Preparation and Properties of High-Temperature-Resistant, Lightweight, Flexible Polyimide Foams with Different Diamine Structures
Abstract
1. Introduction
2. Materials and Experimental
2.1. Materials
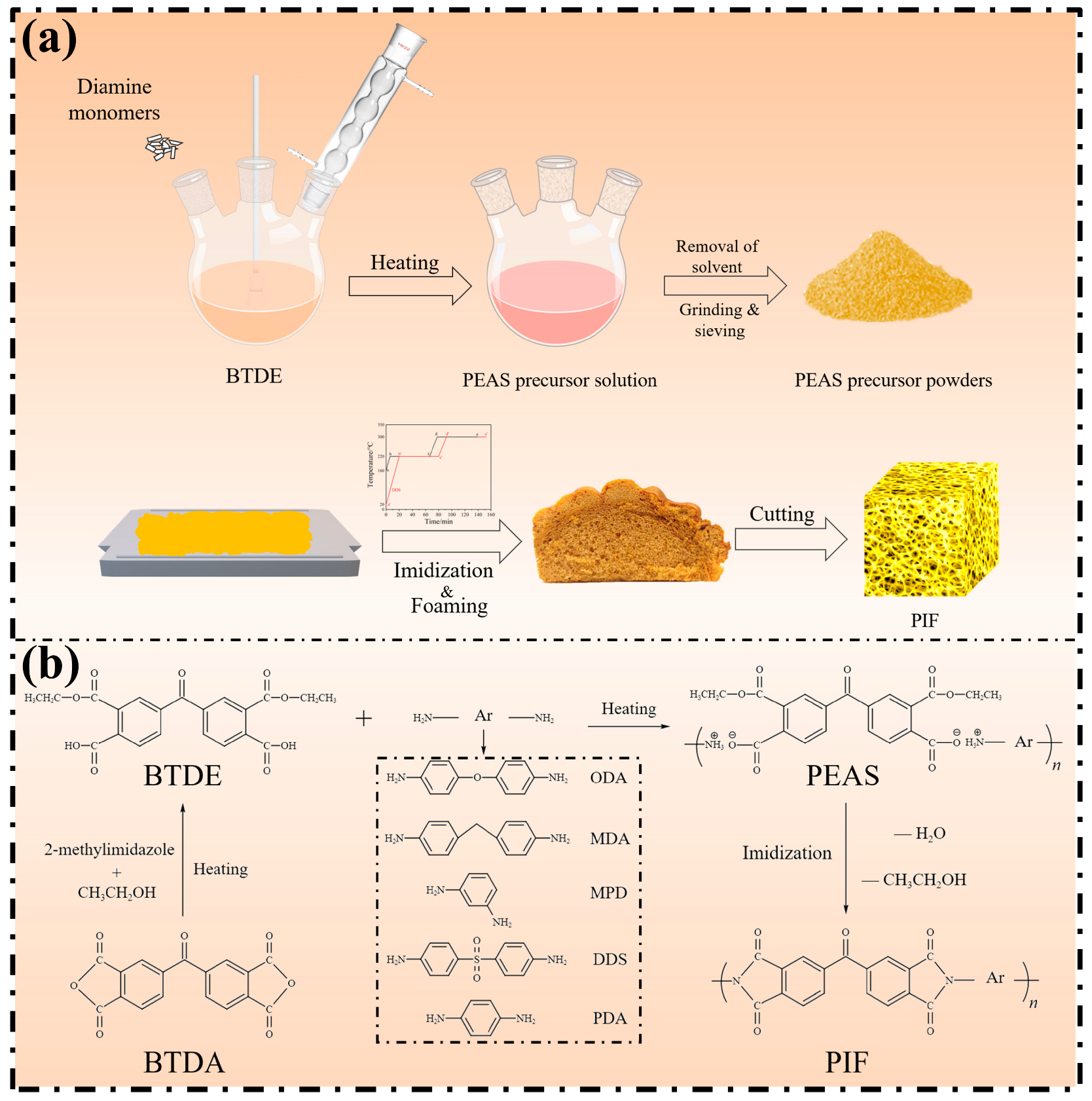
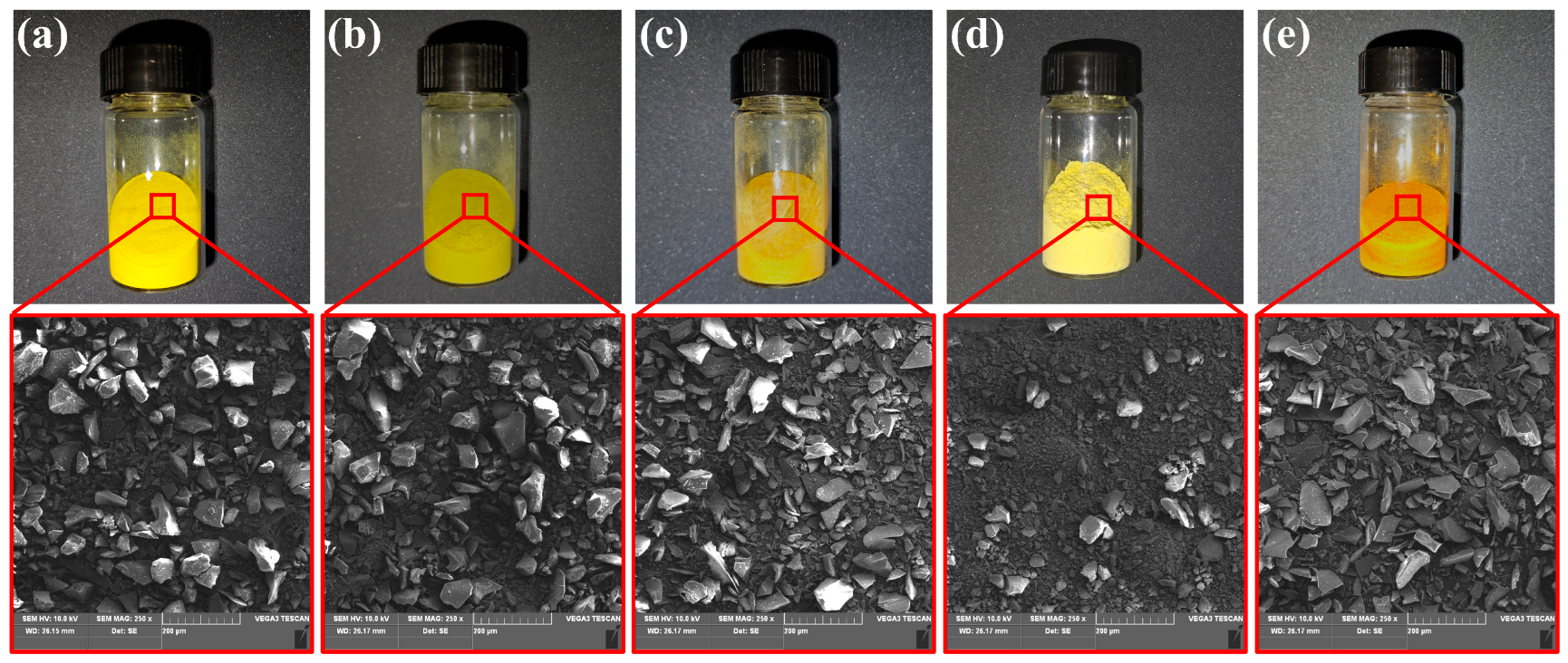
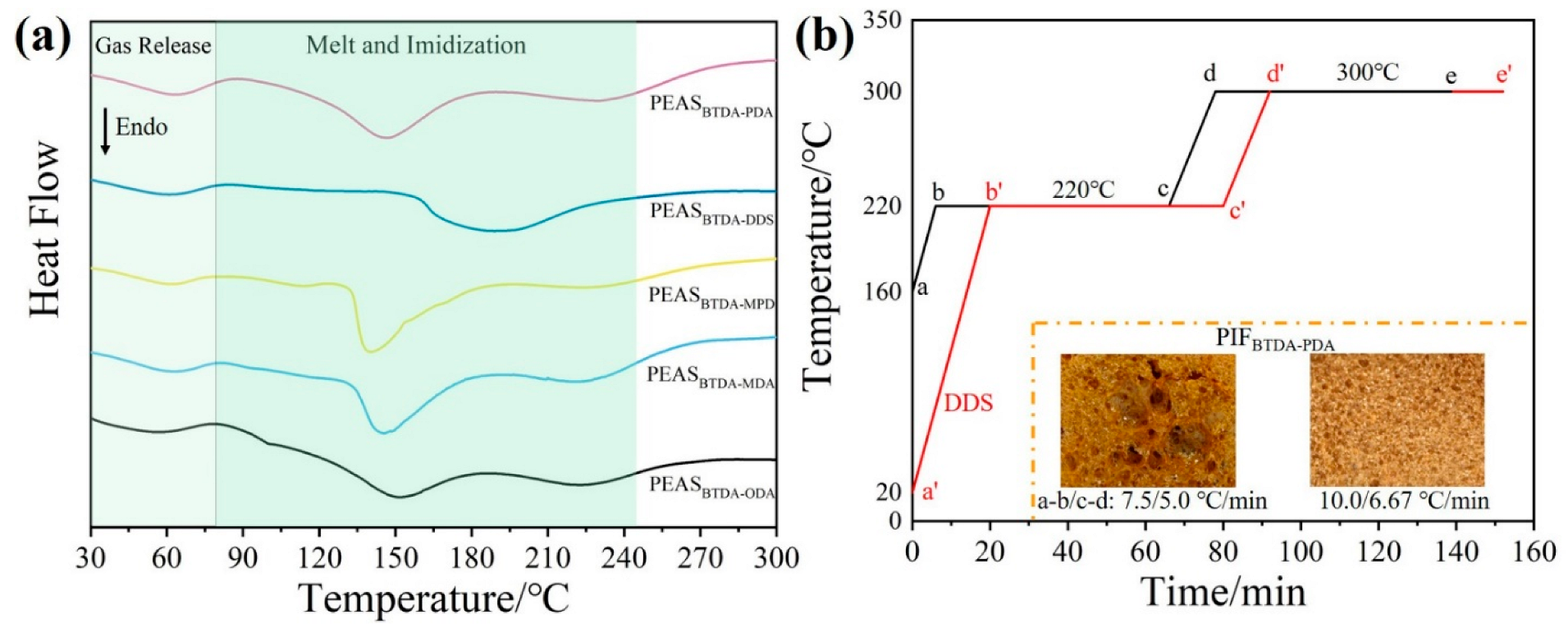
2.2. Synthesis of Polyester Ammonium Precursor Powders and Preparation of Foams
2.3. Characterization
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. Scanning Electron Microscopy (SEM)
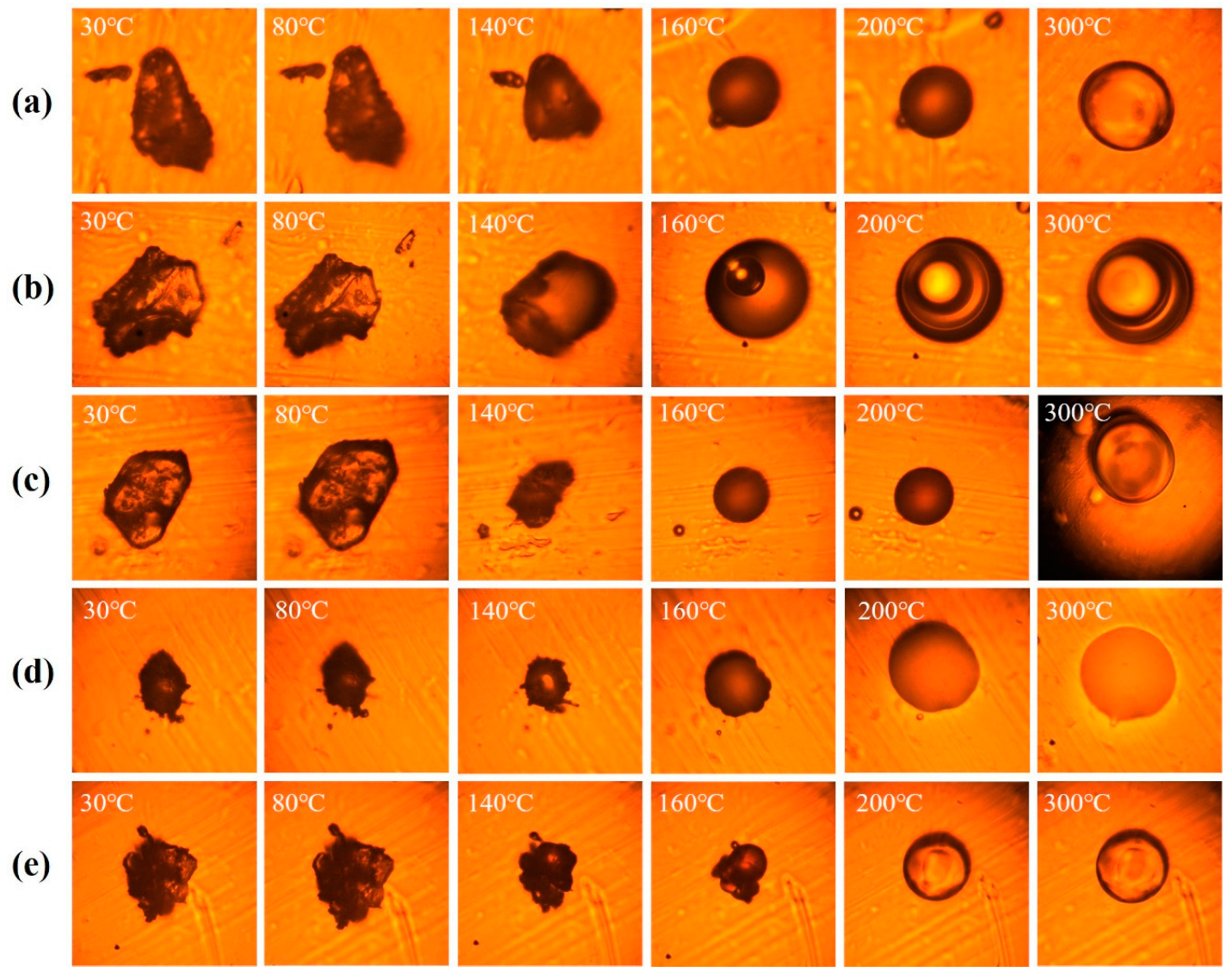
2.3.3. Hot-Stage Optical Microscopy
2.3.4. Compression-Recovery Tests
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Thermogravimetric Analysis (TGA)
2.3.7. Flame-Retardant Property
2.3.8. Thermal Insulation Property
2.3.9. The Residual Solvent Content in the Powder
3. Results and Discussion
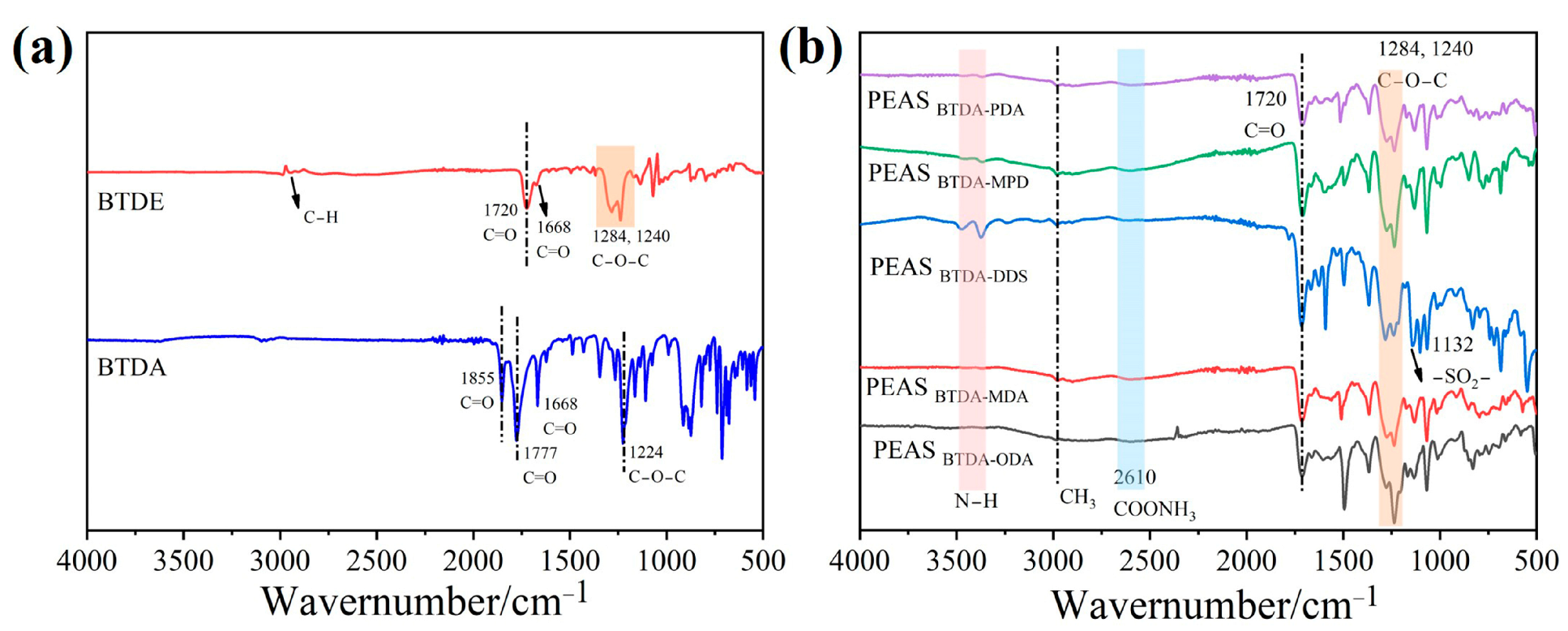
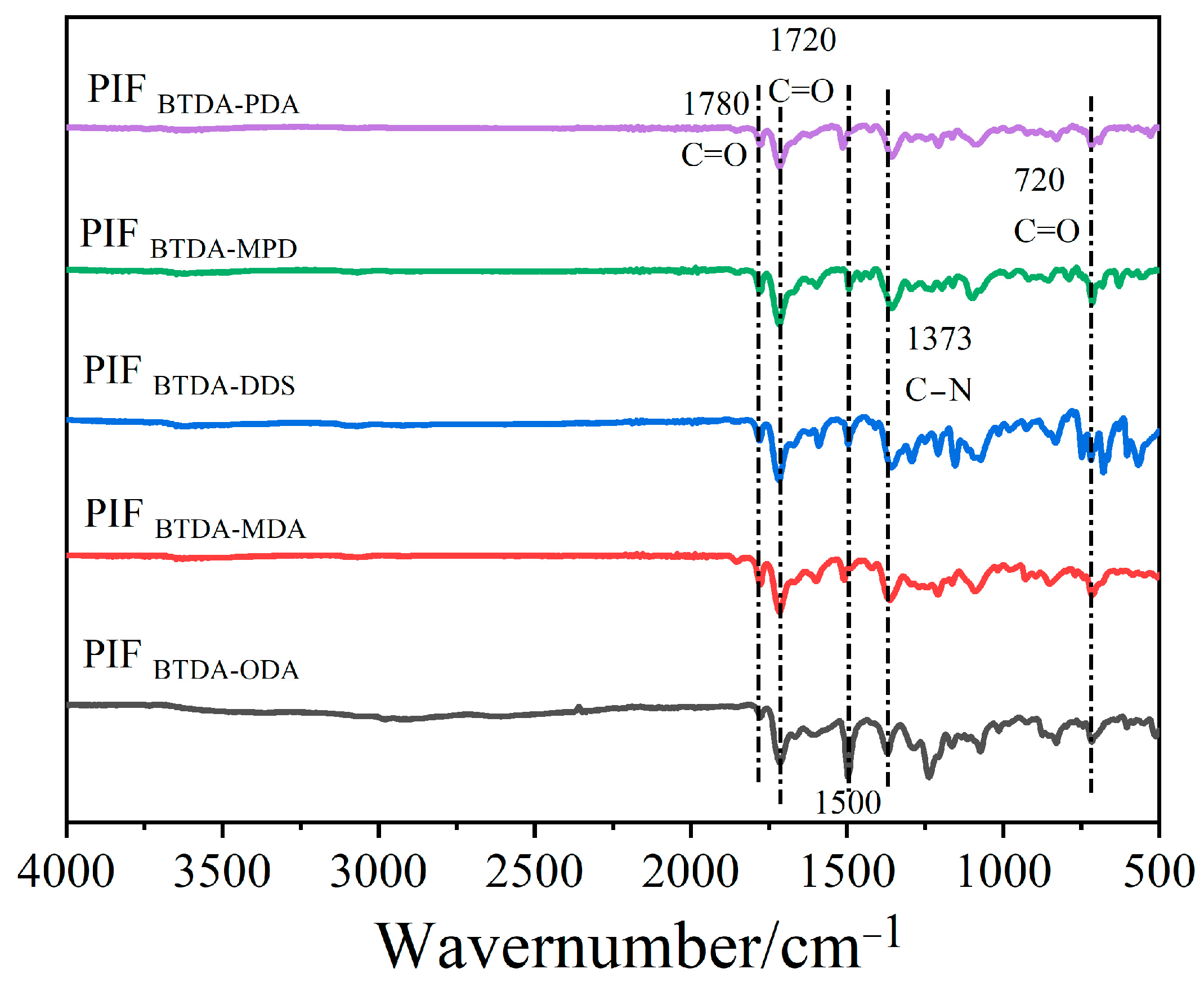
3.1. Characteristic Absorption Peaks in FTIR Spectrum
3.2. Microfoaming Behavior of PEAS and the Optimized Thermo-Foaming Protocol
3.3. Imidization, Foaming, and Cellular Structure of PIFs
3.4. Pore Structure Regularity and Compression–Recovery Properties of PIFs
3.5. Molecular Origin of Pore Structure and Mechanical Performance of PIFs
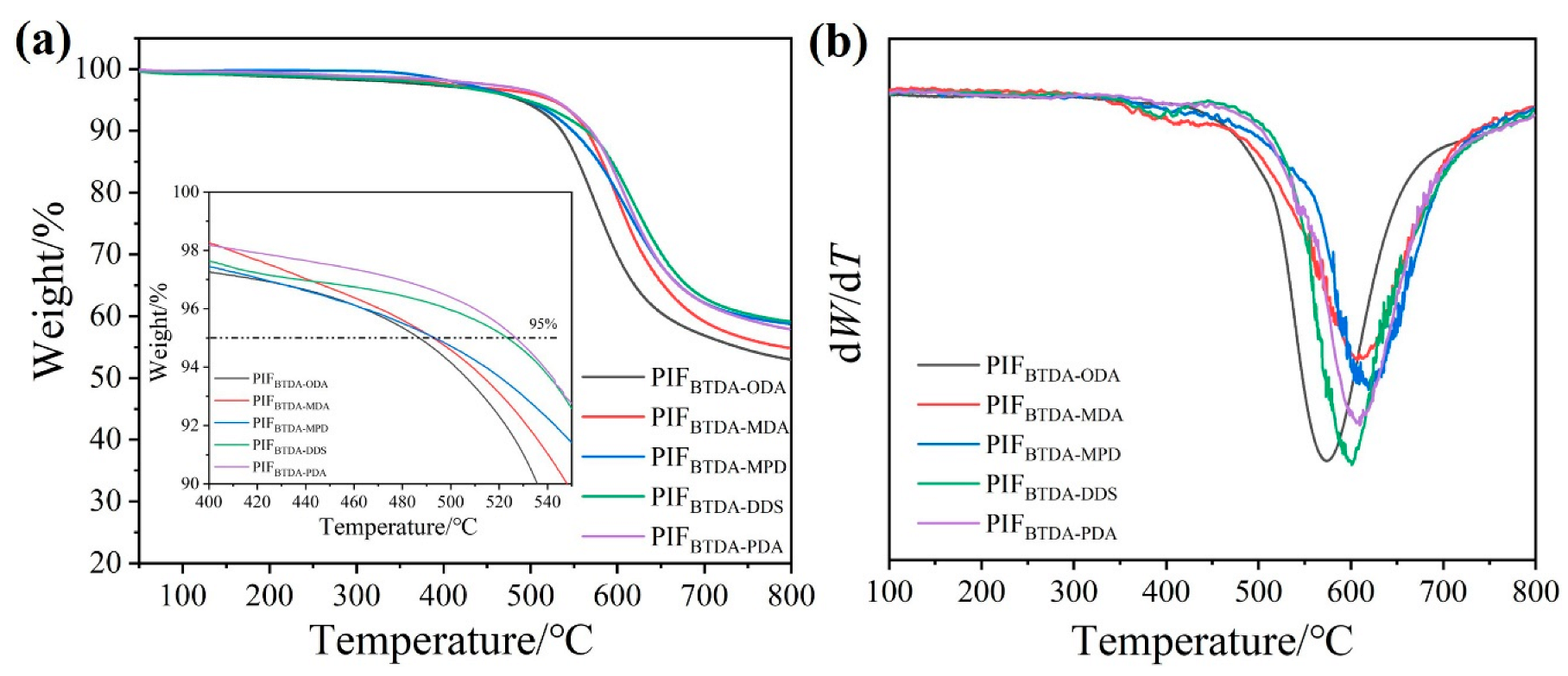
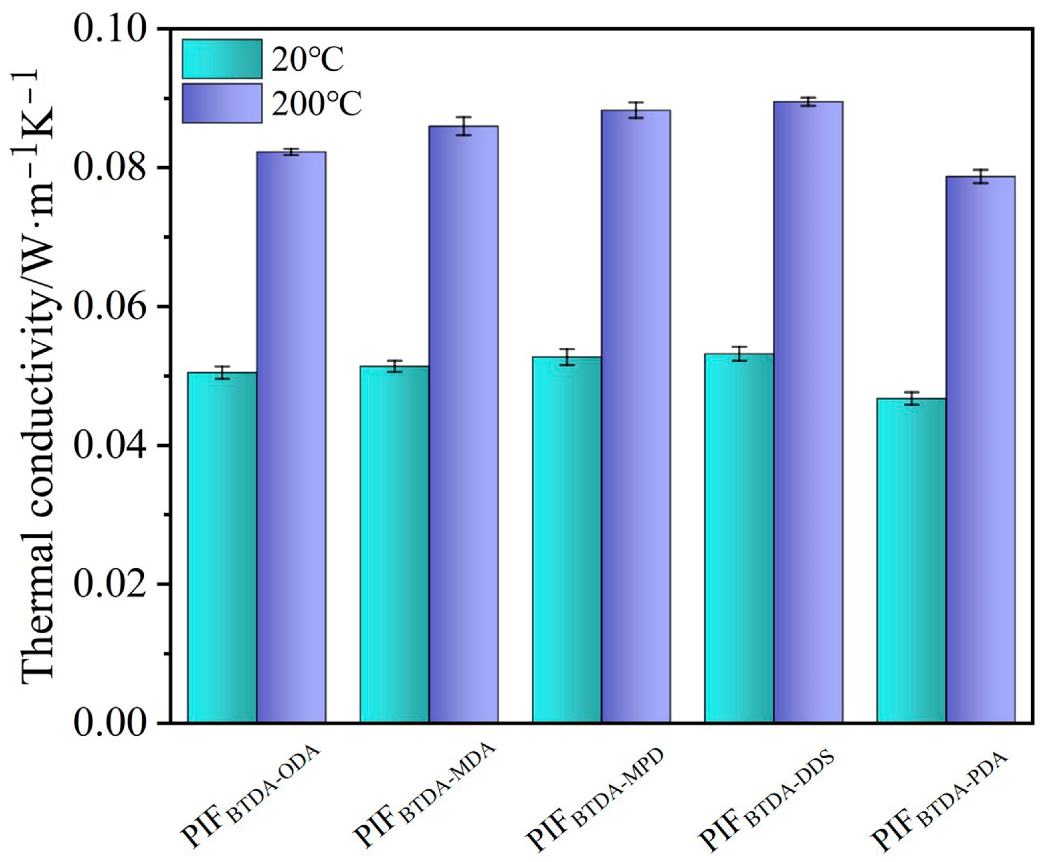
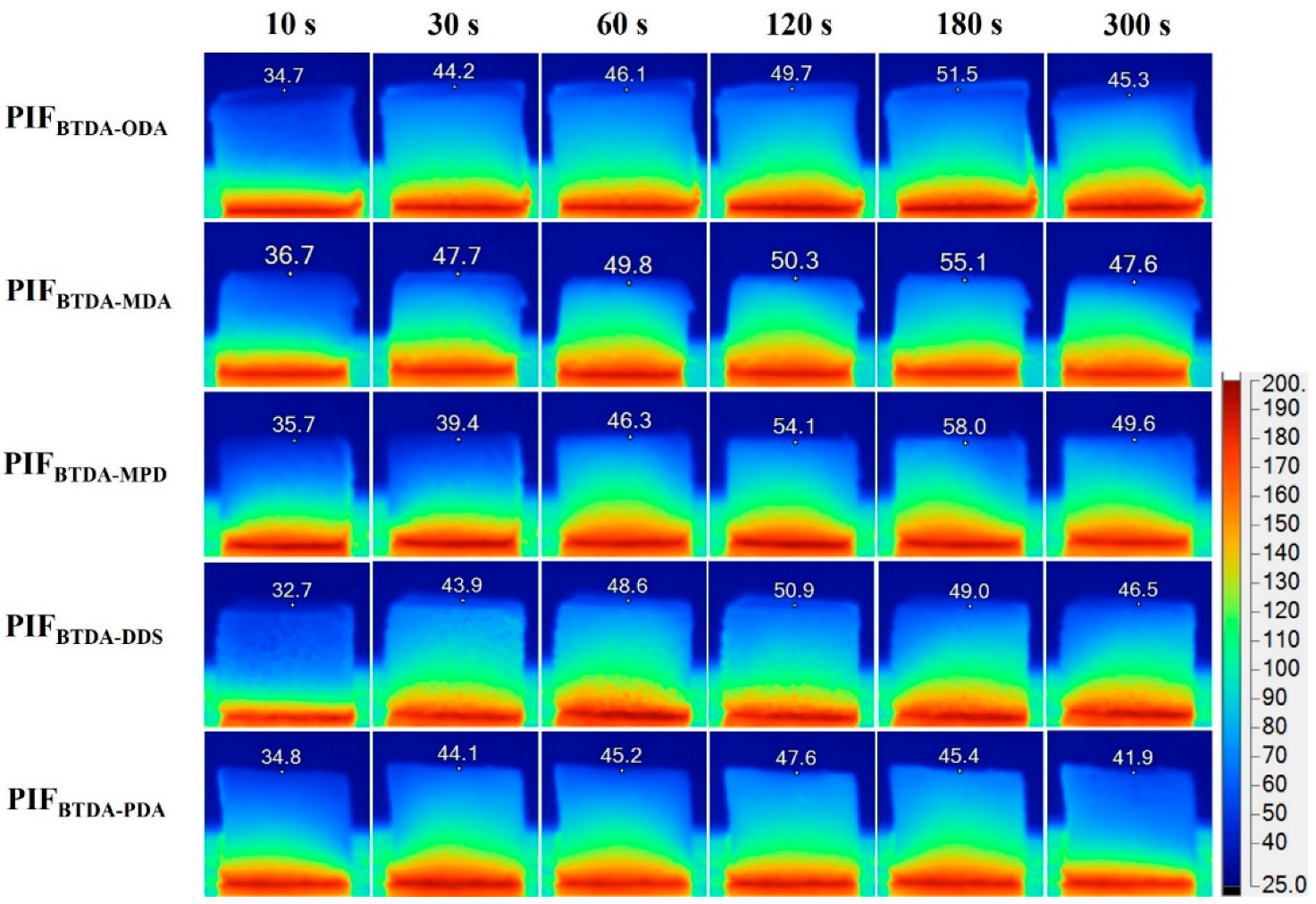
3.6. Thermal Stability and Thermal Insulative Performance of PIFs
3.7. Flame-Retardant Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomin, M.; Kmetty, Á. Polymer foams as advanced energy absorbing materials for sports applications—A review. J. Appl. Polym. Sci. 2021, 139, 51714. [Google Scholar] [CrossRef]
- Ates, M.; Karadag, S.; Eker, A.A.; Eker, B. Polyurethane foam materials and their industrial applications. Polym. Int. 2022, 71, 1157–1163. [Google Scholar] [CrossRef]
- Shi, A.; Zhang, G.; Zhao, C. Study of rigid cross-linked PVC foams with heat resistance. Molecules 2012, 17, 14858–14869. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.D.; Batt, G.S.; Gibert, J.M.; Darby, D.O. Predicting the Effect of Temperature on the Shock Absorption Properties of Polyethylene Foam. Packag. Technol. Sci. 2017, 30, 477–494. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Bai, S.; Wang, Q. Synergistic Effects of Flame Retardants on the Flammability and Foamability of PS Foams Prepared by Supercritical Carbon Dioxide Foaming. ACS Omega 2019, 4, 9306–9315. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, F.; Jiang, S.; Hu, Y.; Song, L.; Hu, W. Mosquito’s eyes inspired, hydrophobic and multifunctional coating on flexible polyurethane (PU) foam: Highly efficient oil spills remediation and exceptional flame-retardant performance. Mater. Today Chem. 2022, 26, 101127. [Google Scholar] [CrossRef]
- Mazzuca, P.; Firmo, J.P.; Correia, J.R.; Garrido, M. Mechanical behaviour in shear and compression of polyurethane foam at elevated temperature. J. Sandw. Struct. Mater. 2021, 24, 1429–1448. [Google Scholar] [CrossRef]
- Mazzuca, P.; Firmo, J.P.; Correia, J.R.; Castilho, E. Mechanical behaviour in shear and compression at elevated temperature of polyethylene terephthalate (PET) foam. J. Build. Eng. 2021, 42, 102526. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Li, J.; Zhou, L.; Jing, Z.; Ma, Z. Preparation and properties of polyimide/chopped carbon fiber composite foams. Polym. Adv. Technol. 2017, 28, 28–34. [Google Scholar] [CrossRef]
- Wang, L.; Hu, A.; Fan, L.; Yang, S. Approach to produce rigid closed-cell polyimide foams. High Perform. Polym. 2013, 25, 956–965. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, X.; Chen, W.; Wang, Y.; Liu, C.; Cui, B.; Li, G.; Song, J.; Zhao, D.; Wang, D.; et al. A simple and green strategy for preparing flexible thermoplastic polyimide foams with exceptional mechanical, thermal-insulating properties, and temperature resistance for high-temperature lightweight composite sandwich structures. Compos. Part B Eng. 2022, 228, 109405. [Google Scholar] [CrossRef]
- Mougel, C.; Garnier, T.; Cassagnau, P.; Sintes-Zydowicz, N. Phenolic foams: A review of mechanical properties, fire resistance and new trends in phenol substitution. Polymer 2019, 164, 86–117. [Google Scholar] [CrossRef]
- Cafiero, L.; Iannace, S.; Sorrentino, L. Microcellular foams from high performance miscible blends based on PEEK and PEI. Eur. Polym. J. 2016, 78, 116–128. [Google Scholar] [CrossRef]
- Rai, N.; Chauhan, I. Multifunctional Aerogels: A comprehensive review on types, synthesis and applications of aerogels. J. Sol Gel Sci. Technol. 2023, 105, 324–336. [Google Scholar] [CrossRef]
- Shi, B.; Ma, B.; Wang, C.; He, H.; Qu, L.; Xu, B.; Chen, Y. Fabrication and applications of polyimide nano-aerogels. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106283. [Google Scholar] [CrossRef]
- Qiu, G.; Ma, W.; Wu, L. Low dielectric constant polyimide mixtures fabricated by polyimide matrix and polyimide microsphere fillers. Polym. Int. 2020, 69, 485–491. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Zhang, J.; Liang, Y.; Chi, H.; Xiao, G. Enhanced toughness and gas permeabilities of polyimide composites derived from polyimide matrix and flower-like polyimide microparticles. Polym. Compos. 2021, 42, 3870–3881. [Google Scholar] [CrossRef]
- Gu, W.; Wang, G.; Zhou, M.; Zhang, T.; Ji, G. Polyimide-Based Foams: Fabrication and Multifunctional Applications. ACS Appl. Mater Interfaces 2020, 12, 48246–48258. [Google Scholar] [CrossRef]
- Ni, L.; Luo, Y.; Peng, X.; Zhou, S.; Zou, H.; Liang, M. Investigation of the properties and structure of semi-rigid closed-cellular polyimide foams with different diamine structures. Polymer 2021, 229, 123957. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Wang, Y.; Chu, W.; Liu, F.; Wang, B. Cross-Linked and Rigid Polyimide Composite Foams with Prominent Fire Resistant, Thermal Insulating, and Wave-Transparent Properties. ACS Appl. Polym. Mater. 2022, 4, 6994–7003. [Google Scholar] [CrossRef]
- Liu, H.; Tian, H.; Yao, Y.; Xiang, A.; Qi, H.; Wu, Q.; Rajulu, A.V. Polyimide foams with outstanding flame resistance and mechanical properties by the incorporation of noncovalent bond modified graphene oxide. New J. Chem. 2020, 44, 12068–12078. [Google Scholar] [CrossRef]
- Teo, N.; Gu, Z.; Jana, S.C. Polyimide-based aerogel foams, via emulsion-templating. Polymer 2018, 157, 95–102. [Google Scholar] [CrossRef]
- Xiang, A.; Li, Y.; Fu, L.; Chen, Y.; Tian, H.; Rajulu, A.V. Thermal degradation and flame retardant properties of isocyanate-based flexible polyimide foams with different isocyanate indices. Thermochim. Acta 2017, 652, 160–165. [Google Scholar] [CrossRef]
- Sun, G.; Wang, W.; Zhang, C.; Liu, L.; Wei, H.; Han, S. Fabrication of isocyanate-based polyimide foam by a postgrafting method. J. Appl. Polym. Sci. 2017, 134, 44240. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, P.; Zhang, R. Organic–Inorganic Hybrid Isocyanate-Based Polyimide Foams with Excellent Fire Resistance and Thermal Insulation Performance. ACS Appl. Polym. Mater. 2022, 4, 9015–9024. [Google Scholar] [CrossRef]
- Luo, Y.; Ni, L.; Zhang, X.; Jiang, X.; Zou, H.; Zhou, S.; Liang, M.; Liu, P. Fabrication of Rigid Polyimide Foams with Superior Compressive Properties. Ind. Eng. Chem. Res. 2022, 61, 1089–1099. [Google Scholar] [CrossRef]
- Liu, J.N.; Wu, D.Y.; Liang, W.H.; Cao, J.H. Effect of DAPBO segment on the structure and performance enhancement of powder foamed BTDA-ODA polyimide. J. Appl. Polym. Sci. 2020, 138, 49911. [Google Scholar] [CrossRef]
- Ni, L.; Luo, Y.; Qiu, B.; Yan, L.; Zou, H.; Zhou, S.; Liang, M.; Liu, P. Combining Microwave-Assisted Foaming and Post Curing Process to Prepare Lightweight Flexible Polyimide Foams for Thermal Insulation Applications. Macromol. Mater. Eng. 2022, 307, 2100941. [Google Scholar] [CrossRef]
- Ni, L.; Luo, Y.; Qiu, C.; Shen, L.; Zou, H.; Liang, M.; Liu, P.; Zhou, S. Mechanically flexible polyimide foams with different chain structures for high temperature thermal insulation purposes. Mater. Today Phys. 2022, 26, 100720. [Google Scholar] [CrossRef]
- Zhan, F.; Youssef, M.; Shah, B.R.; Li, J.; Li, B. Overview of foam system: Natural material-based foam, stabilization, characterization, and applications. Food Hydrocoll. 2022, 125, 107435. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhang, G.C.; He, Z.; Sun, X.F.; Rajaratnam, M. Visualization Study of Solid Poly (Ester-Amine Salt) Precursor Foam. Process. Cell. Polym. 2010, 29, 45–57. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Yao, Y.; Jing, Z.; Zhou, L.; Ma, Z. Synthesis and properties of polyimide foams containing benzimidazole units. RSC Adv. 2016, 6, 60094–60100. [Google Scholar] [CrossRef]
- Taki, K. Experimental and numerical studies on the effects of pressure release rate on number density of bubbles and bubble growth in a polymeric foaming process. Chem. Eng. Sci. 2008, 63, 3643–3653. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. The nucleation of microcellular thermoplastic foam with additives: Part I: Theoretical considerations. Polym. Eng. Sci. 1987, 27, 485–492. [Google Scholar] [CrossRef]
- Leung, S.N.; Park, C.B.; Xu, D.; Li, H.; Fenton, R.G. Computer Simulation of Bubble-Growth Phenomena in Foaming. Ind. Eng. Chem. Res. 2006, 45, 7823–7831. [Google Scholar] [CrossRef]
- Rahimidehgolan, F.; Altenhof, W. Compressive behavior and deformation mechanisms of rigid polymeric foams: A review. Compos. Part B Eng. 2023, 253, 110513. [Google Scholar] [CrossRef]
- Soloveva, O.V.; Solovev, S.A.; Vankov, Y.V.; Shakurova, R.Z. Experimental Studies of the Effective Thermal Conductivity of Polyurethane Foams with Different Morphologies. Processes 2022, 10, 112257. [Google Scholar] [CrossRef]
- Weiser, E.S.; Johnson, T.F.; St Clair, T.L.; Echigo, Y.; Kaneshiro, H.; Grimsley, B.W. Polyimide Foams for Aerospace Vehicles. High Perform. Polym. 2000, 12, 1–12. [Google Scholar] [CrossRef]
- Ou, A.; Huang, Z.; Qin, R.; Chen, X.; Li, Y.; Liu, Y.; Liu, X.; Wang, X. Preparation of Thermosetting/Thermoplastic Polyimide Foam with Pleated Cellular Structure via In Situ Simultaneous Orthogonal Polymerization. ACS Appl. Polym. Mater. 2019, 1, 2430–2440. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, S.; Li, B.; Hou, W.; Li, G.; Memon, M.A.; Huang, Y.; Geng, J. Graphene Oxide: A Versatile Agent for Polyimide Foams with Improved Foaming Capability and Enhanced Flexibility. Chem. Mater. 2015, 27, 4358–4367. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, G.; Li, J.; Wang, A.; Shi, X. Effects of 4,4’-diaminodiphenyl ether on the structures and properties of isocyanate-based polyimide foams. J. Appl. Polym. Sci. 2018, 135, 46029. [Google Scholar] [CrossRef]
- Tian, H.; Yao, Y.; Ma, S.; Fu, L.; Xiang, A.; Rajulu, A.V. Improved mechanical, thermal and flame resistant properties of flexible isocyanate-based polyimide foams by graphite incorporation. High Perform. Polym. 2017, 30, 1130–1138. [Google Scholar] [CrossRef]



| Powders Samples | Residual Solvent Content/% | Heating Rate of a-b/°C·min−1 | Heating Rate of c-d/°C·min−1 |
|---|---|---|---|
| PEASBTDA-ODA | 12.65 | 7.5 | 5.0 |
| PEASBTDA-MDA | 13.65 | 7.5 | 5.0 |
| PEASBTDA-MPD | 13.38 | 10.0 | 5.0 |
| PEASBTDA-DDS | 17.62 | 10.0 | 6.67 |
| PEASBTDA-PDA | 12.95 | 10.0 | 6.67 |
| Foam Samples | Density/kg∙m−3 | Compressive Strength at 25%/kPa | Residual Strain after the First Compression/% | SR/% |
|---|---|---|---|---|
| PIFBTDA-ODA | 15.60 | 41.47 | 5.71 | 87 |
| PIFBTDA-MDA | 17.11 | 49.90 | 6.91 | 89 |
| PIFBTDA-MPD | 16.45 | 50.63 | 6.74 | 90 |
| PIFBTDA-DDS | 19.96 | 38.67 | 8.14 | 85 |
| PIFBTDA-PDA | 16.26 | 51.17 | 6.18 | 91 |
| Foam Samples | Tg/°C | T5%/°C | T10%/°C | Tmax/°C | R800/%-Nitrogen | LOI/% |
|---|---|---|---|---|---|---|
| PIFBTDA-ODA | 271.3 | 487.1 | 535.7 | 573.5 | 53.0 | 41.8 |
| PIFBTDA-MDA | 277.9 | 492.7 | 548.1 | 607.3 | 58.8 | 40.1 |
| PIFBTDA-MPD | 302.8 | 493.3 | 564.3 | 615.3 | 59.1 | 42.5 |
| PIFBTDA-DDS | 327.1 | 523.3 | 564.4 | 602.3 | 54.9 | 42.6 |
| PIFBTDA-PDA | 340.4 | 526.9 | 569.3 | 609.6 | 57.9 | 42.8 |
| Foam Samples | Density/kg∙m−3 | Compressive Strength/kPa | Tg/°C | T5%/°C | LOI/% | Thermal Conductivity/W·m−1∙K−1 at RT | Thermal Conductivity/W·m−1∙K−1 at 200 °C | Ref. |
|---|---|---|---|---|---|---|---|---|
| PIFBTDA-ODA | 15.60 | 41.47 (25%) | 271.3 | 487.1 | 41.8 | 0.0505 | 0.0823 | This work |
| PIFBTDA-MDA | 17.11 | 49.90 (25%) | 277.9 | 492.7 | 40.1 | 0.0514 | 0.0859 | This work |
| PIFBTDA-MPD | 16.45 | 50.63 (25%) | 302.8 | 493.3 | 42.5 | 0.0527 | 0.0883 | This work |
| PIFBTDA-DDS | 19.96 | 38.67 (25%) | 327.1 | 523.3 | 42.6 | 0.0532 | 0.0895 | This work |
| PIFBTDA-PDA | 16.26 | 51.17 (25%) | 340.4 | 526.9 | 42.8 | 0.0467 | 0.0787 | This work |
| PIFBTDA-MDA | - | 25.00 (10%) | 255.9 | 493.0 | - | 0.0496 | 0.134 | [39] |
| PIFBTDA-ODA/graphene | 75 | 20.00 (10%) | 260.0 | 480.0 | - | - | - | [40] |
| PIF(Isocyanate index 0.89–1.18) | 5.7–8.7 | 7.5–11.4 (20%) | - | 288.5–320.0 | 25.3–28.3 | - | - | [23] |
| Isocyanate-based PIFODA/PMDA | 13.7–16.49 | 33.0–45.7 (15%) | - | 270.11–273.18 | - | 0.051–0.055 | - | [41] |
| Isocyanate-based PIF at graphite (0–3.25%) | 7.8–11.88 | 11.0–25.0 | - | 406.9–452.1 (T10%) | 31.0–34.8 | - | - | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, S.; Sheng, X.; Wang, S.; Miao, X.; Shi, X.; Zhao, Y.; Qin, J.; Zhang, G. Preparation and Properties of High-Temperature-Resistant, Lightweight, Flexible Polyimide Foams with Different Diamine Structures. Polymers 2023, 15, 2609. https://doi.org/10.3390/polym15122609
Yun S, Sheng X, Wang S, Miao X, Shi X, Zhao Y, Qin J, Zhang G. Preparation and Properties of High-Temperature-Resistant, Lightweight, Flexible Polyimide Foams with Different Diamine Structures. Polymers. 2023; 15(12):2609. https://doi.org/10.3390/polym15122609
Chicago/Turabian StyleYun, Shuhuan, Xianzhe Sheng, Shengli Wang, Xing Miao, Xuetao Shi, Yongsheng Zhao, Jianbin Qin, and Guangcheng Zhang. 2023. "Preparation and Properties of High-Temperature-Resistant, Lightweight, Flexible Polyimide Foams with Different Diamine Structures" Polymers 15, no. 12: 2609. https://doi.org/10.3390/polym15122609
APA StyleYun, S., Sheng, X., Wang, S., Miao, X., Shi, X., Zhao, Y., Qin, J., & Zhang, G. (2023). Preparation and Properties of High-Temperature-Resistant, Lightweight, Flexible Polyimide Foams with Different Diamine Structures. Polymers, 15(12), 2609. https://doi.org/10.3390/polym15122609