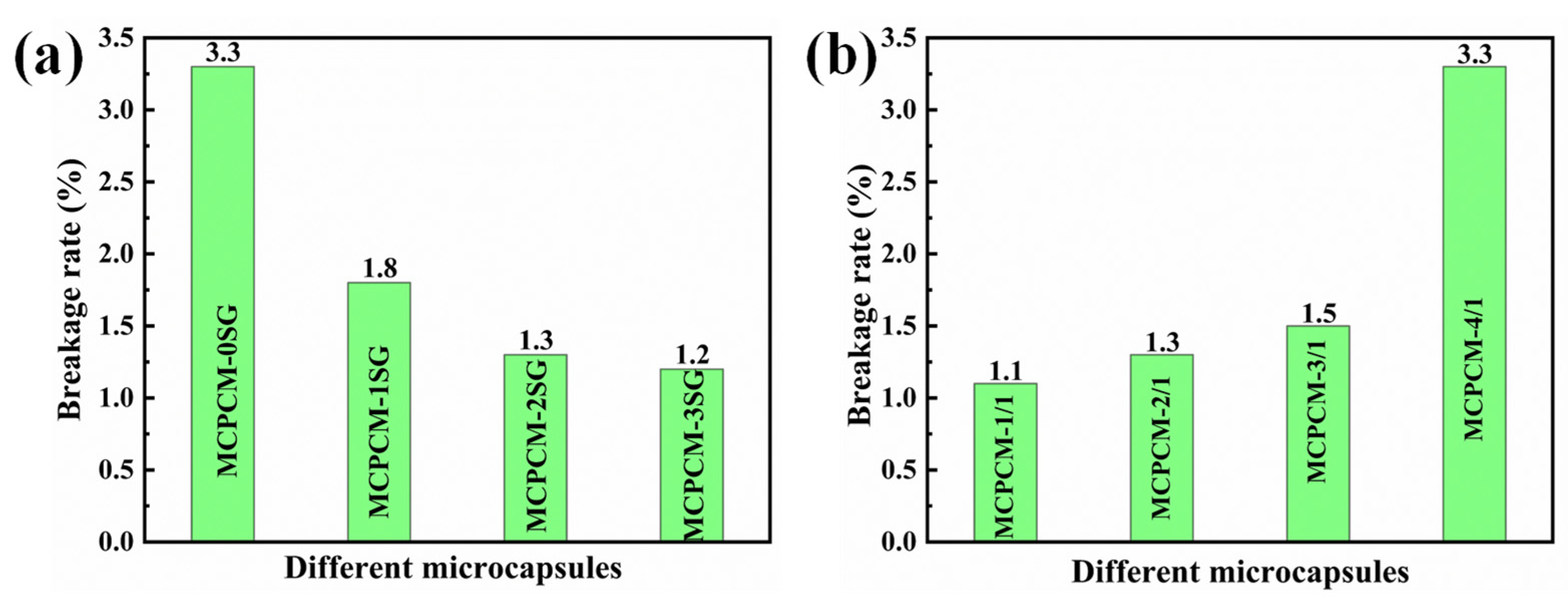
3.7. Mechanical Strength of MCPCM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, Characterization and Applications of Microencapsulated Phase Change Materials in Thermal Energy Storage: A Review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Miró, L.; Gasia, J.; Cabeza, L.F. Thermal Energy Storage (TES) for Industrial Waste Heat (IWH) Recovery: A Review. Appl. Energy 2016, 179, 284–301. [Google Scholar] [CrossRef]
- Sharif, M.A.; Al-Abidi, A.A.; Mat, S.; Sopian, K.; Ruslan, M.H.; Sulaiman, M.Y.; Rosli, M.A.M. Review of the Application of Phase Change Material for Heating and Domestic Hot Water Systems. Renew. Sustain. Energy Rev. 2015, 42, 557–568. [Google Scholar] [CrossRef]
- Zhan, S.; Chen, S.; Chen, L.; Hou, W. Preparation and Characterization of Polyurea Microencapsulated Phase Change Material by Interfacial Polycondensation Method. Powder Technol. 2016, 292, 217–222. [Google Scholar] [CrossRef]
- Lu, S.; Shen, T.; Xing, J.; Song, Q.; Shao, J.; Zhang, J.; Xin, C. Preparation and Characterization of Cross-Linked Polyurethane Shell Microencapsulated Phase Change Materials by Interfacial Polymerization. Mater. Lett. 2018, 211, 36–39. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Sun, R.; Ban, B.; Li, J.; Chen, J. Nano-Encapsulated Phase Change Materials Prepared by One-Step Interfacial Polymerization for Thermal Energy Storage. Mater. Chem. Phys. 2019, 231, 244–251. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Yang, Y.; Zhao, L.; Song, G.; Tang, G. Fabrication and Characterization of Microcapsulated Phase Change Materials with an Additional Function of Thermochromic Performance. Sol. Energy 2016, 139, 591–598. [Google Scholar] [CrossRef]
- Parvate, S.; Singh, J.; Dixit, P.; Vennapusa, J.R.; Maiti, T.K.; Chattopadhyay, S. Titanium Dioxide Nanoparticle-Decorated Polymer Microcapsules Enclosing Phase Change Material for Thermal Energy Storage and Photocatalysis. ACS Appl. Polym. Mater. 2021, 3, 1866–1879. [Google Scholar] [CrossRef]
- Xu, R.; Xia, X.; Wang, W.; Yu, D. Infrared Camouflage Fabric Prepared by Paraffin Phase Change Microcapsule with Good Thermal Insulting Properties. Colloids Surf. Physicochem. Eng. Asp. 2020, 591, 124519. [Google Scholar] [CrossRef]
- Kumar, G.N.; Al-Aifan, B.; Parameshwaran, R.; Ram, V.V. Facile Synthesis of Microencapsulated 1-Dodecanol/Melamine-Formaldehyde Phase Change Material Using in-Situ Polymerization for Thermal Energy Storage. Colloids Surf. Physicochem. Eng. Asp. 2021, 610, 125698. [Google Scholar] [CrossRef]
- Zhao, Q.; He, F.; Zhang, Q.; Fan, J.; He, R.; Zhang, K.; Yan, H.; Yang, W. Microencapsulated Phase Change Materials Based on Graphene Pickering Emulsion for Light-to-Thermal Energy Conversion and Management. Sol. Energy Mater. Sol. Cells 2019, 203, 110204. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Chang, T.; Wang, J.; Wang, X.; Zhou, G. Phase Change Material Microcapsules with Melamine Resin Shell via Cellulose Nanocrystal Stabilized Pickering Emulsion In-Situ Polymerization. Chem. Eng. J. 2022, 428, 131164. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Wang, H.; Du, Q. Encapsulated Phase Change Materials Stabilized by Modified Graphene Oxide. J. Mater. Chem. A 2014, 2, 5304–5314. [Google Scholar] [CrossRef]
- Yin, D.; Liu, H.; Ma, L.; Zhang, Q. Fabrication and Performance of Microencapsulated Phase Change Materials with Hybrid Shell by in Situ Polymerization in Pickering Emulsion. Polym. Adv. Technol. 2015, 26, 613–619. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Kannan, A.M. Recent Developments in Phase Change Materials for Energy Storage Applications: A Review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on Thermal Conductivity Enhancement, Thermal Properties and Applications of Phase Change Materials in Thermal Energy Storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Gulfam, R.; Zhang, P.; Meng, Z. Advanced Thermal Systems Driven by Paraffin-Based Phase Change Materials–A Review. Appl. Energy 2019, 238, 582–611. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Alva, G.; Fang, G. Microencapsulation and Thermal Properties of Myristic Acid with Ethyl Cellulose Shell for Thermal Energy Storage. Appl. Energy 2018, 231, 494–501. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Gao, Q.; Yang, J.-M.; Wang, Y.; Yang, J.-Y.; Zhang, X.; Feng, G.-Z.; Cheng, Z.-Q.; Wang, S.-J.; Su, H. Fabrication and Release Behavior of Nitrapyrin Microcapsules: Using Modified Melamine-Formaldehyde Resin as Shell Material. Sci. Total Environ. 2020, 704, 135394. [Google Scholar] [CrossRef]
- Döğüşcü, D.K.; Altıntaş, A.; Sarı, A.; Alkan, C. Polystyrene Microcapsules with Palmitic-Capric Acid Eutectic Mixture as Building Thermal Energy Storage Materials. Energy Build. 2017, 150, 376–382. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Koh, W.-G. Magnetic Nanoparticle-Embedded PCM Nanocapsules Based on Paraffin Core and Polyurea Shell. Colloids Surf. Physicochem. Eng. Asp. 2014, 450, 46–51. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Ji, J.; Zheng, L. Research Progress on Preparation, Characterization, and Application of Nanoparticle-Based Microencapsulated Phase Change Materials. Int. J. Energy Res. 2021, 45, 9831–9857. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Liu, X.; Dai, E.; Li, B.; Fang, S.; Li, D. Synthesis and Performances of Phase Change Microcapsules with a Polymer/Diatomite Hybrid Shell for Thermal Energy Storage. Polymers 2018, 10, 601. [Google Scholar] [CrossRef]
- Fu, Y.; Hansson, J.; Liu, Y.; Chen, S.; Zehri, A.; Samani, M.K.; Wang, N.; Ni, Y.; Zhang, Y.; Zhang, Z.-B. Graphene Related Materials for Thermal Management. 2D Mater. 2020, 7, 012001. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal Properties of Graphene and Nanostructured Carbon Materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Liang, S.; Chen, K.; Tian, C.; Wang, J.; Luo, X.; Zhang, L. Graphene/SiO2/n-Octadecane Nanoencapsulated Phase Change Material with Flower like Morphology, High Thermal Conductivity, and Suppressed Supercooling. Appl. Energy 2019, 250, 98–108. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, W.; Jiang, Z.; He, F.; Zhang, K.; Fan, J.; Wu, J. Graphene Oxide-Modified Microencapsulated Phase Change Materials with High Encapsulation Capacity and Enhanced Leakage-Prevention Performance. Appl. Energy 2017, 197, 354–363. [Google Scholar] [CrossRef]
- Maithya, O.M.; Li, X.; Feng, X.; Sui, X.; Wang, B. Microencapsulated Phase Change Material via Pickering Emulsion Stabilized by Graphene Oxide for Photothermal Conversion. J. Mater. Sci. 2020, 55, 7731–7742. [Google Scholar] [CrossRef]
- Luo, J.; Chen, Y.; Zheng, Y.; Wang, C.; Wei, W.; Liu, X. Hollow Graphene-Polyaniline Hybrid Spheres Using Sulfonated Graphene as Pickering Stabilizer for High Performance Supercapacitors. Electrochim. Acta 2018, 272, 221–232. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Cui, J.; Qiu, H.; Yang, G.; Zheng, S.; Yang, J. Dispersion and Parallel Assembly of Sulfonated Graphene in Waterborne Epoxy Anticorrosion Coatings. J. Mater. Chem. A 2019, 7, 17937–17946. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Wu, S.; Zhang, D.; Oliver, S.; Li, X.; Chen, X.; Lei, L.; Shi, S. Micron-Dimensional Sulfonated Graphene Sheets Co-Stabilized Emulsion Polymerization to Prepare Acrylic Latex Used for Reinforced Anticorrosion Coatings. Prog. Org. Coat. 2022, 165, 106762. [Google Scholar] [CrossRef]
- Radnia, H.; Rashidi, A.; Nazar, A.R.S.; Eskandari, M.M.; Jalilian, M. A Novel Nanofluid Based on Sulfonated Graphene for Enhanced Oil Recovery. J. Mol. Liq. 2018, 271, 795–806. [Google Scholar] [CrossRef]
- Dao, T.D.; Erdenedelger, G.; Jeong, H.M. Water-Dispersible Graphene Designed as a Pickering Stabilizer for the Suspension Polymerization of Poly (Methyl Methacrylate)/Graphene Core–Shell Microsphere Exhibiting Ultra-Low Percolation Threshold of Electrical Conductivity. Polymer 2014, 55, 4709–4719. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Lei, S.; Song, Y. Graphene-Based Polyaniline Nanocomposites: Preparation, Properties and Applications. J. Mater. Chem. A 2014, 2, 4491–4509. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Nadeem, M.; Naz, M.Y.; Tan, W.L.; Shariff, A.M. Synthesis and Thermal Degradation Studies of Melamine Formaldehyde Resins. Sci. World J. 2014, 2014, 940502. [Google Scholar] [CrossRef]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N. Recent Progress in Ultra-Low Formaldehyde Emitting Adhesive Systems and Formaldehyde Scavengers in Wood-Based Panels: A Review. Wood Mater. Sci. Eng. 2022, 2, 1–20. [Google Scholar] [CrossRef]
- Ullah, H.; Azizli, K.A.M.; Man, Z.B.; Ismail, M.B.C.; Khan, M.I. The Potential of Microencapsulated Self-Healing Materials for Microcracks Recovery in Self-Healing Composite Systems: A Review. Polym. Rev. 2016, 56, 429–485. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Tailoring of Bifunctional Microencapsulated Phase Change Materials with CdS/SiO2 Double-Layered Shell for Solar Photocatalysis and Solar Thermal Energy Storage. Appl. Therm. Eng. 2018, 134, 603–614. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Z.; Ma, W. Fabrication and Characterization of a Novel Polyurethane Microencapsulated Phase Change Material for Thermal Energy Storage. Prog. Org. Coat. 2021, 151, 106006. [Google Scholar] [CrossRef]
- Cataldo, F. Structural Analogies and Differences between Graphite Oxide and C60 and C70 Polymeric Oxides (Fullerene Ozopolymers). Fuller. Nanotub. Carbon Nanostruct. 2003, 11, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, T.; Wu, S.; Zhou, H. Sulfonated Graphene Oxide: The New and Effective Material for Synthesis of Polystyrene-Based Nanocomposites. Colloid Polym. Sci. 2013, 291, 2061–2068. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.-Q.; Tang, R.-F.; Lu, Y.-L. Synthesis and Thermal Properties of Phase-Change Microcapsules Incorporated with Nano Alumina Particles in the Shell. J. Appl. Polym. Sci. 2012, 124, 689–698. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. Phase-Change Characteristics and Thermal Performance of Form-Stable n-Alkanes/Silica Composite Phase Change Materials Fabricated by Sodium Silicate Precursor. Renew. Energy 2015, 74, 689–698. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Wu, D. Design and Synthesis of Magnetic Microcapsules Based on N-Eicosane Core and Fe3O4/SiO2 Hybrid Shell for Dual-Functional Phase Change Materials. Appl. Energy 2014, 134, 456–468. [Google Scholar] [CrossRef]
- Renteria, J.D.; Nika, D.L.; Balandin, A.A. Graphene Thermal Properties: Applications in Thermal Management and Energy Storage. Appl. Sci. 2014, 4, 525–547. [Google Scholar] [CrossRef]
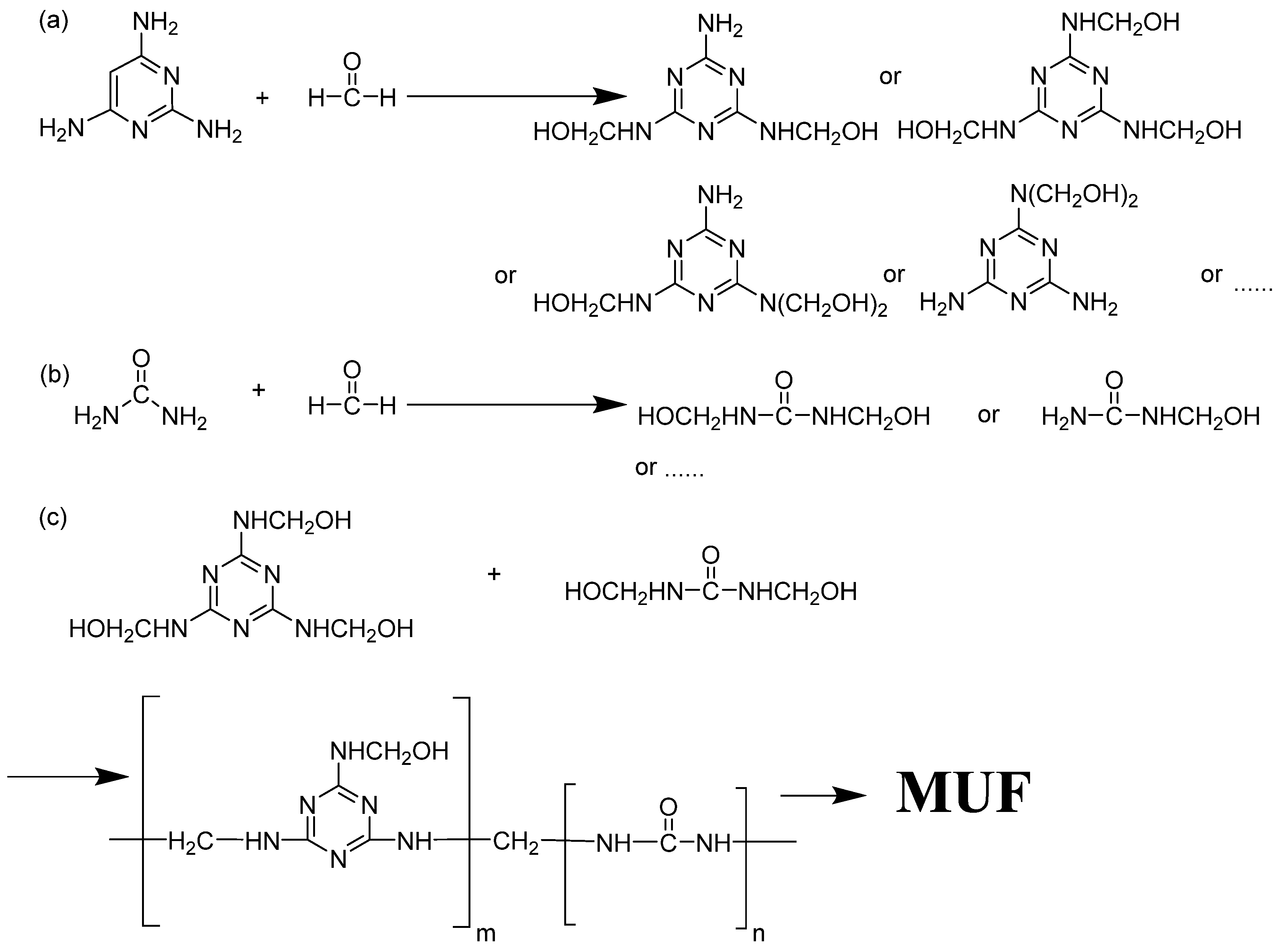



| Sample | C18 (g) | SG Slurry (g) | SDS (g) | Deionized Water (mL) |
|---|---|---|---|---|
| PCMEs-0SG | 15.00 | 0.00 | 1.50 | 90 |
| PCMEs-1SG | 15.00 | 1.50 | 0.00 | 90 |
| PCMEs-2SG | 15.00 | 3.00 | 0.00 | 90 |
| PCMEs-3SG | 15.00 | 4.50 | 0.00 | 90 |
| Sample | C18 (g) | SG Slurry (g) | Melamine (g) | Formaldehyde (g) | Urea (g) | Core/Shell (Mass Ratio) |
|---|---|---|---|---|---|---|
| MCPCM-0SG | 15.00 | 0.00 | 3.81 | 6.89 | 0.92 | 2:1 |
| MCPCM-1SG | 15.00 | 1.50 | 3.81 | 6.89 | 0.92 | 2:1 |
| MCPCM-2SG | 15.00 | 3.00 | 3.81 | 6.89 | 0.92 | 2:1 |
| MCPCM-3SG | 15.00 | 4.50 | 3.81 | 6.89 | 0.92 | 2:1 |
| MCPCM-1/1 | 7.50 | 1.50 | 3.81 | 6.89 | 0.92 | 1:1 |
| MCPCM-2/1 | 15.00 | 3.00 | 3.81 | 6.89 | 0.92 | 2:1 |
| MCPCM-3/1 | 22.50 | 4.50 | 3.81 | 6.89 | 0.92 | 3:1 |
| MCPCM-4/1 | 30.00 | 6.00 | 3.81 | 6.89 | 0.92 | 4:1 |
| Samples | Tm/Peak (°C) | ∆Hm (J/g) | Tc/Peak (°C) | ∆Hc (J/g) | Er (%) |
|---|---|---|---|---|---|
| C18 | 30.14 | 196.04 | 20.87 | 197.26 | - |
| MCPCM-0SG | 28.19 | 177.48 | 21.71 | 177.36 | 90.22 |
| MCPCM-1SG | 28.83 | 119.36 | 11.21 | 119.96 | 60.85 |
| MCPCM-2SG | 28.88 | 140.35 | 17.63 | 139.70 | 71.21 |
| MCPCM-3SG | 28.83 | 157.32 | 11.85 | 157.89 | 80.14 |
| MCPCM-1/1 | 28.60 | 93.20 | 23.53 | 93.31 | 47.42 |
| MCPCM-2/1 | 28.88 | 140.35 | 17.63 | 139.70 | 71.21 |
| MCPCM-3/1 | 29.31 | 174.27 | 15.14 | 174.12 | 88.58 |
| MCPCM-4/1 | 29.47 | 165.52 | 21.10 | 167.23 | 84.60 |
| Time (h) | MCPCM-0SG | MCPCM-1SG | MCPCM-2SG | MCPCM-3SG | |||
|---|---|---|---|---|---|---|---|
| Lr (%) | Lr (%) | Dr1 a (%) | Lr (%) | Dr2 a (%) | Lr (%) | Dr3 a (%) | |
| 2 | 2.7 | 2.4 | 11.1 | 0.9 | 66.7 | 0.41 | 85.2 |
| 4 | 5.0 | 4.1 | 18.0 | 1.6 | 68.0 | 0.95 | 80.0 |
| 6 | 6.9 | 5.3 | 23.2 | 2.1 | 69.6 | 1.31 | 81.2 |
| 8 | 8.3 | 6.8 | 18.1 | 2.5 | 69.9 | 1.62 | 80.7 |
| Time (h) | MCPCM-1/1 Lr (%) | MCPCM-2/1 Lr (%) | MCPCM-3/1 Lr (%) | MCPCM-4/1 Lr (%) |
|---|---|---|---|---|
| 2 | 0.5 | 0.9 | 1.2 | 6.1 |
| 4 | 1.0 | 1.6 | 2.1 | 10.7 |
| 6 | 1.4 | 2.1 | 3.4 | 14.6 |
| 8 | 1.8 | 2.5 | 4.3 | 16.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Mei, D.; Wang, J.; Wu, H.; Wen, S. Preparation of Microencapsulated Phase Change Materials from Sulfonated Graphene Stabilized Pickering Emulsion. Polymers 2023, 15, 2441. https://doi.org/10.3390/polym15112441
Li W, Mei D, Wang J, Wu H, Wen S. Preparation of Microencapsulated Phase Change Materials from Sulfonated Graphene Stabilized Pickering Emulsion. Polymers. 2023; 15(11):2441. https://doi.org/10.3390/polym15112441
Chicago/Turabian StyleLi, Weiping, Dajiang Mei, Jihu Wang, Hui Wu, and Shaoguo Wen. 2023. "Preparation of Microencapsulated Phase Change Materials from Sulfonated Graphene Stabilized Pickering Emulsion" Polymers 15, no. 11: 2441. https://doi.org/10.3390/polym15112441
APA StyleLi, W., Mei, D., Wang, J., Wu, H., & Wen, S. (2023). Preparation of Microencapsulated Phase Change Materials from Sulfonated Graphene Stabilized Pickering Emulsion. Polymers, 15(11), 2441. https://doi.org/10.3390/polym15112441