Modification of the Physical Properties of a Nafion Film Due to Inclusion of n-Dodecyltriethylammonium Cation: Time Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Structural and Chemical Surface Characterization
2.3. Electrical Characterization
2.4. Elastic Characterization
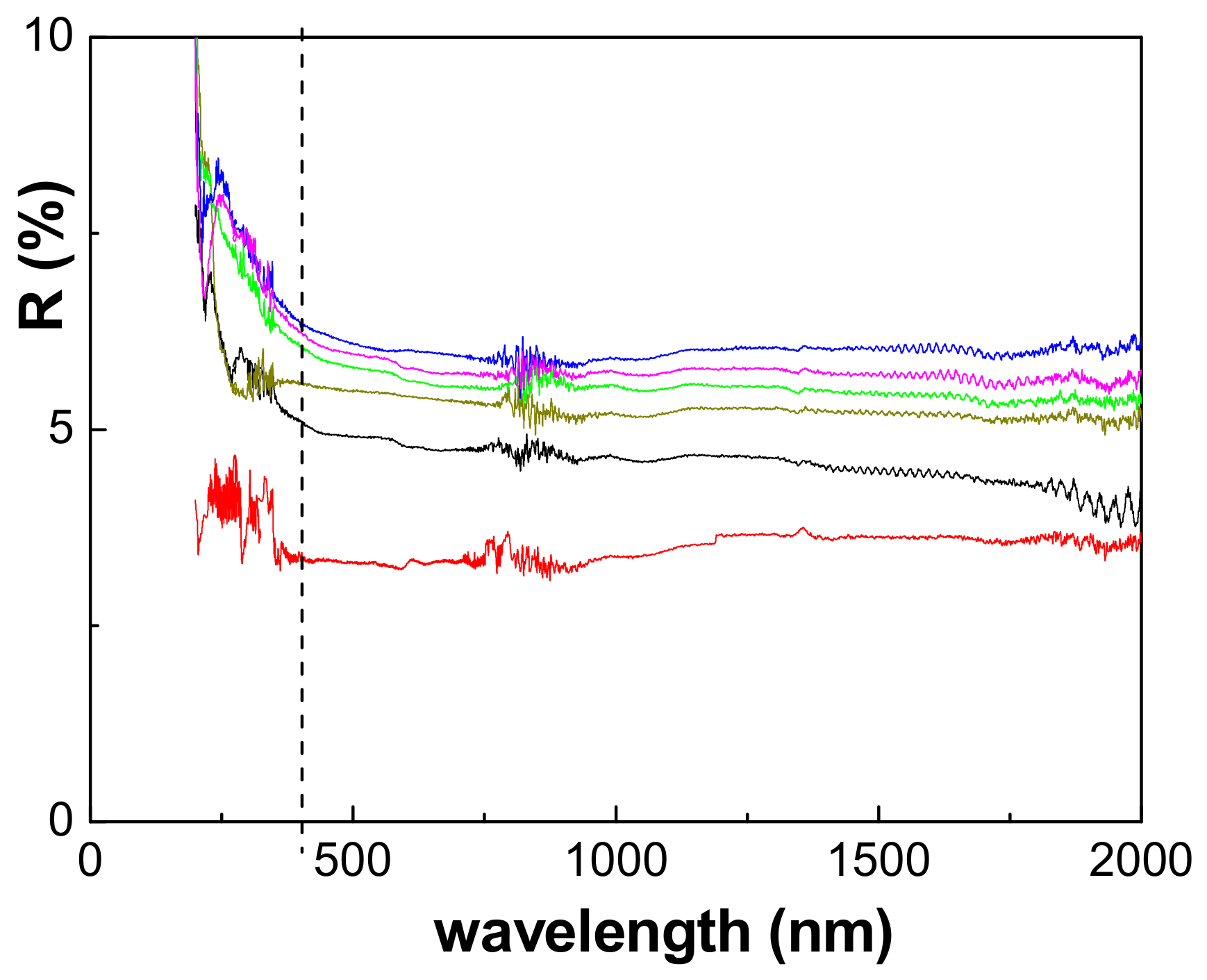
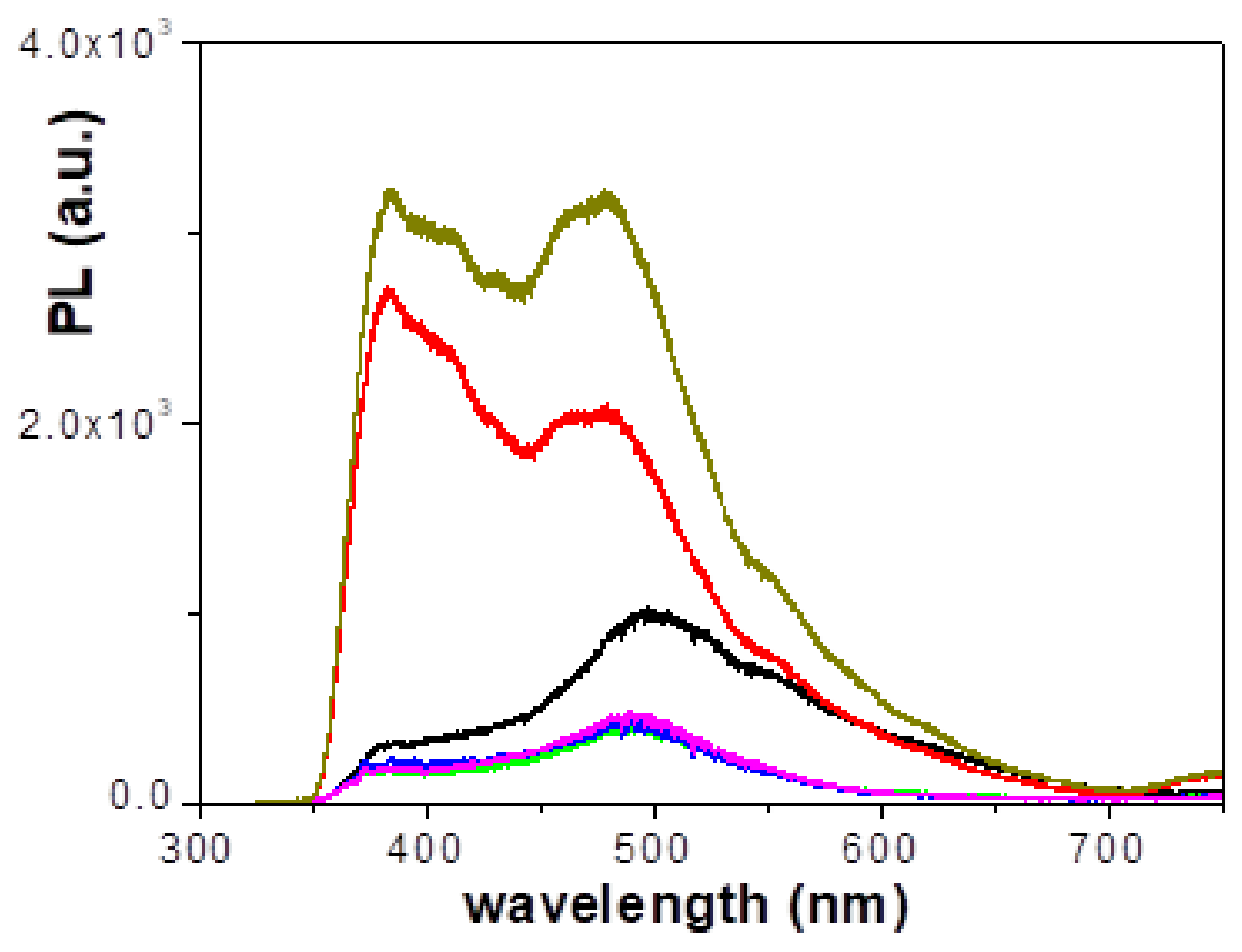
2.5. Optical Characterization
3. Results
3.1. XRD and XPS Characterization
3.2. Physicochemical and Electrical Characterization of the Thin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Quan, N.D.; Hwang, J.M.; Lee, S.D.; Kim, H.; Lee, H.; Kim, H.S. Polymer Electrolyte Membranes for Fuel Cells. J. Ind. Eng. Chem. 2006, 12, 175–183. [Google Scholar] [CrossRef]
- Paul, D.K.; Fraser, A.; Karan, K. Towards the Understanding of Proton Conduction Mechanism in PEMFC Catalyst Layer: Conductivity of Adsorbed Nafion Films. Electrochem. Commun. 2011, 13, 774–777. [Google Scholar] [CrossRef]
- Napoli, L.; Franco, J.; Fasoli, H.; Sanguinetti, A. Conductivity of Nafion® 117 Membrane Used in Polymer Electrolyte Fuel Cells. Int. J. Hydrogen Energy 2014, 39, 8656–8660. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent Approaches to Improve Nafion Performance for Fuel Cell Applications: A Review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Li, Y.C.; Liu, J.; He, J.; Wang, L.Y.; Lei, J. Du Recent Developments in High-Performance Nafion Membranes for Hydrogen Fuel Cells Applications. Pet. Sci. 2022, 19, 1371–1381. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An Overview of Proton Exchange Membranes for Fuel Cells: Materials and Manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Wei, Y.; Li, S.; Zhang, X.; Fu, Y.; Chen, K. Smart Devices Based on the Soft Actuator with Nafion-Polypropylene-PDMS/Graphite Multilayer Structure. Appl. Sci. 2020, 10, 1829. [Google Scholar] [CrossRef]
- Santos, J.S.; Raimundo, I.M.; Cordeiro, C.M.B.; Biazoli, C.R.; Gouveia, C.A.J.; Jorge, P.A.S. Characterisation of a Nafion Film by Optical Fibre Fabry-Perot Interferometry for Humidity Sensing. Sens. Actuators B Chem. 2014, 196, 99–105. [Google Scholar] [CrossRef]
- Li, L.; Tian, X.; Meng, D.; Collins, M.; Wang, J.; Patterson, R.; Nguyen, L.; Yi, X. Processing, Characterization, and Impact of Nafion Thin Film on Photonic Nanowaveguides for Humidity Sensing. Adv. Photonics Res. 2022, 3, 2100181. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Shkirin, A.V.; Kozlov, V.A.; Ninham, B.W.; Uspenskaya, E.V.; Gudkov, S.V. Near-Surface Structure of Nafion in Deuterated Water. J. Chem. Phys. 2018, 149, 164901. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Bashkina, U.A.; Bolikov, N.G.; Bereza, I.S.; Molchanov, I.I.; Kozlov, V.A. Study of the Luminescence from Polymeric Membrane Swollen in Water with Various Content of Deuterium; Isotopic Effects. J. Phys. Conf. Ser. 2019, 1348, 012030. [Google Scholar] [CrossRef]
- Martínez De Yuso, M.V.; Neves, L.A.; Coelhoso, I.M.; Crespo, J.G.; Benavente, J.; Rodríguez-Castellõn, E. A Study of Chemical Modifications of a Nafion Membrane by Incorporation of Different Room Temperature Ionic Liquids. Fuel Cells 2012, 12, 606–613. [Google Scholar] [CrossRef]
- Satterfield, M.B.; Majsztrik, P.W.; Ota, H.; Benziger, J.B.; Bocarsly, A.B. Mechanical Properties of Nafion and Titania/Nafion Composite Membranes for Polymer Electrolyte Membrane Fuel Cells. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2327–2345. [Google Scholar] [CrossRef]
- Simonov, A.S.; Kondratenko, M.S.; Elmanovich, I.V.; Sizov, V.E.; Kharitonova, E.P.; Abramchuk, S.S.; Nikolaev, A.Y.; Fedosov, D.A.; Gallyamov, M.O.; Khokhlov, A.R. Modification of Nafion with Silica Nanoparticles in Supercritical Carbon Dioxide for Electrochemical Applications. J. Memb. Sci. 2018, 564, 106–114. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hossain, O.; Chaggar, J.; Steinberger-Wilckens, R.; El-Kharouf, A. GO-Nafion Composite Membrane Development for Enabling Intermediate Temperature Operation of Polymer Electrolyte Fuel Cell. Int. J. Hydrogen Energy 2020, 45, 5526–5534. [Google Scholar] [CrossRef]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Ṋemavhola, F.; Nonjola, P.F.; Msomi, P.F. The Proton Conductivity and Mechanical Properties of Nafion®/ ZrP Nanocomposite Membrane. Heliyon 2019, 5, e02240. [Google Scholar] [CrossRef]
- Bertoncello, P.; Peruffo, M.; Unwin, P.R. Formation and Evaluation of Electrochemically-Active Ultra-Thin Palladium-Nafion Nanocomposite Films. Chem. Commun. 2007, 16, 1597–1599. [Google Scholar] [CrossRef]
- Domènech, B.; Romero, V.; Vázquez, M.I.; Avila, M.; Benavente, J.; Muñoz, M.; MacAnás, J. Chemical and Electrochemical Characterization of Nafion Containing Silver Nanoparticles in a Stripe-like Distribution. RSC Adv. 2016, 6, 9923–9931. [Google Scholar] [CrossRef]
- Neves, L.A.; Coelhoso, I.M.; Crespo, J.G. Methanol and Gas Crossover through Modified Nafion Membranes by Incorporation of Ionic Liquid Cations. J. Memb. Sci. 2010, 360, 363–370. [Google Scholar] [CrossRef]
- Yuyama, K.; Masuda, G.; Yoshida, H.; Sato, T. Ionic Liquids Containing the Tetrafluoroborate Anion Have the Best Performance and Stability for Electric Double Layer Capacitor Applications. J. Power Sources 2006, 162, 1401–1408. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-Liquid Materials for the Electrochemical Challenges of the Future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bostrom, T. Application of Ionic Liquids in Solar Cells and Batteries: A Review. Curr. Org. Chem. 2015, 19, 556–566. [Google Scholar] [CrossRef]
- Algarra, M.; López Escalante, M.C.; Martínez de Yuso, M.V.; Soto, J.; Cuevas, A.L.; Benavente, J. Nanoporous Alumina Support Covered by Imidazole Moiety–Based Ionic Liquids: Optical Characterization and Application. Nanomaterials 2022, 12, 4131. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. [Google Scholar] [CrossRef]
- Schäfer, T.; Di Paolo, R.E.; Franco, R.; Crespo, J.G. Elucidating Interactions of Ionic Liquids with Polymer Films Using Confocal Raman Spectroscopy. Chem. Commun. 2005, 20, 2594–2596. [Google Scholar] [CrossRef]
- Neves, L.A.; Benavente, J.; Coelhoso, I.M.; Crespo, J.G. Design and Characterisation of Nafion Membranes with Incorporated Ionic Liquids Cations. J. Memb. Sci. 2010, 347, 42–52. [Google Scholar] [CrossRef]
- De Yuso, M.D.V.M.; Cuberes, M.T.; Romero, V.; Neves, L.; Coelhoso, I.; Crespo, J.G.; Rodríguez-Castellón, E.; Benavente, J. Modification of a Nafion Membrane by N-Dodecyltrimethylammonium Cation Inclusion for Potential Application in DMFC. Int. J. Hydrogen Energy 2014, 39, 4023–4029. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Minneapolis, MN, USA, 1992. [Google Scholar]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Istomina, A.S.; Yaroslavtseva, T.V.; Reznitskikh, O.G.; Kayumov, R.R.; Shmygleva, L.V.; Sanginov, E.A.; Dobrovolsky, Y.A.; Bushkova, O.V. Li-Nafion Membrane Plasticised with Ethylene Carbonate/Sulfolane: Influence of Mixing Temperature on the Physicochemical Properties. Polymers 2021, 13, 1150. [Google Scholar] [CrossRef]
- Ostrowska, J.; Narębska, A. Infrared Study of Hydration and Association of Functional Groups in a Perfluormated Nation Membrane.—Part 2. Colloid Polym. Sci. 1984, 262, 305–310. [Google Scholar] [CrossRef]
- Hwang, T.; Palmre, V.; Nam, J.; Lee, D.C.; Kim, K.J. A New Ionic Polymer-Metal Composite Based on Nafion/Poly(Vinyl Alcohol-Co-Ethylene) Blends. Smart Mater. Struct. 2015, 24, 105011. [Google Scholar] [CrossRef]
- Zakharova, J.A.; Novoskoltseva, O.A.; Pyshkina, O.A.; Karpushkin, E.A.; Sergeyev, V.G. Controlled Modification of Nafion Membrane with Cationic Surfactant. Colloid Polym. Sci. 2018, 296, 835–846. [Google Scholar] [CrossRef]
- Ariza, M.J.; Benavente, J.; Rodríguez-Castellón, E. The Capability of X-Ray Photoelectron Spectroscopy in the Characterization of Membranes: Correlation between Surface Chemical and Transport Properties in Polymeric Membranes. In Handbook of Membranes: Properties, Performance and Applications; Nova Science: New York, NY, USA, 2009. [Google Scholar]
- Fontàs, C.; Vera, R.; Anticó, E.; de Yuso, M.D.V.M.; Rodríguez-Castellón, E.; Benavente, J. New Insights on the Effects of Water on Polymer Inclusion Membranes Containing Aliquat 336 Derivatives as Carriers. Membranes 2022, 12, 192. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Johnson, W.B. Fundamentals of Impedance Spectroscopy in Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; Wiley Online Library: Hoboken, NJ, USA, 2018. [Google Scholar]
- Benavente, J. Use of Impedance Spectroscopy for Characterization of Membranes and the Effect of Different Modifications. In Membrane Modification: Technology and Applications; Hilal, N., Khayet, M., Wright, C.J., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 21–40. [Google Scholar]
- Marrero-López, D.; Ruiz-Morales, J.C.; Peña-Martínez, J.; Martín-Sedeño, M.C.; Ramos-Barrado, J.R. Influence of Phase Segregation on the Bulk and Grain Boundary Conductivity of LSGM Electrolytes. Solid State Ion. 2011, 186, 44–52. [Google Scholar] [CrossRef]
- González, A.S.; Vega, V.; Cuevas, A.L.; de Yuso, M.D.V.M.; Prida, V.M.; Benavente, J. Surface Modification of Nanoporous Anodic Alumina during Self-Catalytic Atomic Layer Deposition of Silicon Dioxide from (3-Aminopropyl)Triethoxysilane. Materials 2021, 14, 5052. [Google Scholar] [CrossRef]
- West, A.; Irvine, J.; Sinclair, D. Electroceramics: Characterization by Impedance Spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar]
- Yuso, M.; Cuevas, A.L.; Benavente, J. Physicochemical Characterization of a Cellulosic Film Modified with Two Room-Temperature Ionic Liquids. Appl. Sci. 2022, 12, 10290. [Google Scholar] [CrossRef]
- Shi, S.; Chen, G.; Wang, Z.; Chen, X. Mechanical Properties of Nafion 212 Proton Exchange Membrane Subjected to Hygrothermal Aging. J. Power Sources 2013, 238, 318–323. [Google Scholar] [CrossRef]
- Algarra, M.; Cuevas, A.L.; de Yuso, M.V.M.; Romero, R.; Alonso, B.; Casado, C.M.; Benavente, J. Optical and Physicochemical Characterizations of a Cellulosic/CdSe-QDs@S-DAB5 Film. Nanomaterials 2022, 12, 484. [Google Scholar] [CrossRef] [PubMed]
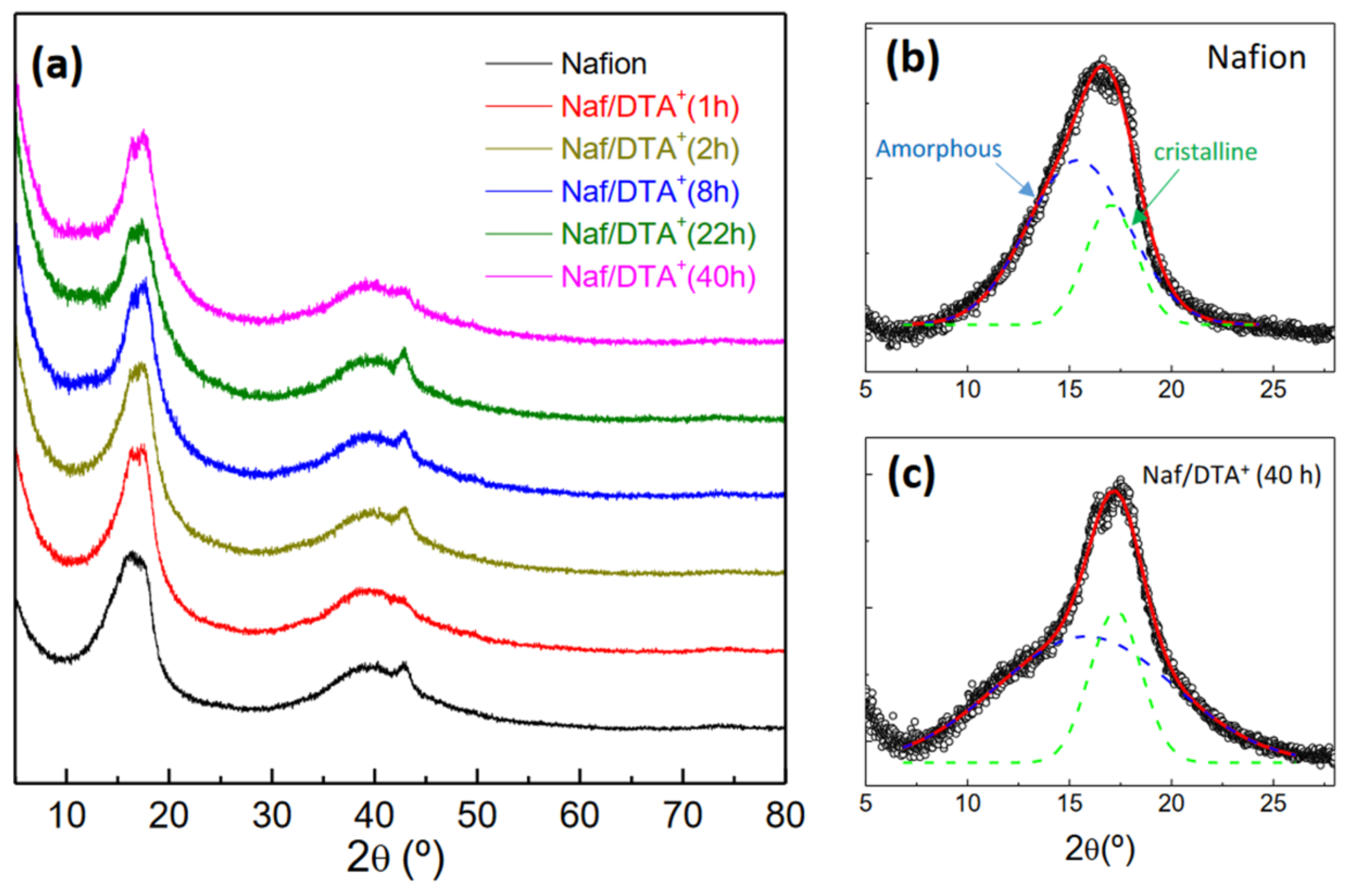

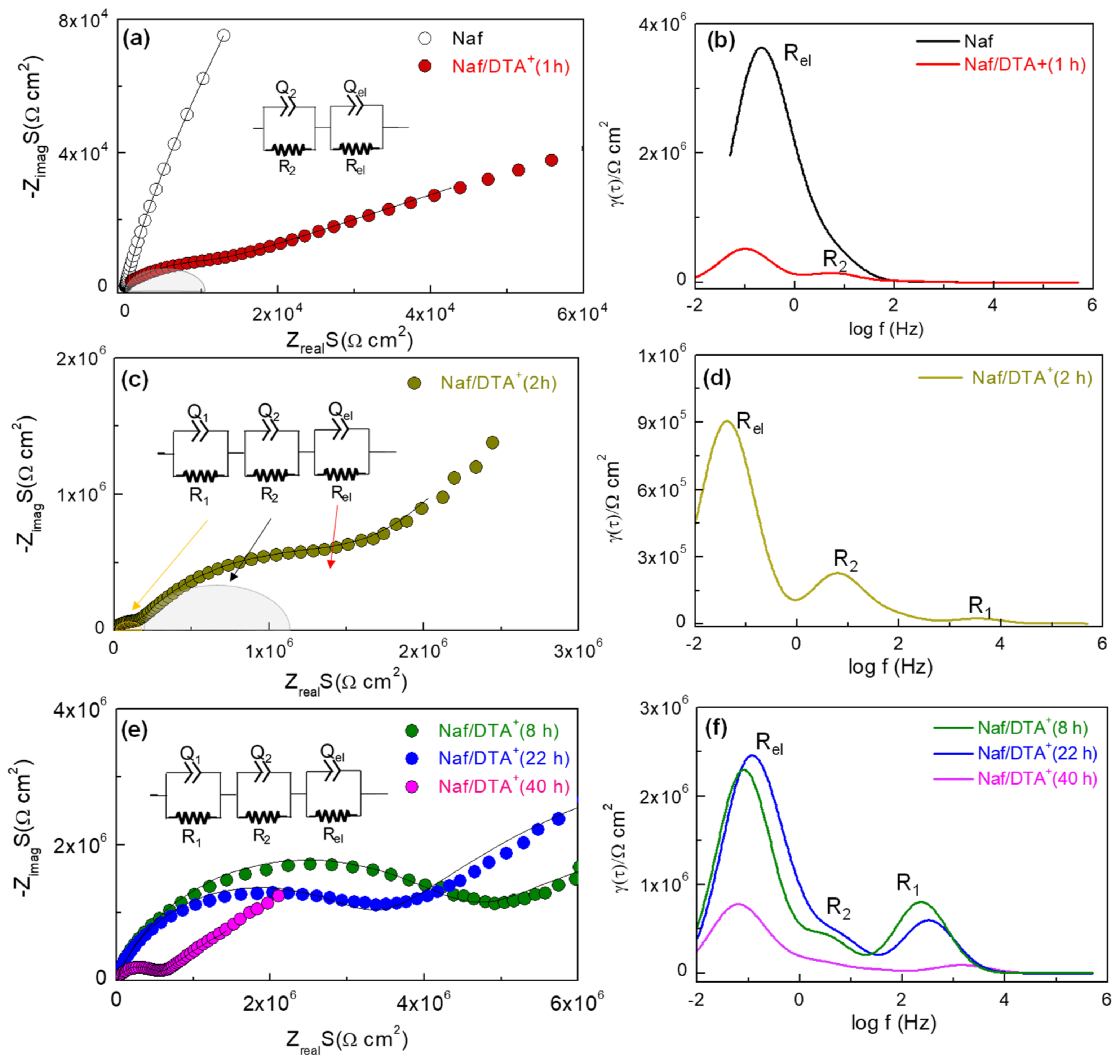

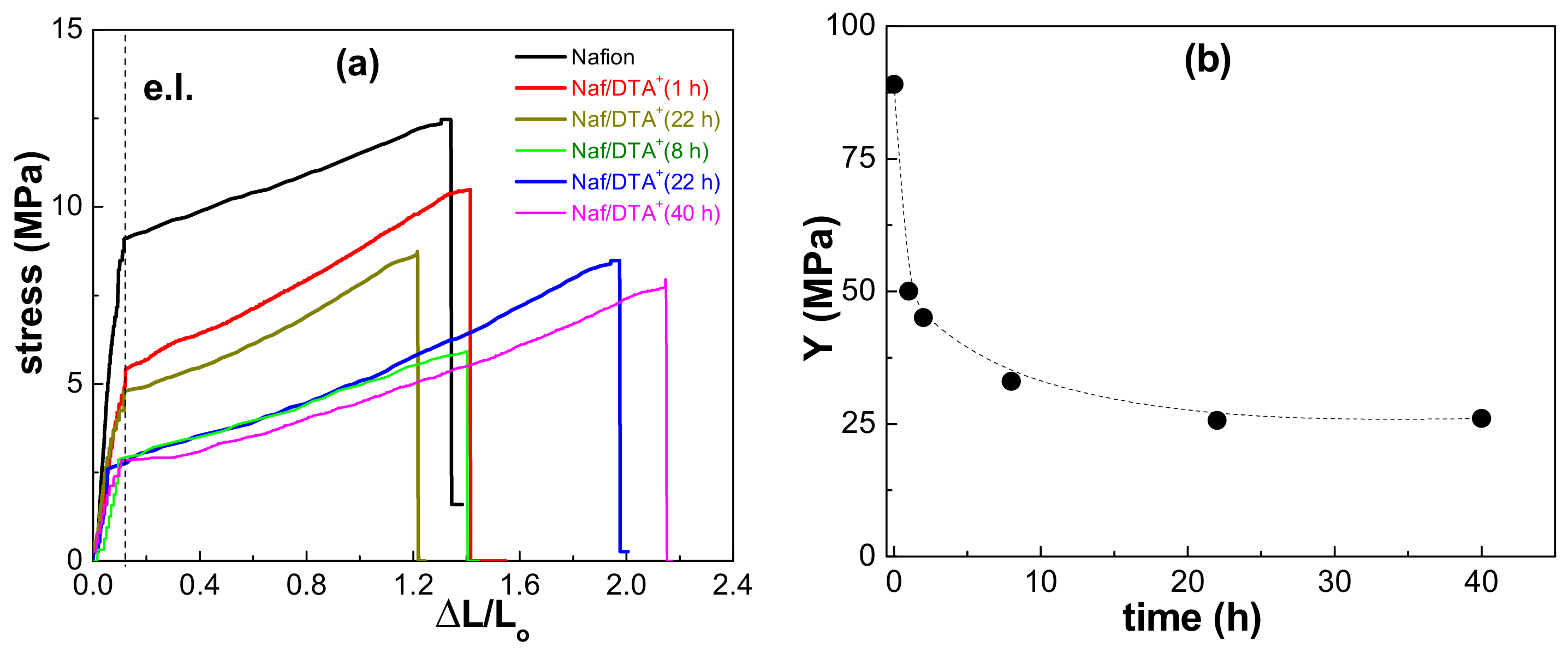
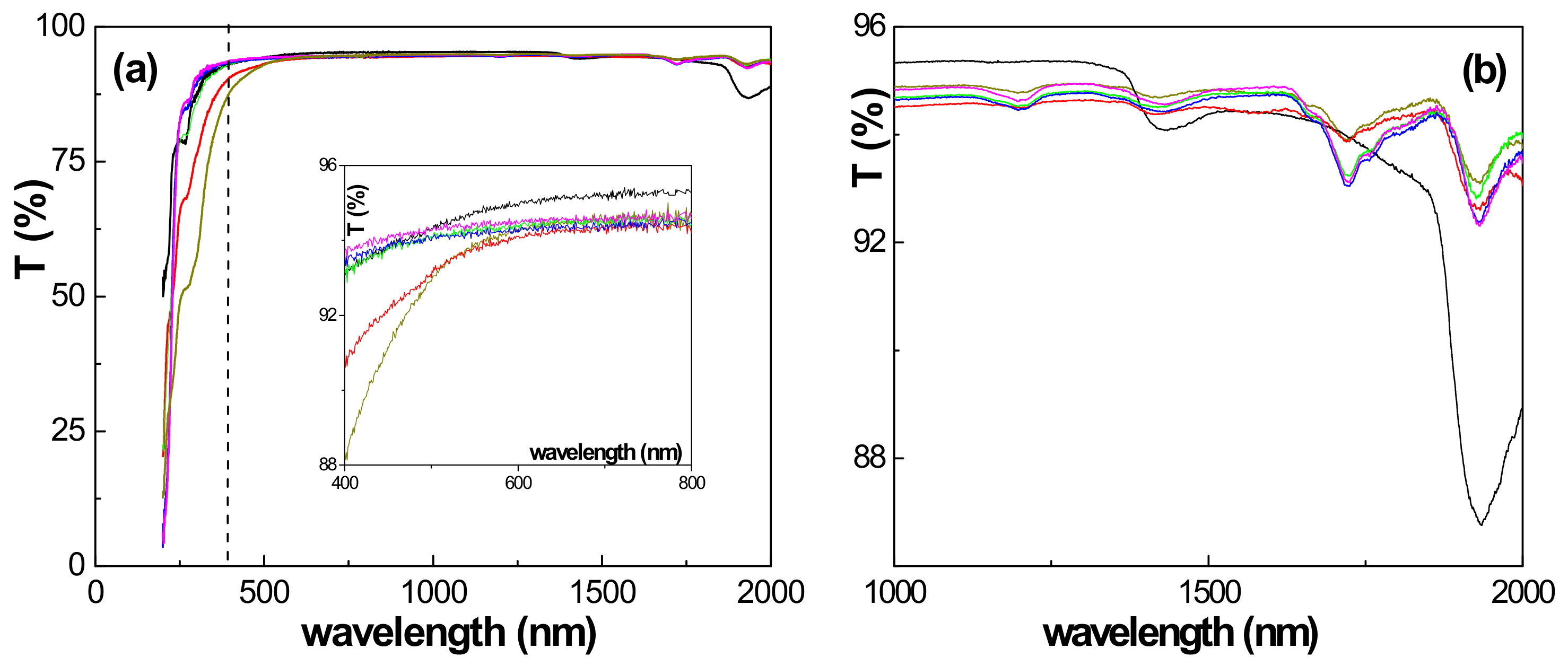
| Sample | F 1s (%) | C 1s (%) | O 1s (%) | S 2p (%) | N 1s (%) |
|---|---|---|---|---|---|
| Nafion-212 | 50.4 | 41.8 | 6.9 | 0.84 | - |
| Naf/DTA+ (1 h) | 29.5 | 56.8 | 9.7 | 0.95 | 1.0 |
| Naf/DTA+ (2 h) | 29.7 | 58.5 | 8.8 | 1.11 | 1.5 |
| Naf/DTA+ (8 h) | 36.3 | 49.4 | 8.7 | 1.26 | 1.9 |
| Naf/DTA+ (22 h) * | 39.0 | 47.2 | 8.2 | 1.14 | 1.6 |
| Sample | CA (%) | CB (%) | CC (%) | CD (%) | CE (%) | OA (%) | OB (%) | NA (%) | NB (%) |
|---|---|---|---|---|---|---|---|---|---|
| Nafion | 24.3 | 9.3 | 1.5 | 3.1 | 3.2 | 0.37 | 0.43 | - | - |
| Naf/DTA+ (1 h) | 12.0 | 37.9 | 4.7 | 1.3 | 0.9 | 0.21 | 0.79 | 1.0 | - |
| Naf/DTA+ (2 h) | 11.3 | 36.7 | 6.9 | 1.3 | 1.1 | 0.25 | 0.85 | 1.2 | 0.3 |
| Naf/DTA+ (8 h) | 15.1 | 24.1 | 7.4 | 1.8 | 1.1 | 0.26 | 1.03 | 1.5 | 0.4 |
| Naf/DTA+ (22 h) | 18.3 | 19.6 | 6.3 | 2.0 | 1.0 | 0.24 | 0.76 | 1.0 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamudio-García, J.; Martínez de Yuso, M.V.; Cuevas, A.L.; Marrero-López, D.; Benavente, J. Modification of the Physical Properties of a Nafion Film Due to Inclusion of n-Dodecyltriethylammonium Cation: Time Effect. Polymers 2023, 15, 2527. https://doi.org/10.3390/polym15112527
Zamudio-García J, Martínez de Yuso MV, Cuevas AL, Marrero-López D, Benavente J. Modification of the Physical Properties of a Nafion Film Due to Inclusion of n-Dodecyltriethylammonium Cation: Time Effect. Polymers. 2023; 15(11):2527. https://doi.org/10.3390/polym15112527
Chicago/Turabian StyleZamudio-García, Javier, María V. Martínez de Yuso, Ana L. Cuevas, David Marrero-López, and Juana Benavente. 2023. "Modification of the Physical Properties of a Nafion Film Due to Inclusion of n-Dodecyltriethylammonium Cation: Time Effect" Polymers 15, no. 11: 2527. https://doi.org/10.3390/polym15112527
APA StyleZamudio-García, J., Martínez de Yuso, M. V., Cuevas, A. L., Marrero-López, D., & Benavente, J. (2023). Modification of the Physical Properties of a Nafion Film Due to Inclusion of n-Dodecyltriethylammonium Cation: Time Effect. Polymers, 15(11), 2527. https://doi.org/10.3390/polym15112527








