Synthesis and Characterization of a New Alginate/Carrageenan Crosslinked Biopolymer and Study of the Antibacterial, Antioxidant, and Anticancer Performance of Its Mn(II), Fe(III), Ni(II), and Cu(II) Polymeric Complexes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization of the Polymer Complexes
2.3. Preparation of the Crosslinked Polymeric Ligand (Poly-AG/CTR/CAR)
2.4. Preparation of Polymer/Metal Complexes
2.4.1. Preparation of the Poly-AG/CTR/CAR/Mn(II) Complex
2.4.2. Preparation of the Poly-AG/CTR/CAR/Fe(III) Complex
2.4.3. Preparation of the Poly-AG/CTR/CAR/Ni(II) Complex
2.4.4. Preparation of the Poly-AG/CTR/CAR/Cu (II) Complex
2.5. Biological Analysis
2.5.1. Antimicrobial Activity
2.5.2. Antioxidant Activity
2.5.3. Cell Viability and Anticancer Assays
3. Results and Discussion
3.1. Preparation of the Different Polymeric Complexes
3.2. FT-IR Analysis
3.3. UV–Vis Spectrophotometric Analysis
3.4. Magnetic Moments
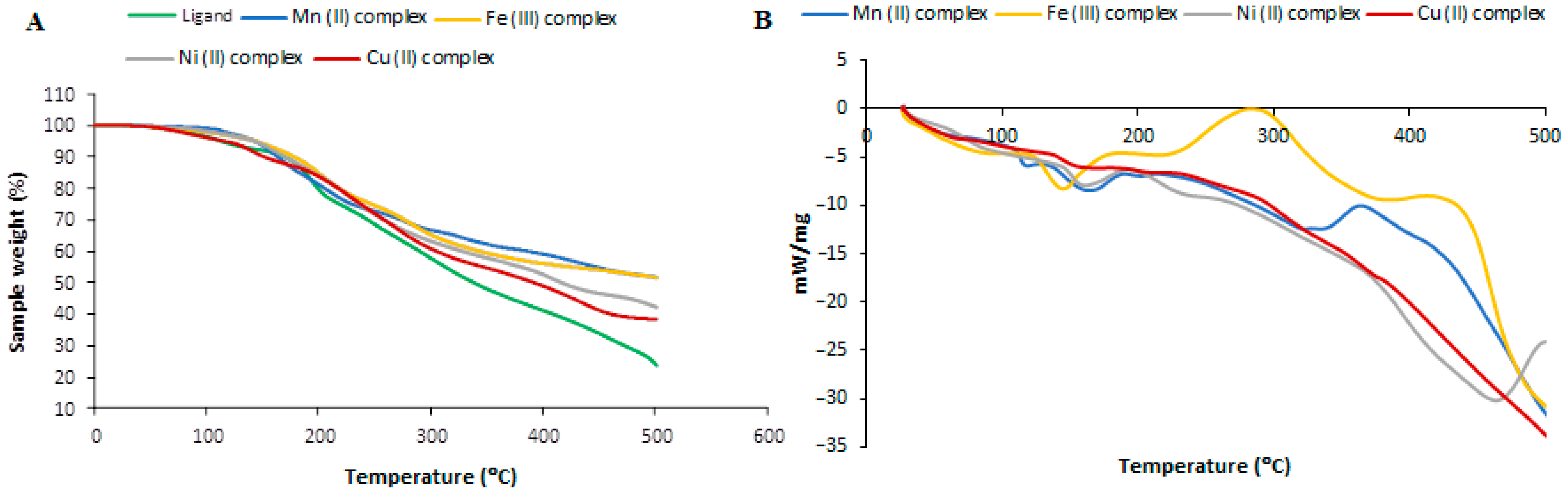
3.5. Thermogravimetric Analysis
3.6. XRD Analysis of the Polymeric Complexes
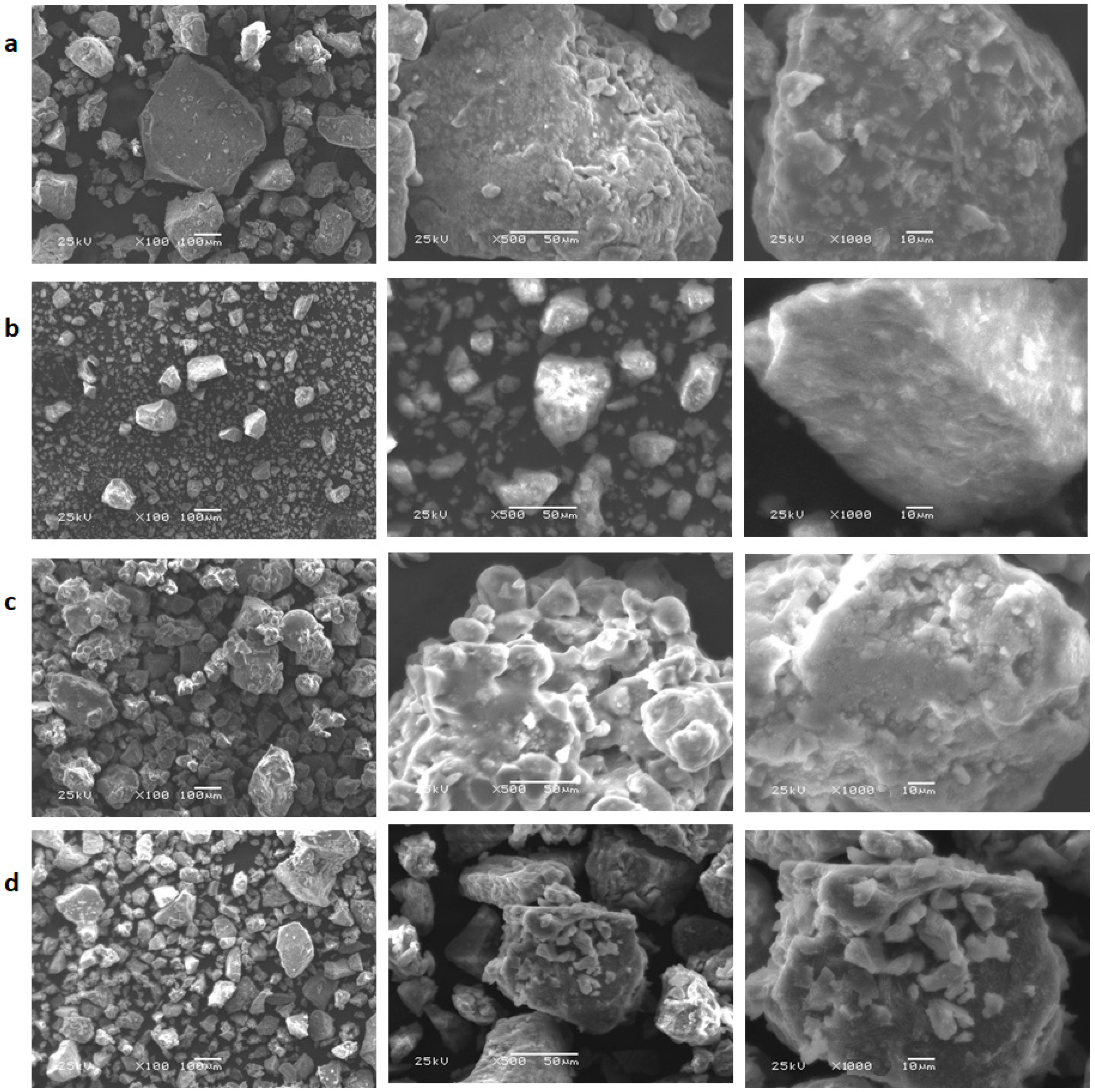
3.7. SEM Morphological Analysis
3.8. Biological Activity of the Different Polymeric Complexes
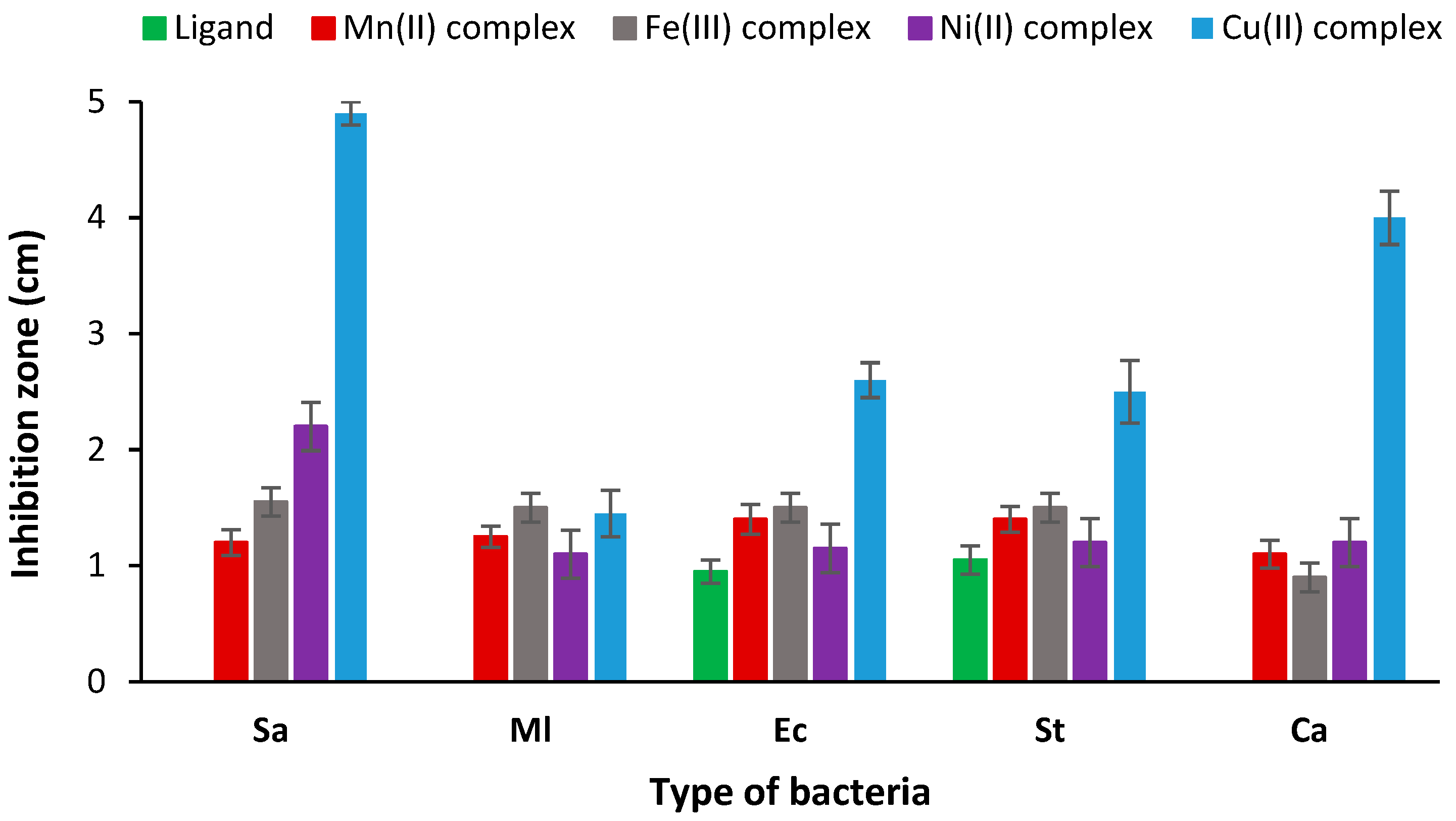
3.8.1. Microbial Activity
3.8.2. Antioxidant Activity
3.8.3. In Vitro Anticancer Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Ciurli, A.; Chiellini, C.; Di Caprio, F.; Concas, A.; Dunford, N.T. Latest developments in wastewater treatment and biopolymer production by microalgae. J. Environ. Chem. Eng. 2021, 9, 104926. [Google Scholar] [CrossRef]
- Parsons, S.; Allen, M.J.; Chuck, C.J. Coproducts of algae and yeast-derived single cell oils: A critical review of their role in improving biorefinery sustainability. Bioresour. Technol. 2020, 303, 122862. [Google Scholar] [CrossRef] [PubMed]
- Ammar, C.; Alminderej, F.M.; EL-Ghoul, Y.; Jabli, M.; Shafiquzzaman, M. Preparation and Characterization of a New Polymeric Multi-Layered Material Based K-Carrageenan and Alginate for Efficient Bio-Sorption of Methylene Blue Dye. Polymers 2021, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef]
- EL-Ghoul, Y.; Ammar, C.; Alminderej, F.M.; Shafiquzzaman, M. Design and Evaluation of a New Natural Multi-Layered Biopolymeric Adsorbent System-Based Chitosan/Cellulosic Nonwoven Material for the Biosorption of Industrial Textile Effluents. Polymers 2021, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview of the Alternative Use of Seaweeds to Produce Safe and Sustainable Bio-Packaging. Appl. Sci. 2022, 12, 3123. [Google Scholar] [CrossRef]
- Uthaya Kumar, U.S.; Abdulmadjid, S.N.; Olaiya, N.G.; Amirul, A.A.; Rizal, S.; Rahman, A.A.; Alfatah, T.; Mistar, E.M.; Abdul Khalil, H.P.S. Extracted Compounds from Neem Leaves as Antimicrobial Agent on the Physico-Chemical Properties of Seaweed-Based Biopolymer Films. Polymers 2020, 12, 1119. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Mohamad, S.E.; Lee, K.X.; Teow, S.-Y. Green Synthesized Montmorillonite/Carrageenan/Fe3O4 Nanocomposites for pH-Responsive Release of Protocatechuic Acid and Its Anticancer Activity. Int. J. Mol. Sci. 2020, 21, 4851. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Utomo, D.P.; Raemas, A.F.A.; Kusworo, T.D.; Jos, B.; Djaeni, M. Alginate/κ-Carrageenan-Based Edible Films Incorporated with Clove Essential Oil: Physico-Chemical Characterization and Antioxidant-Antimicrobial Activity. Polymers 2021, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Postolović, K.S.; Antonijević, M.D.; Ljujić, B.; Radenković, S.; Miletić Kovačević, M.; Hiezl, Z.; Pavlović, S.; Radojević, I.; Stanić, Z. Curcumin and Diclofenac Therapeutic Efficacy Enhancement Applying Transdermal Hydrogel Polymer Films, Based on Carrageenan, Alginate and Poloxamer. Polymers 2022, 14, 4091. [Google Scholar] [CrossRef] [PubMed]
- Alminderej, F.M.; Ammar, C.; El-Ghoul, Y. Functionalization, characterization and microbiological performance of new biocompatible cellulosic dressing grafted chitosan and Suaeda fruticosa polysaccharide extract. Cellulose 2021, 28, 9821–9835. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Lorbeer, A.J.; Lahnstein, J.; Bulone, V.; Franco, C.M.M.; Zhang, W. Enzyme-assisted extraction of carbohydrates from the brown alga Ecklonia radiata: Effect of enzyme type, pH and buffer on sugar yield and molecular weight profiles. Process Biochem. 2016, 51, 1503–1510. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Fan, W.; Liu, Y.; Wang, Q.; Weng, L. Fabrication, Property and Application of Calcium Alginate Fiber: A Review. Polymers 2022, 14, 3227. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Li, S.; Fan, M.; Deng, S.; Tao, N. Characterization and Application in Packaging Grease of Gelatin–Sodium Alginate Edible Films Cross-Linked by Pullulan. Polymers 2022, 14, 3199. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Ma, Q.; Mu, J.; Li, X.; Liu, H. Preparation and Characterization of Yellow Peach Peel/Sodium Alginate/Glycerol Antioxidant Film Applicable for Oil Package. Polymers 2022, 14, 1693. [Google Scholar] [CrossRef]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.T.; Cuong, D.X.; Thuy, L.H.; Thuan, P.T.; Tuyen, D.T.T.; Mo, V.T.; Dong, D.H. Carrageenan of Red Algae Eucheuma gelatinae: Extraction, Antioxidant Activity, Rheology Characteristics, and Physicochemistry Characterization. Molecules 2022, 27, 1268. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, D.; Zhang, J.; Li, J.; Lai, D.; Lin, S.; Hu, J. Preparation and Characterization of Biodegradable κ-Carrageenan Based Anti-Bacterial Film Functionalized with Wells-Dawson Polyoxometalate. Foods 2022, 11, 586. [Google Scholar] [CrossRef]
- Panwar, S.; Panjagari, N.R.; Singh, A.K.; Deshwal, G.K.; Badola, R.; Minz, P.S.; Goksen, G.; Rusu, A.; Trif, M. Electrospun Smart Oxygen Indicating Tag for Modified Atmosphere Packaging Applications: Fabrication, Characterization and Storage Stability. Polymers 2022, 14, 2108. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, U.; Sohail, M.; Usman Minhas, M.; Khan, S.; Hussain, Z.; Kazi, M.; Ahmed Shah, S.; Mahmood, A.; Maniruzzaman, M. Biofunctional Hyaluronic Acid/κ-Carrageenan Injectable Hydrogels for Improved Drug Delivery and Wound Healing. Polymers 2022, 14, 376. [Google Scholar] [CrossRef] [PubMed]
- Vernaya, O.I.; Ryabev, A.N.; Shabatina, T.I.; Karlova, D.L.; Shabatin, A.V.; Bulatnikova, L.N.; Semenov, A.M.; Melnikov, M.Y.; Lozinsky, V.I. Cryostructuring of Polymeric Systems: 62 Preparation and Characterization of Alginate/Chondroitin Sulfate Cryostructurates Loaded with Antimicrobial Substances. Polymers 2022, 14, 3271. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Punia Bangar, S.; Thakur, N.; Trif, M. Recent Advancements in Smart Biogenic Packaging: Reshaping the Future of the Food Packaging Industry. Polymers 2022, 14, 829. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.V.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review. Nanomaterials 2022, 12, 1006. [Google Scholar] [CrossRef]
- Zemskova, L.; Silant’ev, V.; Tokar, E.; Egorin, A. Synthesis of Inorganic Compounds in the Matrix of Polysaccharide Chitosan. Biomimetics 2021, 6, 45. [Google Scholar] [CrossRef]
- Zharmenov, A.; Yefremova, S.; Satbaev, B.; Shalabaev, N.; Satbaev, S.; Yermishin, S.; Kablanbekov, A. Production of Refractory Materials Using a Renewable Source of Silicon Dioxide. Minerals 2022, 12, 1010. [Google Scholar] [CrossRef]
- Pileni, M.P. 1998. Fabrication and properties of nanosized material made by using colloidal assemblies as templates. Crystal Research and Technology. J. Exp. Ind. Crystallogr. 1998, 33, 1155–1186. [Google Scholar]
- Mann, S.; Archibald, D.D.; Didymus, J.M.; Douglas, T.; Heywood, B.R.; Meldrum, F.C.; Reeves, N.J. Crystallization at inorganic-organic interfaces: Biominerals and biomimetic synthesis. Science 1993, 261, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Y.; Li, F.-L.; Zhang, Y.-W.; Gupta, R.K.; Patel, S.K.S.; Lee, J.-K. Recent Strategies for the Immobilization of Therapeutic Enzymes. Polymers 2022, 14, 1409. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Virych, P.A.; Chumachenko, V.A.; Kuziv, Y.I.; Marinin, A.I.; Cheng, L.; Nie, G. Thermoresponsive Zinc TetraPhenylPorphyrin Photosensitizer/Dextran Graft Poly(N-IsoPropylAcrylAmide) Copolymer/Au Nanoparticles Hybrid Nanosystem: Potential for Photodynamic Therapy Applications. Nanomaterials 2022, 12, 2655. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Saravanakumar, G.; Sobha, S.; Oh, T.H. Dextran Sulfate Nanocarriers: Design, Strategies and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 355. [Google Scholar] [CrossRef]
- Gyurcsik, B.; Nagy, L. Carbohydrates as ligands: Coordination equilibria and structure of the metal complexes. Coord. Chem. Rev. 2000, 203, 81–149. [Google Scholar] [CrossRef]
- Jones, F.; Colfen, H.; Antonietti, M. Interaction of kappa-carrageenan with nickel, cobalt, and iron hydroxides. Biomacromolecules 2000, 1, 556–563. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Rocchetti, R. Enhanced capacity of chitosan for transition-metal ions in sulfate-sulphuric acid solutions. Talanta 1974, 21, 1137–1143. [Google Scholar] [CrossRef]
- Dbeibia, A.; Ben Taheur, F.; Altammar, K.A.; Haddaji, N.; Mahdhi, A.; Amri, Z.; Mzoughi, R.; Jabeur, C. Control of Staphylococcus aureus methicillin resistant isolated from auricular infections using aqueous and methanolic extracts of Ephedra alata. Saudi J. Biol. Sci. 2021, 29, 1021–1028. [Google Scholar] [CrossRef]
- Mahdhi, A.; Leban, N.; Chakroun, I.; Chaouch, M.A.; Hafsa, J.; Fdhila, K.; Mahdouani, K.; Majdoub, H. Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb. Pathog. 2017, 109, 2014–2220. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; AlSalhi, M.; Alrokayan, S.A. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 2011, 283, 101–108. [Google Scholar] [CrossRef]
- Kasaai, M.R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Rochas, C.; Lahaye, M.; Yaphe, W. Sulfate Content of Carrageenan and Agar Determined by Infrared Spectroscopy. Bot. Mar. 1986, 29, 335–340. [Google Scholar] [CrossRef]
- Lee, T.W.; Lau, J.P.K.; Wong, W.T. Synthesis and characterization of coordination polymers of Zn (II) with 1, 3-bis (4-pyridyl) propane and 4, 4′-pyridine ligands. Polyhedron 2004, 23, 999–1002. [Google Scholar] [CrossRef]
- Bargujar, S.; Chandra, S.; Chauhan, R.; Rajor, H.K.; Bhardwaj, J. Synthesis, spectroscopic evaluation, molecular modelling, thermal study and biological evaluation of manganese(II) complexes derived from bidentate N,O and N,S donor Schiff base ligands. Appl. Organomet. Chem. 2021, 32, e4149. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, P.K.; Sinha, N.; Chaudhari, S.; Sharma, S. Spectroscopic characterization of metal complexes with tetradentate ligand. J. Phys. Sci. 2018, 29, 1–11. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Alsaedi, R.O.; Amiri, N.; Allazzam, G.A. Synthesis, Characterization, and Antimicrobial of MnO and CdO Nanoparticles by Using a Calcination Method. Coatings 2022, 12, 215. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S. Synthesis, Properties and Biological Effectiveness of Iron Oxide Nanoparticles Via Calcinations Method. Orient. J. Chem. 2020, 36, 174–178. [Google Scholar] [CrossRef]
- Moustafa, I.M.I.; Magda, H. Synthesis, Spectroscopic Studies and Biological Evaluation of Co(II), Ni(II), Cu(II) and Zr(IV) Complexes of Azo Dyes and Thiamine Hydrochloride as Antimicrobial Agents. Mod. Chem. Appl. 2017, 5, 1000202. [Google Scholar]
- Al-Fakeh, M.S.; Alsikhan, M.A.; Alnawmasi, J.S. Physico-Chemical Study of Mn(II), Co(II), Cu(II), Cr(III), and Pd(II) Complexes with Schiff-Base and Aminopyrimidyl Derivatives and Anti-Cancer, Antioxidant, Antimicrobial Applications. Molecules 2023, 28, 2555. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Geetha Bai, R.; Tuvikene, R. Potential Antiviral Properties of Industrially Important Marine Algal Polysaccharides and Their Significance in Fighting a Future Viral Pandemic. Viruses 2021, 13, 1817. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, F.; Xing, Z.; Fan, L.; Li, Y.; Wang, S.; Ling, J.; Ouyang, X.-K. Efficient Delivery of Curcumin by Alginate Oligosaccharide Coated Aminated Mesoporous Silica Nanoparticles and In Vitro Anticancer Activity against Colon Cancer Cells. Pharmaceutics 2022, 14, 1166. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef] [PubMed]
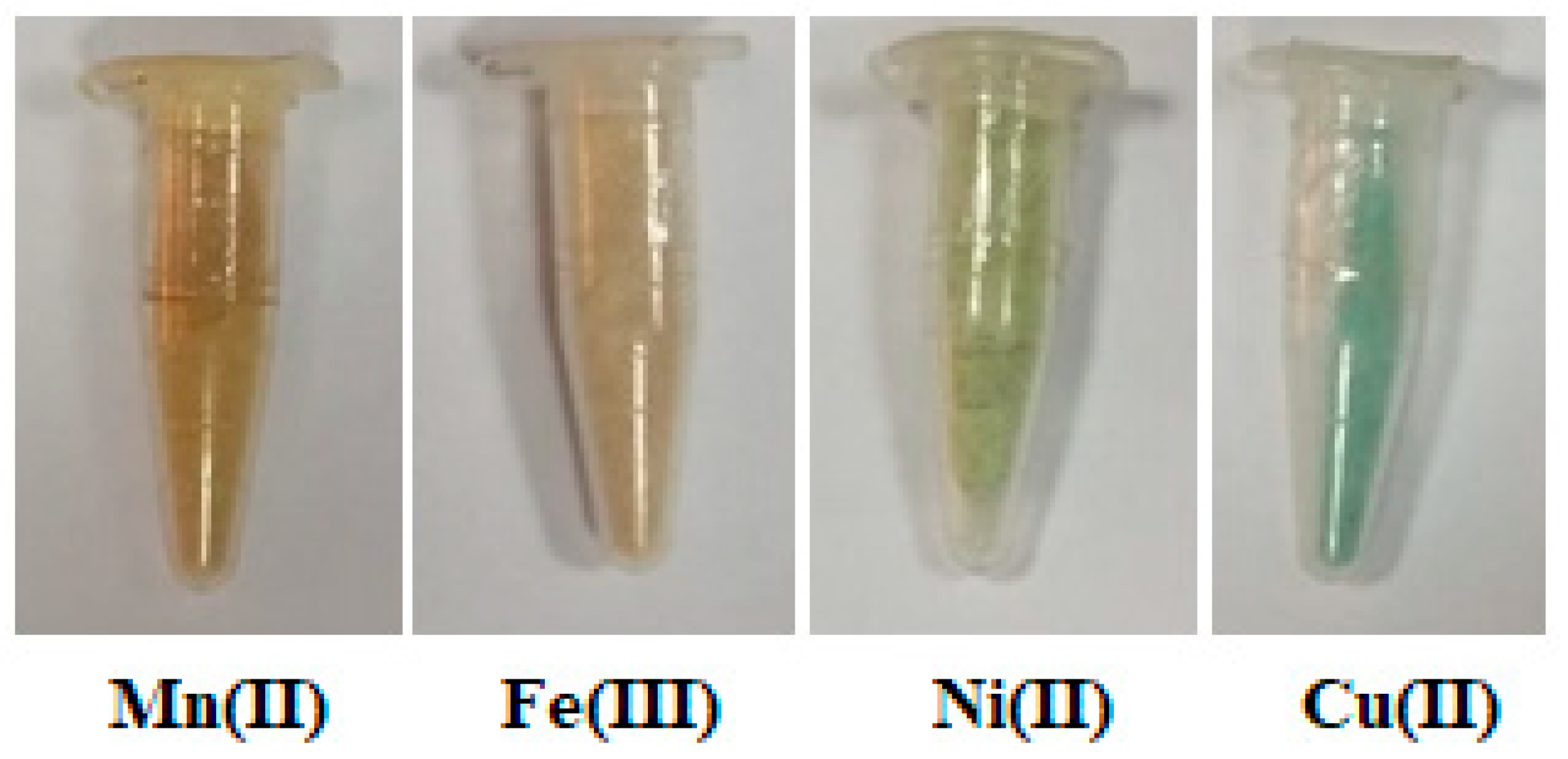






| Complex | M.F (M.Wt) | Color | Found (Calculated %) | m.p.C° Decomp. | Λm Scm2 mol−1 | ||
|---|---|---|---|---|---|---|---|
| C | H | S | |||||
| Ligand (poly-AG/CTR/CAR) | C24H27O26S2 | Dark | 36.92 | 3.76 | 8.52 | 110 | - |
| (795.37) | Beige | (36.23) | (3.42) | (8.06) | |||
| [Mn(AG/CTR/CAR)(H2O)2] | C24H29O28S2Mn | Light-Brown | 33.05 | 3.62 | 7.98 | 150 | 2.58 |
| (884.54) | (32.59) | (3.30) | (7.25) | ||||
| [Fe(AG/CTR/CAR)(H2O)3Cl] | C24H31O29S2FeCl | Light-Orange | 29.86 | 3.11 | 6.93 | 155 | 2.08 |
| (938.90) | (30.69) | (3.32) | (6.82) | ||||
| [Ni(AG/CTR/CAR)(H2O)2] | C24H29O28S2Ni | Light- Green | 34.07 | 4.06 | 7.86 | 162 | 6.78 |
| (888.29) | (32.45) | (3.29) | (7.22) | ||||
| [Cu(AG/CTR/CAR)(H2O)2] | C24H29O28S2Cu | Light-Blue | 33.12 | 3.90 | 7.75 | 166 | 2.95 |
| (892.95) | (32.27) | (3.27) | (7.18) | ||||
| Compound | ᵛ(O-H) | ᵛ(C-H) | ᵛ(C=O) | ᵛ(C=C) | ᵛ(S=O) | ᵛ(C-O) | ᵛ(=C-H) | ᵛ(-C-H) | ᵛ(M-O) |
|---|---|---|---|---|---|---|---|---|---|
| Ligand (poly-AG/CTR/CAR) | 3431 | 2177 | 1725 | - | 1260 | 1035 | 783 | 560 | - |
| [Mn(AG/CTR/CAR)(H2O)2] | 3315 | - | 1699 | 1598 | 1206 | 1026 | 928 | 700 | 508 |
| [Fe(AG/CTR/CAR)(H2O)3Cl] | 3380 | - | 1716 | 1610 | 1214 | 1027 | 926 | 840 | 516 |
| [Ni(AG/CTR/CAR)(H2O)2] | 3260 | 2917 | 1715 | 1614 | 1395 | 1025 | 926 | 787 | 510 |
| [Cu(AG/CTR/CAR)(H2O)2] | 3353 | 2917 | 1715 | 1583 | 1214 | 1025 | 927 | 864 | 505 |
| Complex | Vmax (nm) | Assignment | µeff. (BM) |
|---|---|---|---|
| Ligand (poly-AG/CTR/CAR) | 24.213 | n→π* | - |
| 39.215 | π→π* | ||
| [Mn(AG/CTR/CAR)(H2O)2] | 29.761 | n→π* | 5.72 |
| 35.842 | π→π* | ||
| 21.551 | d–d transition | ||
| [Fe(AG/CTR/CAR)(H2O)3Cl] | 29.411 | n→π* | 5.92 |
| 32.573 | π→π* | ||
| 21.598 | d–d transition | ||
| [Ni(AG/CTR/CAR)(H2O)2] | 23.202 | n→π* | 2.20 |
| 34.072 | π→π* | ||
| 21.978 | d–d transition | ||
| [Cu(AG/CTR/CAR)(H2O)2] | 24,331 | n→π* | 1.84 |
| 36,221 | π→π* | ||
| 21,739 | d–d transition |
| Complex | Temp. Range (°C) | TGA (Wt. Loss%) | Assignment | |||
|---|---|---|---|---|---|---|
| Ti | Tm | Tf | Calculated | Found | ||
| Mn(II) complex | 66 | 110 | 127 | 4.06 | 3.10 | Loss of two water molecules. |
| 112 | 200 | 222 | 19.78 | 18.63 | Loss of ligand (AG). | |
| 202 | 350 | 313 | 70.09 | 70.01 | Decomposition of the rest of the organic ligands with the formation of MnO. | |
| 352 | 500 | 550 | 8.01 | 7.81 | ||
| Fe(III) complex | 72 | 112 | 146 | 5.75 | 4.55 | Loss of three water molecules. |
| 114 | 206 | 240 | 3.77 | 3.42 | Loss of chloride atom. | |
| 208 | 500 | 550 | 8.50 | 7.28 | Decomposition of the rest of the organic ligands with the formation of (½ Fe2O3). | |
| Ni(II) complex | 76 | 118 | 150 | 4.05 | 4.03 | Loss of two water molecules. |
| 120 | 198 | 248 | 19.70 | 18.86 | Loss of ligand (AG). | |
| 200 | 500 | 550 | 8.40 | 8.22 | Decomposition of the rest of the organic ligands with the formation of NiO. | |
| Cu(II) complex | 65 | 108 | 128 | 4.03 | 4.01 | Loss of two water molecules. |
| 110 | 196 | 181 | 19.59 | 18.45 | Loss of ligand (AG). | |
| 198 | 500 | 550 | 8.90 | 7.95 | Decomposition of the rest of the organic ligands with the formation of CuO. | |
| Parameters | Mn(II) Complex | Fe(III) Complex | Ni(II) Complex | Cu(II) Complex |
|---|---|---|---|---|
| Empirical formula | C24H29O28S2Mn | C24H31O29S2FeCl | C24H29O28S2Ni | C24H29O28S2Cu |
| Formula weight | 884.54 | 938.90 | 888.29 | 892.95 |
| Crystal system | Monoclinic | Cubic | Cubic | Cubic |
| a (Å) | 11.197 | 3.9908 | 3.9940 | 5.628 |
| b (Å) | 9.520 | 3.9908 | 3.9940 | 5.628 |
| c (Å) | 6.195 | 3.9908 | 3.9940 | 5.628 |
| Alfa (°) | 90.00 | 90.00 | 90.00 | 90.00 |
| Beta (°) | 99.74 | 90.00 | 90.00 | 90.00 |
| Gamma (°) | 90.00 | 90.00 | 90.00 | 90.00 |
| Volume of unit cell (Å3) | 650.9 | 63.56 | 63.71 | 178.29 |
| Antimicrobial Activity (Inhibition Zone (cm)) | Antioxidant | |||||
|---|---|---|---|---|---|---|
| Sa | Ml | Ec | St | Ca | ||
| Ligand | 0 | 0 | 0.95 | 1.05 | 0 | 95±1.4 |
| Mn(II) complex | 1.2 | 1.25 | 1.4 | 1.4 | 1.1 | 82.5±0.7 |
| Fe(III) complex | 1.55 | 1.5 | 1.5 | 1.5 | 0.9 | 94±2.8 |
| Ni(II) complex | 2.2 | 1.1 | 1.15 | 1.2 | 1.2 | 83±1.4 |
| Cu(II) complex | 4.9 | 1.45 | 2.6 | 2.5 | 4 | 73±2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
EL-Ghoul, Y.; Al-Fakeh, M.S.; Al-Subaie, N.S. Synthesis and Characterization of a New Alginate/Carrageenan Crosslinked Biopolymer and Study of the Antibacterial, Antioxidant, and Anticancer Performance of Its Mn(II), Fe(III), Ni(II), and Cu(II) Polymeric Complexes. Polymers 2023, 15, 2511. https://doi.org/10.3390/polym15112511
EL-Ghoul Y, Al-Fakeh MS, Al-Subaie NS. Synthesis and Characterization of a New Alginate/Carrageenan Crosslinked Biopolymer and Study of the Antibacterial, Antioxidant, and Anticancer Performance of Its Mn(II), Fe(III), Ni(II), and Cu(II) Polymeric Complexes. Polymers. 2023; 15(11):2511. https://doi.org/10.3390/polym15112511
Chicago/Turabian StyleEL-Ghoul, Yassine, Maged S. Al-Fakeh, and Nora S. Al-Subaie. 2023. "Synthesis and Characterization of a New Alginate/Carrageenan Crosslinked Biopolymer and Study of the Antibacterial, Antioxidant, and Anticancer Performance of Its Mn(II), Fe(III), Ni(II), and Cu(II) Polymeric Complexes" Polymers 15, no. 11: 2511. https://doi.org/10.3390/polym15112511





