Polyurethane Recycling: Thermal Decomposition of 1,3-Diphenyl Urea to Isocyanates
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. DPU Solubility
2.3. Reaction Setup
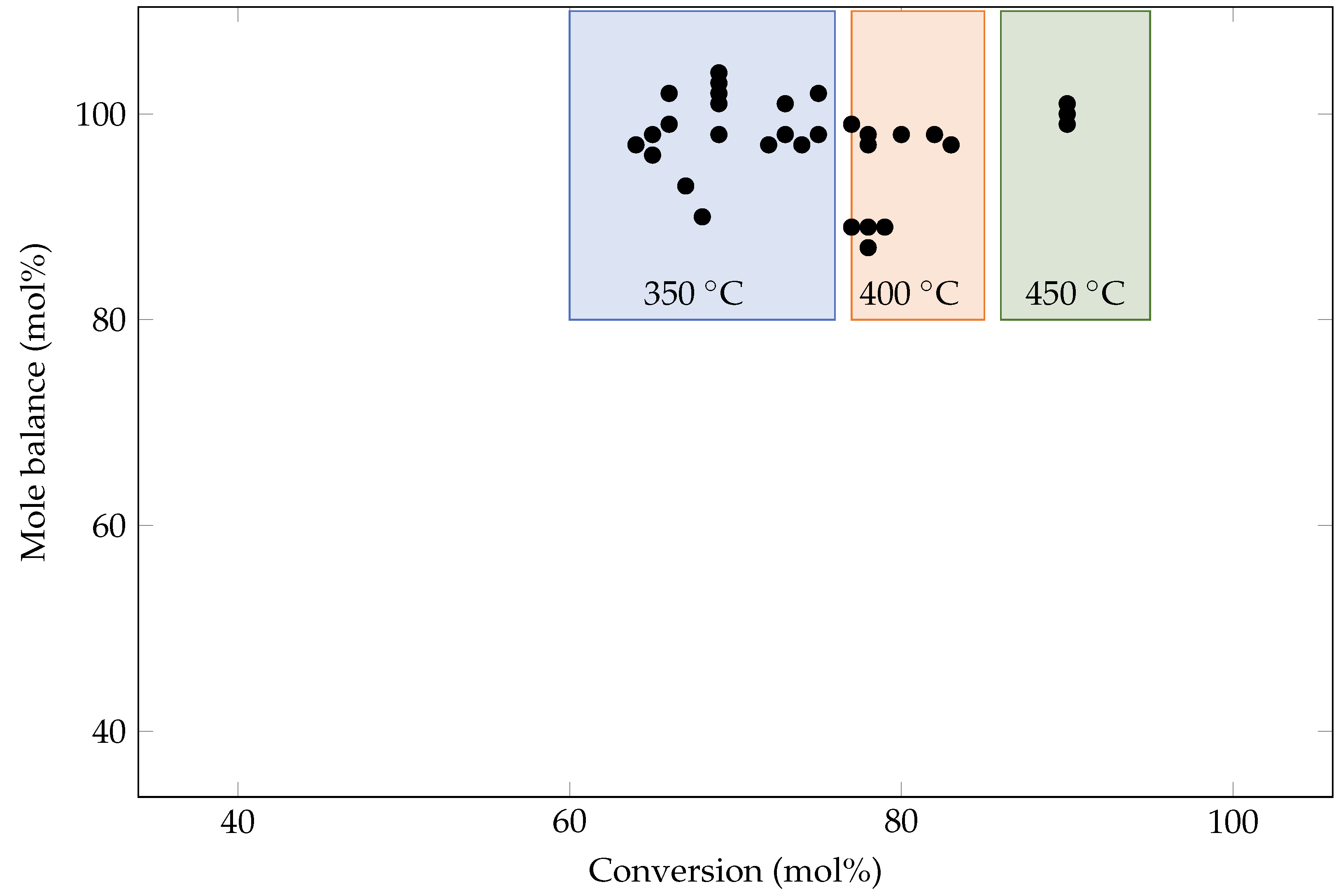
2.4. Analysis and Calculations
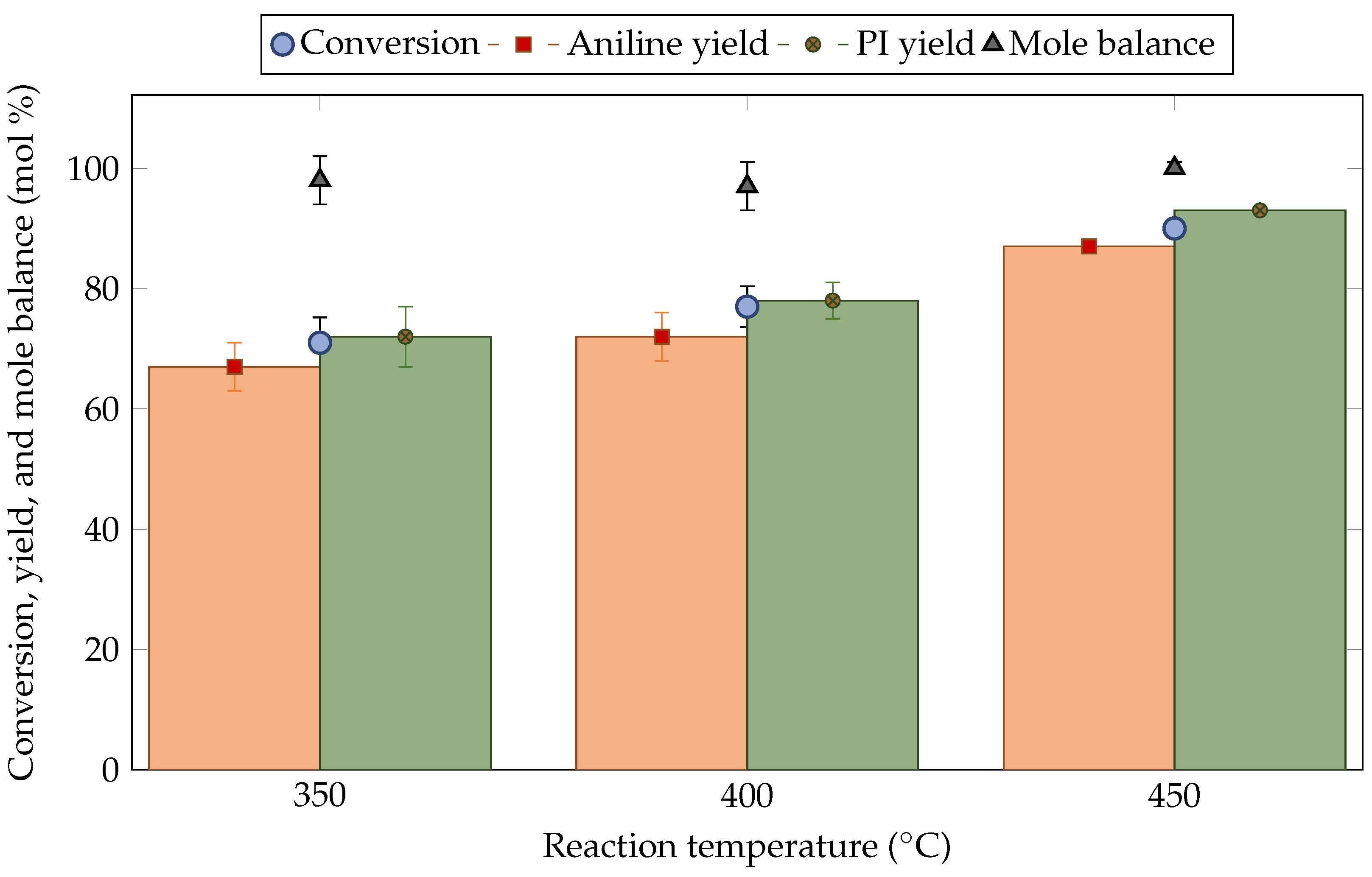
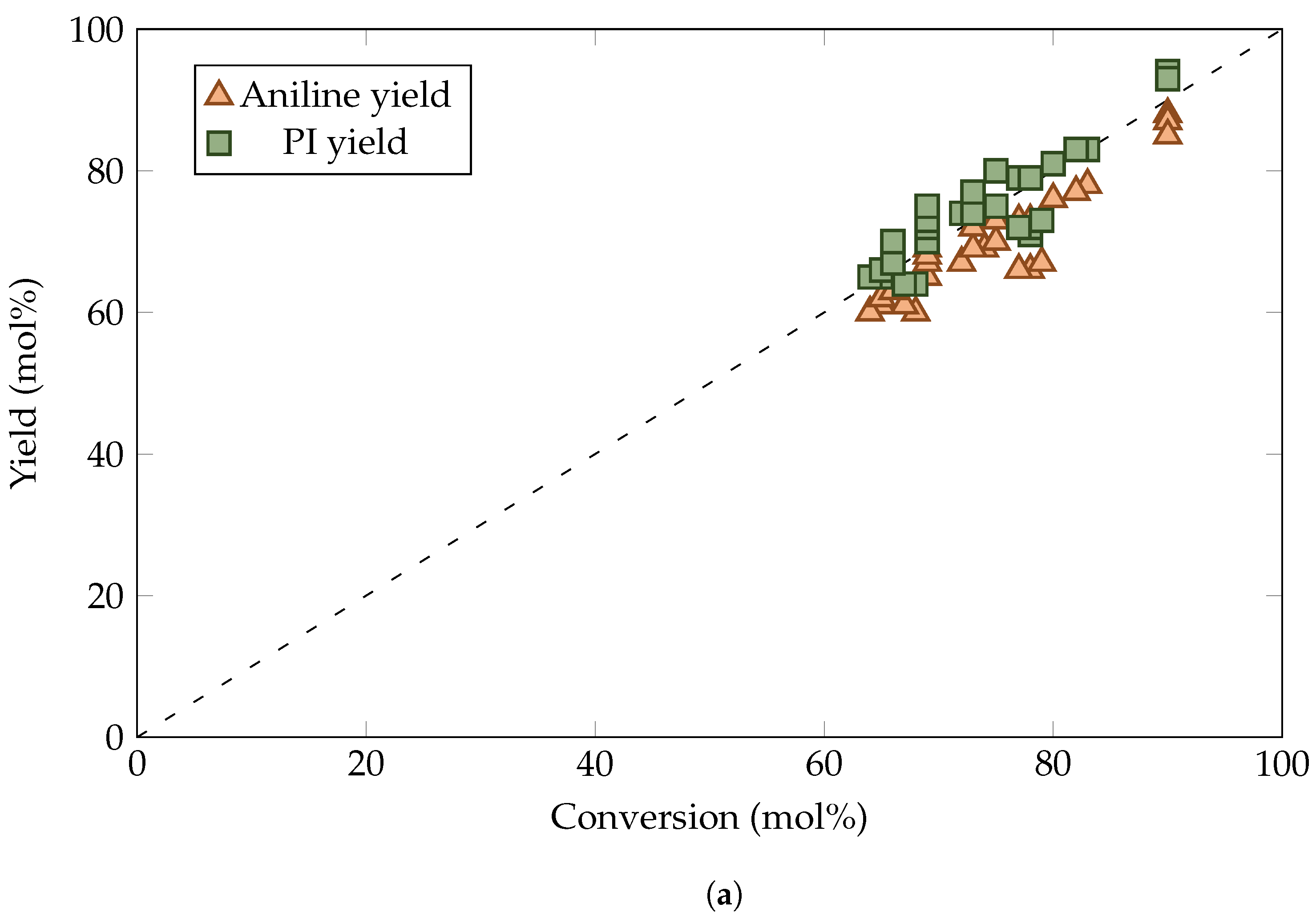
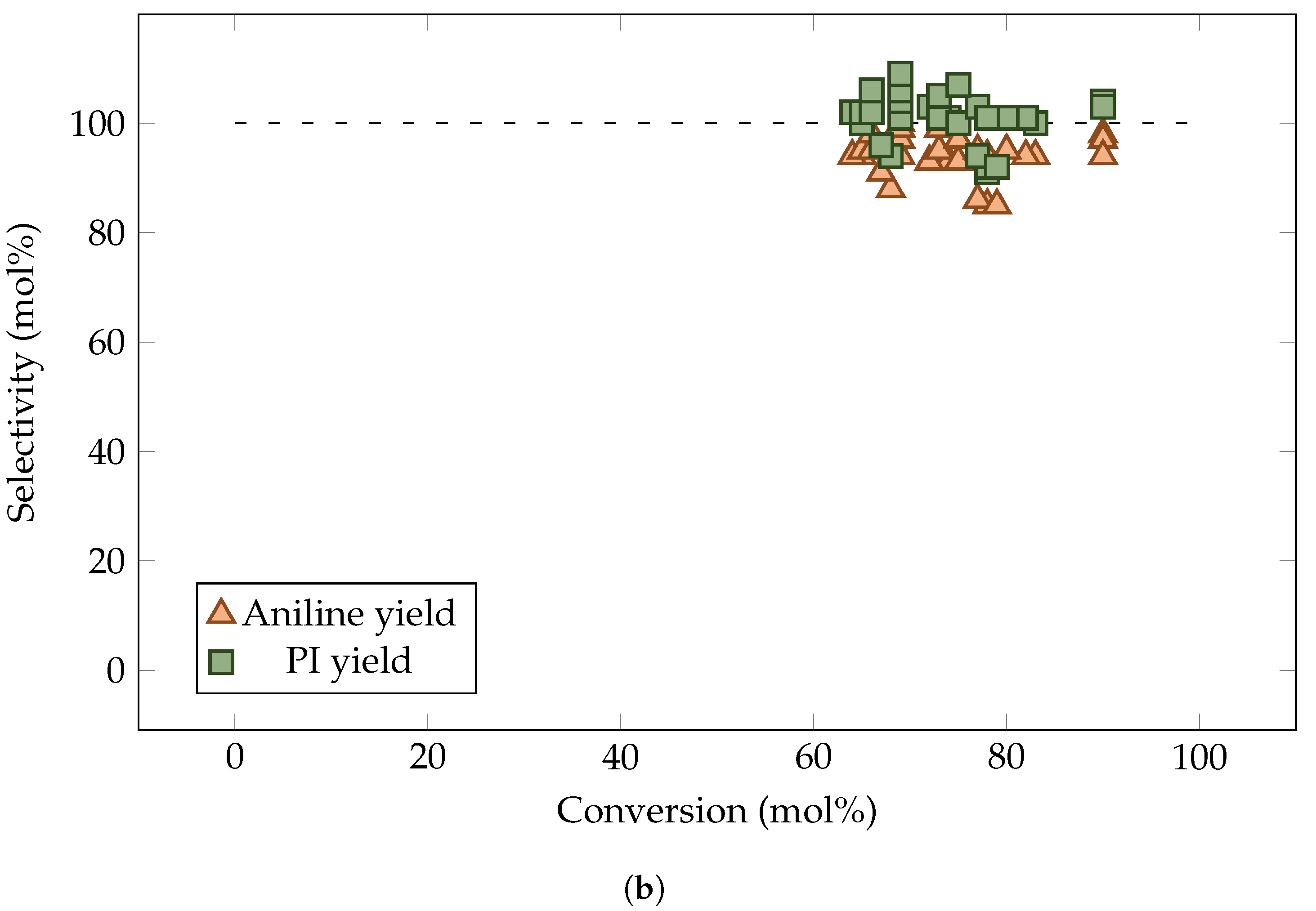
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMSO | Dimethyl sulfoxide |
| DPU | 1,3-Diphenylurea |
| EU | European Union |
| GVL | -Valerolactone |
| HPLC | High-performance liquid chromatography |
| LC | Liquid chromatography |
| MDI | Methyl diphenyl diisocyanate |
| MPC | Methyl N-phenyl carbamate |
| MS | Mass spectrometer |
| PFR | Plug flow reactor |
| PI | Phenyl isocyanate |
| PP | 1-(2-Pyridyl)piperazine |
| PUR | Polyurethanes |
| TDI | Toluene diisocyanate |
| UV | Ultraviolet |
| wt.% | Weight percent |
Appendix A
Appendix A.1. Calibration Curves
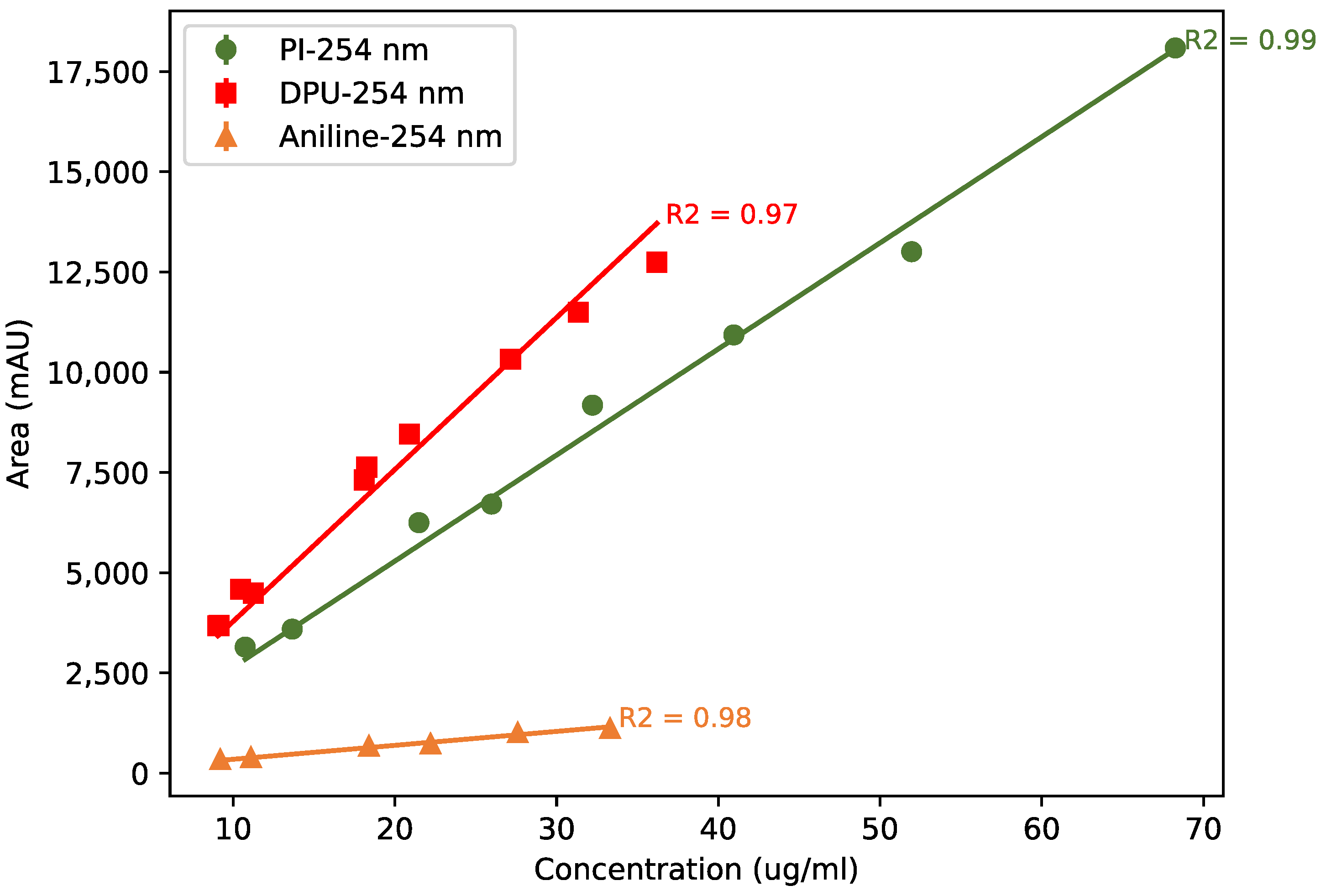
Appendix A.2. LC-UV Chromatograms



References
- Fernández, L. Market Volume of Polyurethane Worldwide from 2015 to 2020, with a Forecast for 2021 to 2026. 2021. Available online: https://www.statista.com/statistics/720449/global-polyurethane-market-size-forecast (accessed on 16 May 2023).
- Plastics Europe. Plastics—The Facts 2021; Plastics Europe: Brussels, Belgium, 2021. [Google Scholar]
- Kemona, A.; Piotrowska, M. Polyurethane recycling and disposal: Methods and prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841–1876. [Google Scholar] [CrossRef]
- Zevenhoven, R. Treatment and Disposal of Polyurethane Wastes: Options for Recovery and Recycling; Technical Report; Helsinki University of Technology: Helsinki, Finland, 2004. [Google Scholar]
- Akindoyo, J.O.; Beg, M.D.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications: A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Valle, V.; Aguirre, C.; Aldás, M.; Pazmiño, M.; Almeida-Naranjo, C.E. Recycled-based thermosetting material obtained from the decomposition of polyurethane foam wastes with castor oil. J. Mater. Cycles Waste Manag. 2020, 22, 1793–1800. [Google Scholar] [CrossRef]
- Lange, J.P. Sustainable development: Efficiency and recycling in chemicals manufacturing. Green Chem. 2002, 4, 546–550. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Ahmad Bhatti, I. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Mohammad Alavi Nikje, M.; Bagheri Garmarudi, A.; Idris, A.B. Polyurethane waste reduction and recycling: From bench to pilot scales. Des. Monomers Polym. 2011, 14, 395–421. [Google Scholar] [CrossRef]
- Kiss, G.; Rusu, G.; Peter, F.; Tanase, I.; Bandur, G. Recovery of flexible polyurethane foam waste for efficient reuse in industrial formulations. Polymers 2020, 12, 1533. [Google Scholar] [CrossRef]
- Beneš, H.; Rösner, J.; Holler, P.; Synková, H.; Kotek, J.; Horák, Z. Glycolysis of flexible polyurethane foam in recycling of car seats. Polym. Adv. Technol. 2007, 18, 149–156. [Google Scholar] [CrossRef]
- Vanbergen, T.; Verlent, I.; De Geeter, J.; Haelterman, B.; Claes, L.; De Vos, D. Recycling of flexible polyurethane foam by split-phase alcoholysis: Identification of additives and alcoholyzing agents to reach higher efficiencies. ChemSusChem 2020, 13, 3835–3843. [Google Scholar] [CrossRef]
- Molero, C.; Ramos, M.J.; De Lucas, A.; Rodríguez, J.F. Chemical recovery of flexible polyurethane foam wastes. WIT Trans. Ecol. Environ. 2010, 140, 69–78. [Google Scholar] [CrossRef]
- PUReSmart. PUReSmart project in a nutshell. Available online: https://www.puresmart.eu (accessed on 16 May 2023).
- Zamani, S.; Lange, J.P.; Kersten, S.R.A.; Ruiz, M.P. Polyurethane Recycling: Conversion of Carbamates—Catalysis, Side-Reactions and Mole Balance. Polymers 2022, 14, 4869. [Google Scholar] [CrossRef]
- Bernhard, A.M.; Peitz, D.; Elsener, M.; Wokaun, A.; Kröcher, O. Hydrolysis and thermolysis of urea and its decomposition byproducts biuret, cyanuric acid and melamine over anatase TiO2. Appl. Catal. B Environ. 2012, 115–116, 129–137. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Dietz, E.; Thielen, D.; Anspach, B.; Brauer, J. Study of the urea thermal decomposition (pyrolysis) reaction and importance to cyanuric acid production. Am. Lab. 1999, 31, 13–21. [Google Scholar]
- Lakra, H.; Dains, F.B. The action of phenyl isocyanate on urethans, ureas and thioureas. J. Am. Chem. Soc. 1929, 51, 2220–2225. [Google Scholar] [CrossRef]
- Davis, T.L.; Blanchard, K.C. The urea dearrangement. II. J. Am. Chem. Soc. 1923, 45, 1816–1820. [Google Scholar] [CrossRef]
- Hegarty, A.F.; Hegarty, C.N.; Scott, F.L. The key role of zwitterionic species in the conversion of ureas into isocyanates in aqueous solution. Hydrolysis of 1-phenylcarbamoylimidazole. J. Chem. Soc. Perkin Trans. 1974, 2, 1258–1268. [Google Scholar] [CrossRef]
- Skuches, G.S.; Carleton, P.S. Correlation of urea structure with thermal stability in model compounds. J. Appl. Polym. Sci. 1984, 29, 3431–3443. [Google Scholar] [CrossRef]
- Tischer, S.; Bö, M.; Amsler, J.; Nter Schoch, G.; Deutschmann, O. Thermodynamics and reaction mechanism of urea decomposition. Phys. Chem. Chem. Phys. 2019, 21, 16785. [Google Scholar] [CrossRef]
- Lundström, A.; Andersson, B.; Olsson, L. Urea thermolysis studied under flow reactor conditions using DSC and FT-IR. Chem. Eng. J. 2009, 150, 544–550. [Google Scholar] [CrossRef]
- Magee, E.M.; Daniels, F. The Kinetics and Carbon-13 Isotope Effect in the Decomposition of Substituted Ureas. J. Am. Chem. Soc. 1957, 79, 829–832. [Google Scholar] [CrossRef]
- Shaw, W.H.; Grushkin, B. Kinetic Studies of Urea Derivatives. I. Methylurea. J. Am. Chem. Soc. 1960, 82, 1022–1024. [Google Scholar] [CrossRef]
- Ozaki, S.; Mukaiyama, T.; Uno, K. On the Thermal Dissociation of Organic Compounds. XIII. The Effect of Ring Size on the Rate of the Thermal Dissociation of Cyclopolymethyleneureas. J. Am. Chem. Soc. 1957, 79, 4358–4360. [Google Scholar] [CrossRef]
- Stradella, L.; Argentero, M. A DSC, TG, IR study of the thermal decomposition of some alkyl- and aryl-ureas. Thermochim. Acta 1995, 268, 1–7. [Google Scholar] [CrossRef]
- Bennet, W.B.; Saunders, J.H.; Hardy, E.E. The Preparation of Isocyanates by the Thermal Decomposition of Substituted Ureas. J. Am. Chem. Soc. 1953, 75, 2101–2103. [Google Scholar] [CrossRef]
- Hoshino, T.; Mukaiyama, T.; Hoshino, H. On the Thermal Dissociation of Organic Compounds. II. The Dissociation of the Urea Linkage in Sym-dimethylurea, Asym-dimethylurea and Asym-phenylethylurea in Butyric Acid. Bull. Chem. Soc. Jpn. 1952, 25, 392–395. [Google Scholar] [CrossRef]
- Špírková, M.; Kubín, M.; Dušek, K. Side reactions in the formation of polyurethanes: Stability of reaction products of phenyl isocyanate. J. Macromol. Sci. Part A-Chem. 1990, 27, 509–522. [Google Scholar] [CrossRef]
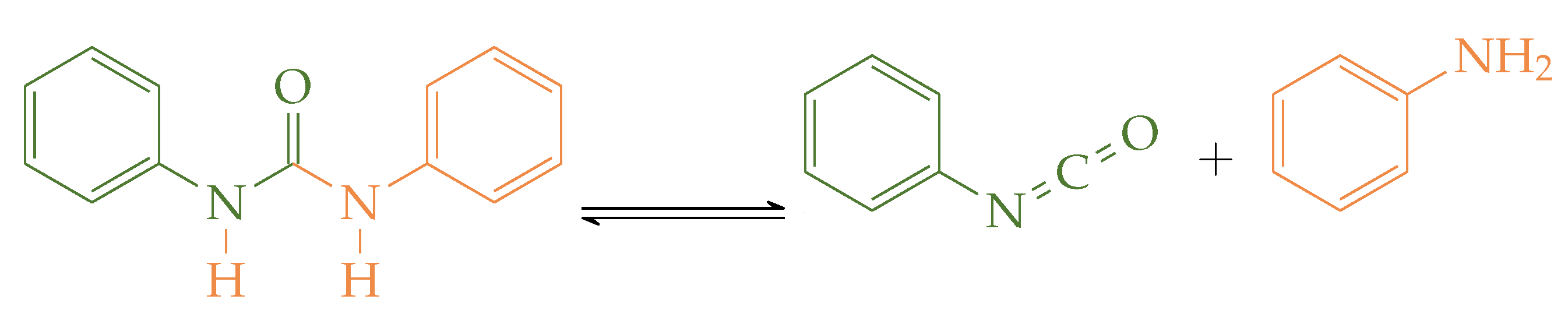
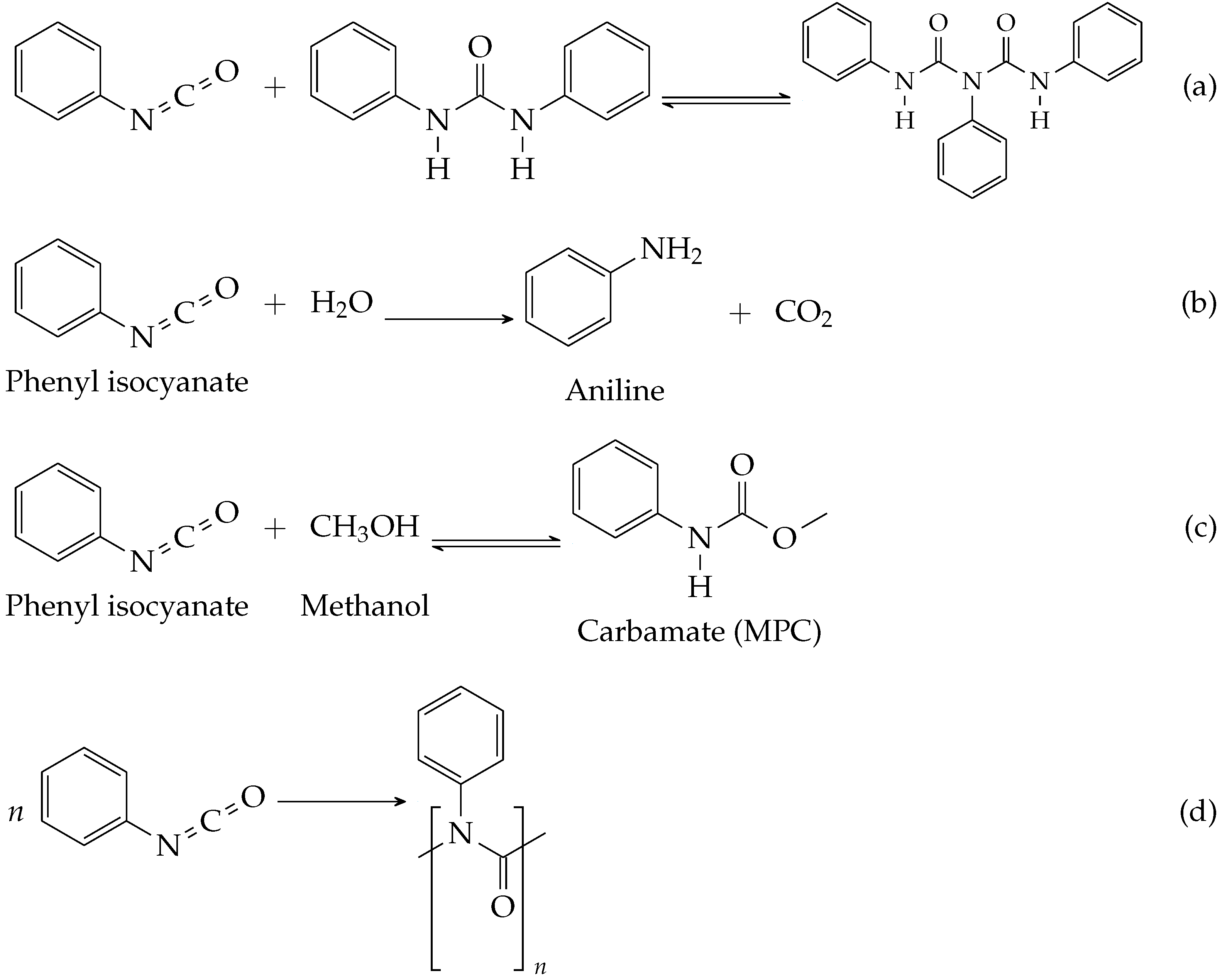
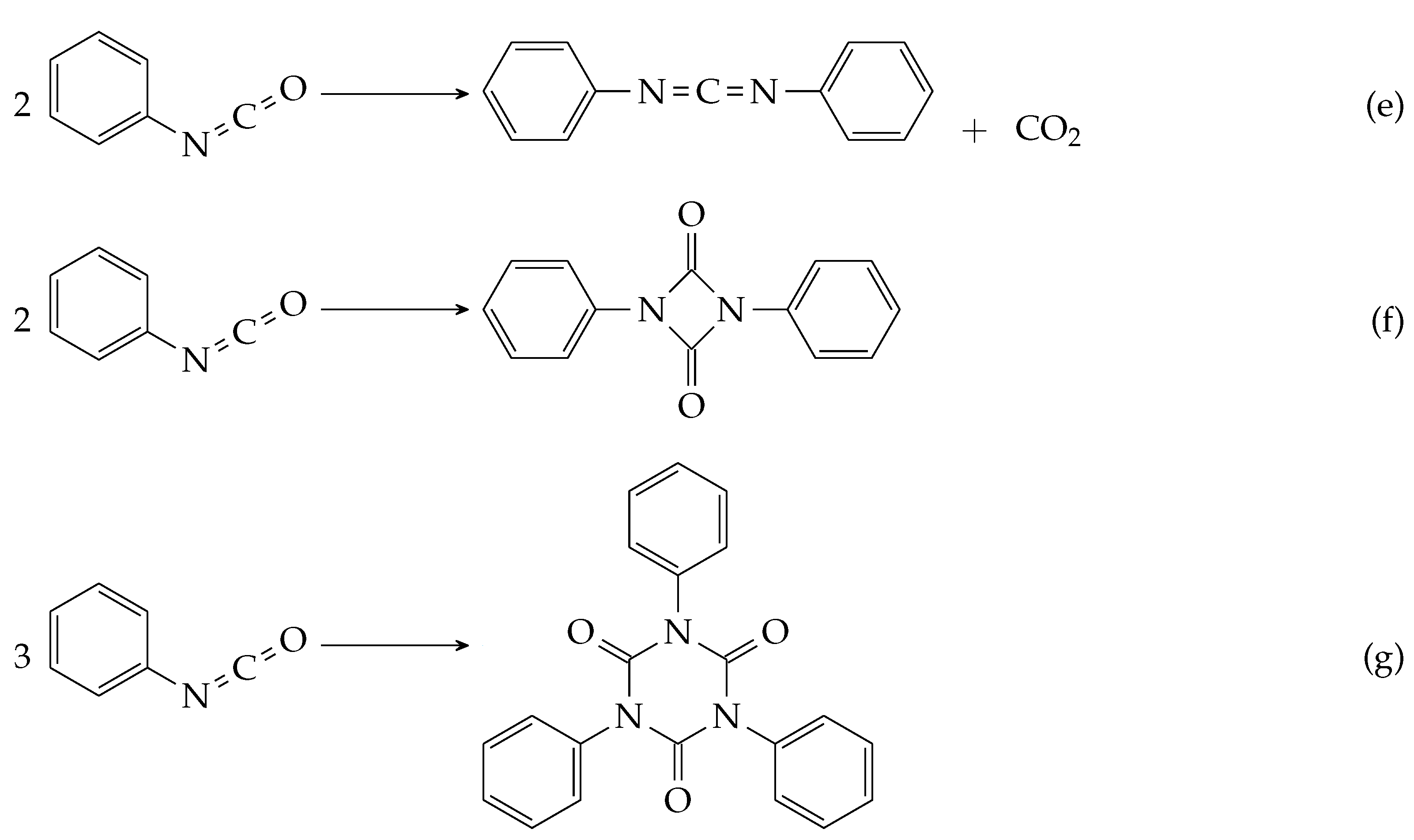
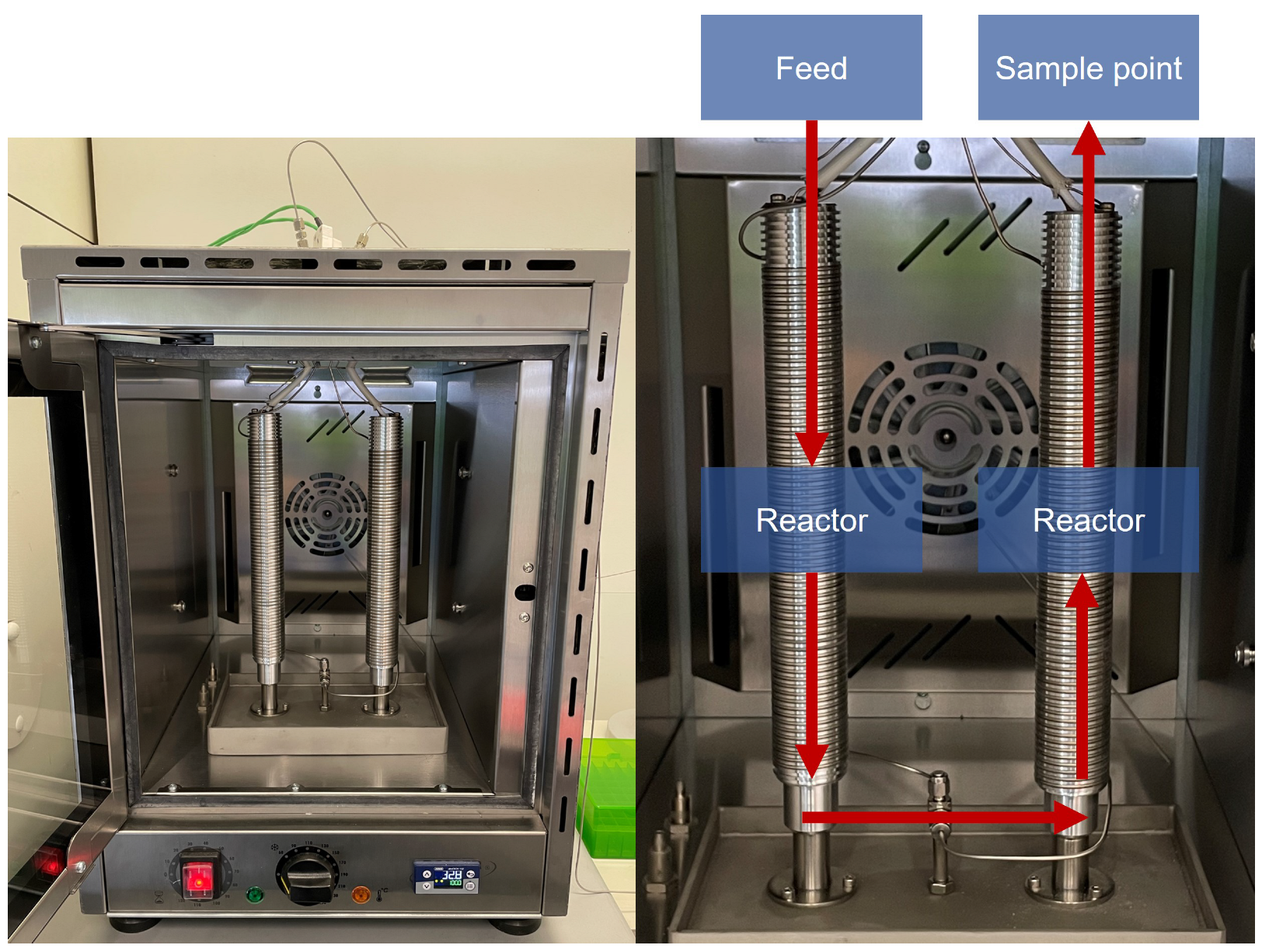
| Source | Solvent | Temperature | Open/Closed | Phenyl Isocyanate |
|---|---|---|---|---|
| System | Formation? | |||
| Stradella and Argentero [28] | – | >240 °C | Closed | Yes |
| Bennet et al. [29] | – | 350–370 °C | Open | Yes (99% DPU conversion; 58% PI yield; 96% aniline yield) |
| Hoshino et al. [30] | Anhydrous acetic acid | 110 °C | Closed | Yes |
| Acetic acid + water | Yes, but it hydrolyzes | |||
| Fatty acids | No | |||
| Špírková et al. [31] | 1,4-dioxane (+ traces of water) | 120 °C | Closed | Yes, but it hydrolyzes |
| Unit | Characteristics | Experimental Conditions |
|---|---|---|
| Pump | Flow rate = 0.1–12 mL/min | Flow rate = 1 mL/min |
| Oven | T = 25–150 °C | T = 100 °C |
| Spirals (Each) | L = 6 m; ID = 0.8763 mm; | - |
| V = 3.62 mL 1 | ||
| Spirals (Each) | T(max) = 500 °C | T = 350–450 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamani, S.; van der Voort, S.H.E.; Lange, J.-P.; Kersten, S.R.A.; Ruiz, M.P. Polyurethane Recycling: Thermal Decomposition of 1,3-Diphenyl Urea to Isocyanates. Polymers 2023, 15, 2522. https://doi.org/10.3390/polym15112522
Zamani S, van der Voort SHE, Lange J-P, Kersten SRA, Ruiz MP. Polyurethane Recycling: Thermal Decomposition of 1,3-Diphenyl Urea to Isocyanates. Polymers. 2023; 15(11):2522. https://doi.org/10.3390/polym15112522
Chicago/Turabian StyleZamani, Shahab, Sterre H. E. van der Voort, Jean-Paul Lange, Sascha R. A. Kersten, and M. Pilar Ruiz. 2023. "Polyurethane Recycling: Thermal Decomposition of 1,3-Diphenyl Urea to Isocyanates" Polymers 15, no. 11: 2522. https://doi.org/10.3390/polym15112522





