Understanding the Mechanical, Surface, and Color Behavior of Oral Bioactive Prosthetic Polymers under Biodegradation Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Resin-Based Polymers Preparation
2.2. Aging Processes
2.3. Resin-Based Polymers Characterization
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krausch-Hofmann, S.; Cuypers, L.; Ivanova, A.; Duyck, J. Predictors of Patient Satisfaction with Removable Denture Renewal: A Pilot Study. J. Prosthodont. 2018, 27, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J. Revisiting the Removable Partial Denture. Dent. Clin. N. Am. 2019, 63, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Fueki, K.; Ohkubo, C.; Yatabe, M.; Arakawa, I.; Arita, M.; Ino, S.; Kanamori, T.; Kawai, Y.; Kawara, M.; Komiyama, O.; et al. Clinical application of removable partial dentures using thermoplastic resin. Part II: Material properties and clinical features of non-metal clasp dentures. J. Prosthodont. Res. 2014, 58, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Lee, J.H.; Yang, T.H.; Kim, Y.J.; Kim, S.C.; Kim, G.R.; Kim, H.R.; Lee, C.J.; Okubo, C. Evaluation of the flexural mechanical properties of various thermoplastic denture base polymers. Dent. Mater. J. 2018, 37, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Marra, J.; Paleari, A.G.; Rodriguez, L.S.; Leite, A.R.; Pero, A.C.; Compagnoni, M.A. Effect of an acrylic resin combined with an antimicrobial polymer on biofilm formation. J. Appl. Oral Sci. 2012, 20, 643–648. [Google Scholar] [CrossRef]
- Figueiral, M.H.; Fonseca, P.; Lopes, M.M.; Pinto, E.; Pereira-Leite, T.; Sampaio-Maia, B. Effect of Denture-Related Stomatitis Fluconazole Treatment on Oral Candida albicans Susceptibility Profile and Genotypic Variability. Open Dent. J. 2015, 9, 46–51. [Google Scholar] [CrossRef]
- Dagistan, S.; Aktas, A.E.; Caglayan, F.; Ayyildiz, A.; Bilge, M. Differential diagnosis of denture-induced stomatitis, Candida, and their variations in patients using complete denture: A clinical and mycological study. Mycoses 2009, 52, 266–271. [Google Scholar] [CrossRef]
- Redding, S.; Bhatt, B.; Rawls, H.R.; Siegel, G.; Scott, K.; Lopez-Ribot, J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 669–672. [Google Scholar] [CrossRef]
- Gomes, A.S.; Sampaio-Maia, B.; Vasconcelos, M.; Fonesca, P.A.; Figueiral, H. In situ evaluation of the microbial adhesion on a hard acrylic resin and a soft liner used in removable prostheses. Int. J. Prosthodont. 2015, 28, 65–71. [Google Scholar] [CrossRef]
- Salim, N.; Moore, C.; Silikas, N.; Satterthwaite, J.D.; Rautemaa, R. Fungicidal amounts of antifungals are released from impregnated denture lining material for up to 28 days. J. Dent. 2012, 40, 506–512. [Google Scholar] [CrossRef]
- Ryalat, S.; Darwish, R.; Amin, W. New form of administering chlorhexidine for treatment of denture-induced stomatitis. Ther. Clin. Risk Manag. 2011, 7, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Salim, N.; Moore, C.; Silikas, N.; Satterthwaite, J.; Rautemaa, R. Candidacidal effect of fluconazole and chlorhexidine released from acrylic polymer. J. Antimicrob. Chemother. 2013, 68, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Amin, W.M.; Al-Ali, M.H.; Salim, N.A.; Al-Tarawneh, S.K. A new form of intraoral delivery of antifungal drugs for the treatment of denture-induced oral candidosis. Eur. J. Dent. 2009, 3, 257–266. [Google Scholar] [CrossRef]
- Costa, J.; Portugal, J.; Neves, C.B.; Bettencourt, A. Should local drug delivery systems be used in dentistry? Drug Deliv. Transl. Res. 2022, 12, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart dental materials for antimicrobial applications. Bioact. Mater. 2022, 24, 1–19. [Google Scholar] [CrossRef]
- Malakhov, A.; Wen, J.; Zhang, B.X.; Wang, H.; Geng, H.; Chen, X.D.; Sun, Y.; Yeh, C.K. Rechargeable anticandidal denture material with sustained release in saliva. Oral Dis. 2016, 22, 391–398. [Google Scholar] [CrossRef]
- Skupien, J.A.; Valentini, F.; Boscato, N.; Pereira-Cenci, T. Prevention and treatment of Candida colonization on denture liners: A systematic review. J. Prosthet. Dent. 2013, 110, 356–362. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Thobity, A.M. Flexural and Surface Properties of PMMA Denture Base Material Modified with Thymoquinone as an Antifungal Agent. J. Prosthodont. 2020, 29, 243–250. [Google Scholar] [CrossRef]
- Brookes, Z.; Bescos, R.; Belfield, L.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef] [PubMed]
- Salim, N.; Silikas, N.; Satterthwaite, J.D.; Moore, C.; Ramage, G.; Rautemaa, R. Chlorhexidine-impregnated PEM/THFM polymer exhibits superior activity to fluconazole-impregnated polymer against Candida albicans biofilm formation. Int. J. Antimicrob. Agents 2013, 41, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, A.F.; Costa, J.; Ribeiro, I.A.C.; Gonçalves, L.; Arias-Moliz, M.T.; Dias, J.R.; Franco, M.; Alves, N.M.; Portugal, J.; Neves, C.B. Development of a chlorhexidine delivery system based on dental reline acrylic resins. Int. J. Pharm. 2023, 631, 122470. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Arias Moliz, T.; Bettencourt, A.; Costa, J.; Martins, F.; Rabadijeva, D.; Rodriguez, D.; Visai, L.; Combes, C.; Farrugia, C.; et al. Standardization of antimicrobial testing of dental devices. Dent. Mater. 2020, 36, e59–e73. [Google Scholar] [CrossRef] [PubMed]
- Barclay, C.W.; Spence, D.; Laird, W.R. Intra-oral temperatures during function. J. Oral Rehabil. 2005, 32, 886–894. [Google Scholar] [CrossRef]
- Seo, R.S.; Murata, H.; Hong, G.; Vergani, C.E.; Hamada, T. Influence of thermal and mechanical stresses on the strength of intact and relined denture bases. J. Prosthet. Dent. 2006, 96, 59–67. [Google Scholar] [CrossRef]
- Neves, C.B.; Costa, J.; Nepomuceno, L.; Madeira, A.; Portugal, J.; Bettencourt, A. Microhardness and Flexural Strength after Chemical Aging of chlorhexidine delivery systems based on acrylic resin. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2019, 60, 104–110. [Google Scholar] [CrossRef]
- Bettencourt, A.F.; Feliz, M.; Sousa, C.; Gonçalves, L.; Neves, C.B. An acrylic reline resin loaded with chlorhexidine: Insights on drug release. Rev. Port. Estomatol. Cir. Maxilofac. 2016, 57, 125–131. [Google Scholar] [CrossRef]
- Moon, J.D.; Seon, E.M.; Son, S.A.; Jung, K.H.; Kwon, Y.H.; Park, J.K. Effect of immersion into solutions at various pH on the color stability of composite resins with different shades. Restor. Dent. Endod. 2015, 40, 270–276. [Google Scholar] [CrossRef]
- Alhotan, A.; Elraggal, A.; Yates, J.; Haider, J.; Jurado, C.A.; Silikas, N. Effect of Different Solutions on the Colour Stability of Nanoparticles or Fibre Reinforced PMMA. Polymers 2022, 14, 1521. [Google Scholar] [CrossRef]
- Goiato, M.C.; Nobrega, A.S.; dos Santos, D.M.; Andreotti, A.M.; Moreno, A. Effect of different solutions on color stability of acrylic resin-based dentures. Braz. Oral Res. 2014, 28, S1806-83242013005000033. [Google Scholar] [CrossRef] [PubMed]
- Vichi, A.; Ferrari, M.; Davidson, C.L. Color and opacity variations in three different resin-based composite products after water aging. Dent. Mater. 2004, 20, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Pero, A.C.; Ignárcio, J.; Giro, G.; Mendoza-Marin, D.O.; Paleari, A.G.; Compagnoni, M.A. Surface properties and color stability of an acrylic resin combined with an antimicrobial polymer. Rev. Odontol. UNESP 2013, 42, 237–242. [Google Scholar] [CrossRef]
- Rijo, I.; Pedro, D.; Costa, J.; Bettencourt, A.F.; Portugal, J.; Neves, C.B. Chlorhexidine loading of acrylic reline resins—Microhardness and flexural strength after thermal aging. Rev. Port. Estomatol. Cir. Maxilofac. 2018, 59, 154–161. [Google Scholar] [CrossRef]
- ISO 20795-1:2013; Dentistry—Base Polymers—Part 1: Denture Base Polymers, 1st Edition. International Organization for Standardization: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/62277.html (accessed on 2 February 2023).
- Costa, J.; Matos, A.; Bettencourt, A.; Portugal, J.; Neves, C.B. Effect of ethanol solutions as post-polymerization treatment on the properties of acrylic reline resins. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2016, 57, 215–222. [Google Scholar] [CrossRef]
- ISO 7491:2000; Dental Materials: Determination of Colour Stability, 1st Edition. International Organization for Standardization: Geneva, Switzerland, 2000. Available online: https://www.iso.org/standard/26857.html (accessed on 10 April 2023).
- Goiato, M.C.; dos Santos, D.M.; Baptista, G.; Moreno, A.; Andreotti, A.M.; Dekon, S.F. Effect of thermal cycling and disinfection on microhardness of acrylic resin denture base. J. Med. Eng. Technol. 2013, 37, 203–207. [Google Scholar] [CrossRef]
- Polychronakis, N.; Sarafianou, A.; Zissis, A.; Papadopoulos, T. The Influence of Thermocycling on the Flexural Strength of a Polyamide Denture Base Material. Acta Stomatol. Croat. 2017, 51, 309–315. [Google Scholar] [CrossRef]
- Hiraishi, N.; Yiu, C.K.; King, N.M.; Tay, F.R.; Pashley, D.H. Chlorhexidine release and water sorption characteristics of chlorhexidine-incorporated hydrophobic/hydrophilic resins. Dent. Mater. 2008, 24, 1391–1399. [Google Scholar] [CrossRef]
- Gong, K.; Braden, M.; Patel, M.P.; Rehman, I.U.; Zhang, Z.; Darr, J.A. Controlled release of chlorhexidine diacetate from a porous methacrylate system: Supercritical fluid assisted foaming and impregnation. J. Pharm. Sci. 2007, 96, 2048–2056. [Google Scholar] [CrossRef]
- Salim, N.; Satterthwaite, J.D.; Rautemaa, R.; Silikas, N. Impregnation with antimicrobials challenge bonding properties and water sorption behaviour of an acrylic liner. J. Dent. 2012, 40, 693–699. [Google Scholar] [CrossRef]
- Bayraktar, G.; Guvener, B.; Bural, C.; Uresin, Y. Influence of polymerization method, curing process, and length of time of storage in water on the residual methyl methacrylate content in dental acrylic resins. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.S.; Paleari, A.G.; Giro, G.; de Oliveira Junior, N.M.; Pero, A.C.; Compagnoni, M.A. Chemical characterization and flexural strength of a denture base acrylic resin with monomer 2-tert-butylaminoethyl methacrylate. J. Prosthodont. 2013, 22, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Salgado, H.; Fialho, J.; Marques, M.; Vaz, M.; Figueiral, M.H.; Mesquita, P. Mechanical and surface properties of a 3D-printed dental resin reinforced with graphene. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2023, 64, 12–19. [Google Scholar] [CrossRef]
- Punset, M.; Brizuela, A.; Pérez-Pevida, E.; Herrero-Climent, M.; Manero, J.M.; Gil, J. Mechanical Characterization of Dental Prostheses Manufactured with PMMA-Graphene Composites. Materials 2022, 15, 5391. [Google Scholar] [CrossRef]
- Lima, J.F.; Maciel, J.G.; Arrais, C.A.; Porto, V.C.; Urban, V.M.; Neppelenbroek, K.H. Effect of incorporating antifungals on the water sorption and solubility of interim resilient liners for denture base relining. J. Prosthet. Dent. 2016, 115, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Koroglu, A.; Dede, D.Ö.; Yilmaz, B. Effect of surface sealant agents on the surface roughness and color stability of denture base materials. J. Prosthet. Dent. 2016, 116, 610–616. [Google Scholar] [CrossRef]
- Salim, N.; Moore, C.; Silikas, N.; Satterthwaite, J.; Rautemaa, R. Chlorhexidine is a highly effective topical broad-spectrum agent against Candida spp. Int. J. Antimicrob. Agents 2013, 41, 65–69. [Google Scholar] [CrossRef]
- Jin, N.Y.; Lee, H.R.; Lee, H.; Pae, A. Wettability of denture relining materials under water storage over time. J. Adv. Prosthodont. 2009, 1, 1–5. [Google Scholar] [CrossRef]
- Barnard, R.G.; Clarke-Farr, P.C.; Latief, A. Factors affecting sorption and solubility of denture base acrylic materials: A review. Ann. Dent. Univ. Malaya 2022, 29, 1–8. [Google Scholar] [CrossRef]
- Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Antifungal, optical, and mechanical properties of polymethylmethacrylate material incorporated with silanized zinc oxide nanoparticles. Int. J. Nanomed. 2017, 12, 2353–2360. [Google Scholar] [CrossRef]
- Waldemarin, R.F.; Terra, P.C.; Pinto, L.R.; Faot, F.; Camacho, G.B. Color change in acrylic resin processed in three ways after immersion in water, cola, coffee, mate and wine. Acta Odontol. Latinoam. 2013, 26, 138–143. [Google Scholar] [PubMed]
- Szaloki, M.; Gall, J.; Bukovinszki, K.; Borbeli, J.; Hegedus, C. Synthesis and characterization of cross-linked polymeric nanoparticles and their composites for reinforcement of photocurable dental resin. React. Funct. Polym. 2013, 73, 465–473. [Google Scholar] [CrossRef]
- Alp, G.; Johnston, W.M.; Yilmaz, B. Optical properties and surfasse roughness of prepolymerized poly (methyl methacrylate) denture base materials. J. Prosthet. Dent. 2019, 121, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, P.; Polychronakis, N.; Lagouvardos, P.; Polyzois, G.; Ngo, H.C. Evaluation of a Realistic Cleansing Protocol for Preventing Discoloration of Denture Resins. J. Prosthodont. 2019, 28, e89–e95. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.C.; Vogt, A.P.; Corneta, C.J.; Velo, M.; Lima, D.; Baron, G.M.M.; Aguiar, F.H.B. Influence of a hydrophobic monomer on the physical and mechanical properties of experimental surface sealants. Braz. Oral Res. 2018, 32, e108. [Google Scholar] [CrossRef]
- Lee, Y.K.; Powers, J.M. Influence of salivary organic substances on the discoloration of esthetic dental materials-a review. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 397–402. [Google Scholar] [CrossRef]
- Pytko-Polonczyk, J.; Jakubik, A.; Przeklasa-Bierowiec, A.; Muszynska, B. Artificial saliva and its use in biological experiments. J. Physiol. Pharmacol. 2017, 68, 807–813. [Google Scholar]
- Agarwalla, S.V.; Malhotra, R.; Rosa, V. Translucency, hardness and strength parameters of PMMA resin containing graphene-like material for CAD/CAM restorations. J. Mech. Behav. Biomed. Mater. 2019, 100, 103388. [Google Scholar] [CrossRef]
- Nowakowska-Toporowska, A.; Raszewski, Z.; Wieckiewicz, W. Color change of soft silicone relining materials after storage in artificial saliva. J. Prosthet. Dent. 2016, 115, 377–380. [Google Scholar] [CrossRef]
- Nezu, T.; Nagano-Takebe, F.; Endo, K. Designing an antibacterial acrylic resin using the cosolvent method -Effect of ethanol on the optical and mechanical properties of a cold-cure acrylic resin. Dent. Mater. J. 2017, 36, 662–668. [Google Scholar] [CrossRef]
| Material | Manufacturer | P/L Ratio (g/mL) | Composition | Curing Cycle |
|---|---|---|---|---|
| K | GC America Inc., Alsip, IL, USA | 1.4/1 | P: PEMA 88–90%, dibenzoyl peroxide 1-<2.5%, silicon and titanium dioxides 5-<10%, cellulose acetate <2.5% L: IBMA 90-<100%, N,N-dimethyl-p-toluidine 1-<2.5% | 10 min 37 °C |
| UFI | VOCO GmbH, Cuxhaven, Germany | 1.77/1 | P: PEMA 90–95%, benzoyl peroxide ≤2.5% L: HDMA 50–100%, hydroxyethyl methacrylate ≤2.5% | 7 min 37 °C |
| PC | Ivoclar Vivadent AG, Schaan, Liechtenstein | 1.5/1 | P: PMMA > 95%, softening agent <1%, benzoyl peroxide 1-<2.5%, catalyst, pigments L: MMA 50–100%, BDMA 2.5-<10%, catalyst | 15 min 40 °C, 4 bar |
| RESIN-BASED POLYMER | CHX LOADING (wt%) | AGING PROCESS | MICROHARDNESS (KHN, kgf mm−2) | FLEXURAL STRENGTH (MPa) | SURFACE FREE ENERGY (mN/m) | |||
|---|---|---|---|---|---|---|---|---|
| MED ± IQR | p-Value | MED ± IQR | p-Value | MED ± IQR | p-Value | |||
| K | 0 | Thermal | 7.9 ± 2.73 | p = 1.000 | 78.9 ± 26.00 | p = 0.054 | 27.9 ± 4.45 | p = 0.222 |
| 2.5 | 7.9 ± 1.60 | 91.1 ± 17.63 | 27.0 ± 1.85 | |||||
| 0 | Chemical | 7.2 ± 2.97 | p = 0.798 | 42.0 ± 14.88 | p = 0.959 | 31.8 ± 2.95 | p = 0.222 | |
| 2.5 | 7.0 ± 3.44 | 42.1 ± 12.89 | 33.4 ± 3.05 | |||||
| UFI | 0 | Thermal | 8.2 ± 1.85 | p = 0.878 | 67.0 ± 20.08 | p = 0.130 | 24.0 ± 5.65 | p = 0.095 |
| 5 | 8.4 ± 0.54 | 75.6 ± 13.60 | 32.0 ± 6.50 | |||||
| 0 | Chemical | 7.6 ± 2.25 | p = 0.878 | 36.5 ± 6.51 | p = 0.645 | 41.8 ± 2.70 | p = 0.548 | |
| 5 | 7.9 ± 1.94 | 37.4 ± 5.60 | 42.5 ± 0.28 | |||||
| PC | 0 | Thermal | 13.5 ± 0.58 | p = 0.010 | 180.0 ± 57.31 | p = 0.038 | 26.3 ± 0.40 | p = 0.008 |
| 5 | 12.8 ± 0.53 | 124.6 ± 38.75 | 30.5 ± 2.40 | |||||
| 0 | Chemical | 13.1 ± 4.17 | p = 0.195 | 87.3 ± 19.04 | p = 0.021 | 37.2 ± 3.30 | p = 0.841 | |
| 5 | 12.4 ± 3.94 | 65.6 ± 9.74 | 36.6 ± 2.25 | |||||
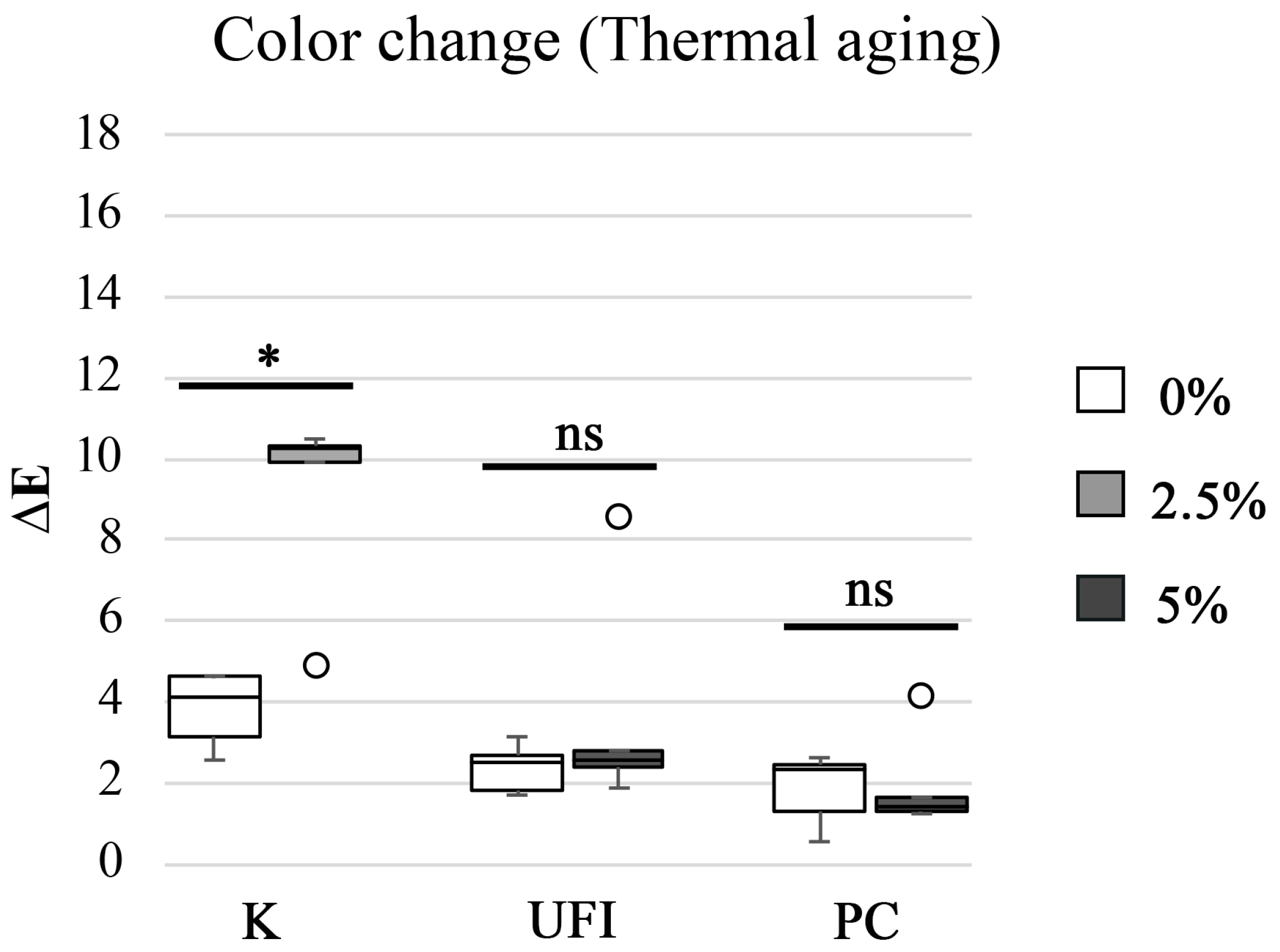
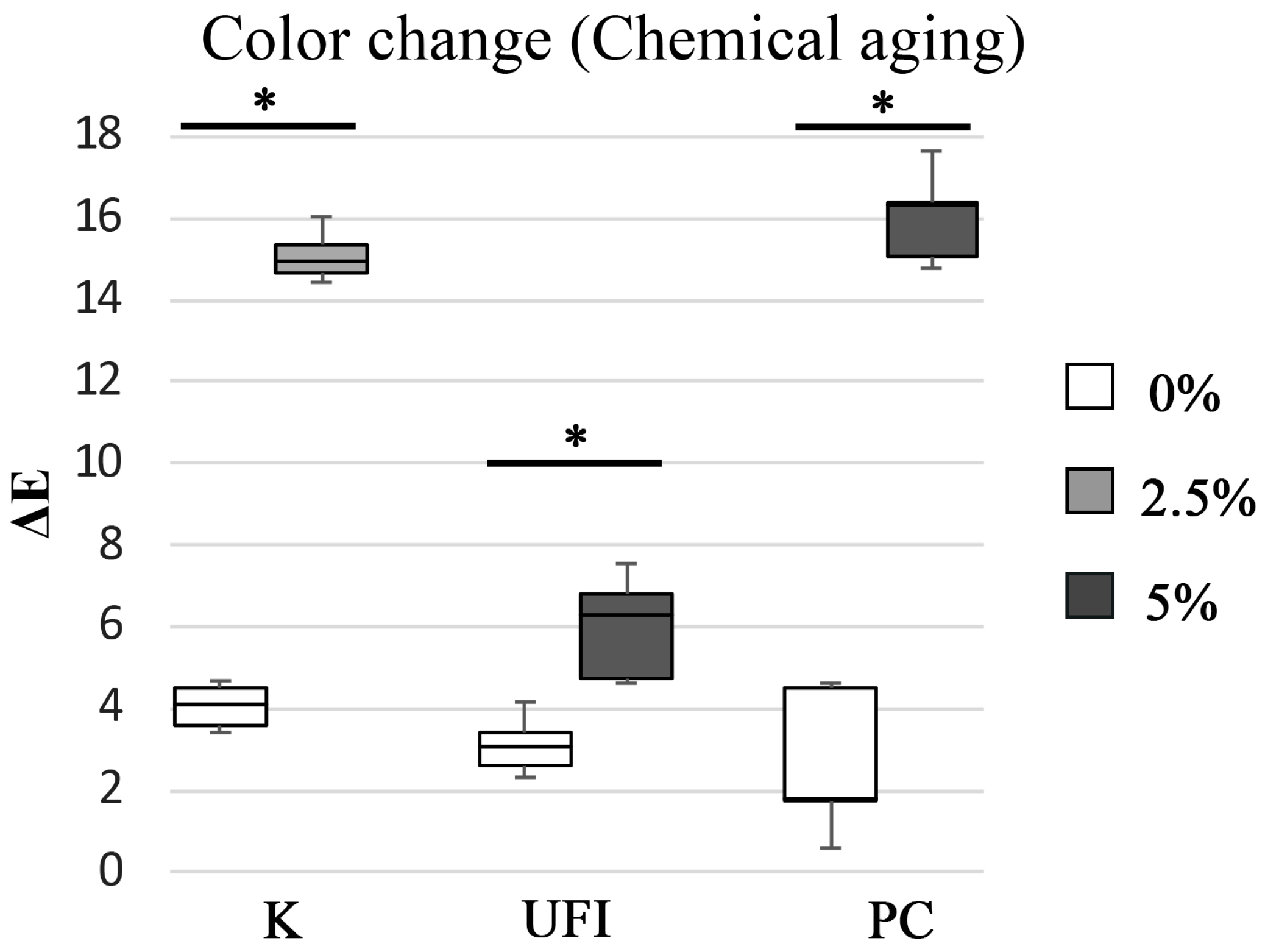
| POLYMER RESIN | CHX LOADING wt% | AGING PROCESS | M ± SD NBS Units | Color Differences |
|---|---|---|---|---|
| K | 0 | Thermal | 3.5 ± 0.85 | Appreciable change |
| 2.5 | 8.5 ± 2.21 | Much appreciable | ||
| 0 | Chemical | 3.7 ± 0.50 | Appreciable change | |
| 2.5 | 13.9 ± 0.58 | Change to another color | ||
| UFI | 0 | Thermal | 2.2 ± 0.56 | Perceivable change |
| 5 | 3.4 ± 2.58 | Appreciable change | ||
| 0 | Chemical | 2.9 ± 0.66 | Perceivable change | |
| 5 | 5.5 ± 1.19 | Appreciable change | ||
| PC | 0 | Thermal | 1.7 ± 0.82 | Perceivable change |
| 5 | 1.8 ± 1.15 | Perceivable change | ||
| 0 | Chemical | 2.4 ± 1.65 | Perceivable change | |
| 5 | 14.8 ± 1.08 | Change to another color |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, C.B.; Costa, J.; Portugal, J.; Bettencourt, A.F. Understanding the Mechanical, Surface, and Color Behavior of Oral Bioactive Prosthetic Polymers under Biodegradation Processes. Polymers 2023, 15, 2549. https://doi.org/10.3390/polym15112549
Neves CB, Costa J, Portugal J, Bettencourt AF. Understanding the Mechanical, Surface, and Color Behavior of Oral Bioactive Prosthetic Polymers under Biodegradation Processes. Polymers. 2023; 15(11):2549. https://doi.org/10.3390/polym15112549
Chicago/Turabian StyleNeves, Cristina B., Joana Costa, Jaime Portugal, and Ana F. Bettencourt. 2023. "Understanding the Mechanical, Surface, and Color Behavior of Oral Bioactive Prosthetic Polymers under Biodegradation Processes" Polymers 15, no. 11: 2549. https://doi.org/10.3390/polym15112549
APA StyleNeves, C. B., Costa, J., Portugal, J., & Bettencourt, A. F. (2023). Understanding the Mechanical, Surface, and Color Behavior of Oral Bioactive Prosthetic Polymers under Biodegradation Processes. Polymers, 15(11), 2549. https://doi.org/10.3390/polym15112549







