Surface-Enhanced Raman Scattering (SERS) Substrates Based on Ag-Nanoparticles and Ag-Nanoparticles/Poly (methyl methacrylate) Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PMMA Microspheres
2.3. Synthesis of Ag-Nanoparticles
2.4. Preparation of SERS Substrates
2.5. Preparation of Ag/PMMA Composites
2.6. Materials Characterization
2.7. SERS Measurements
3. Results and Discussion
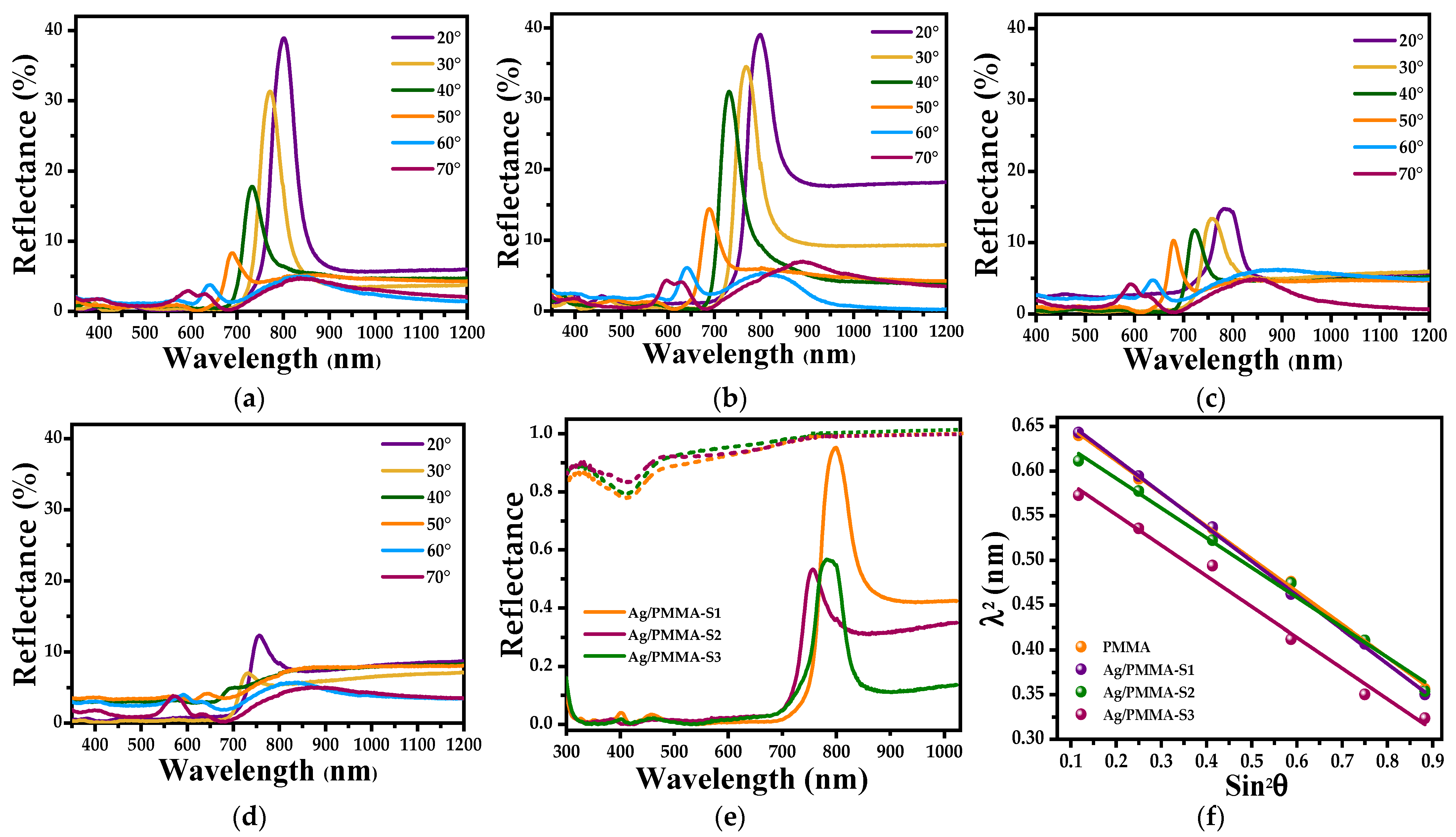
3.1. Characterization of SERS Substrates
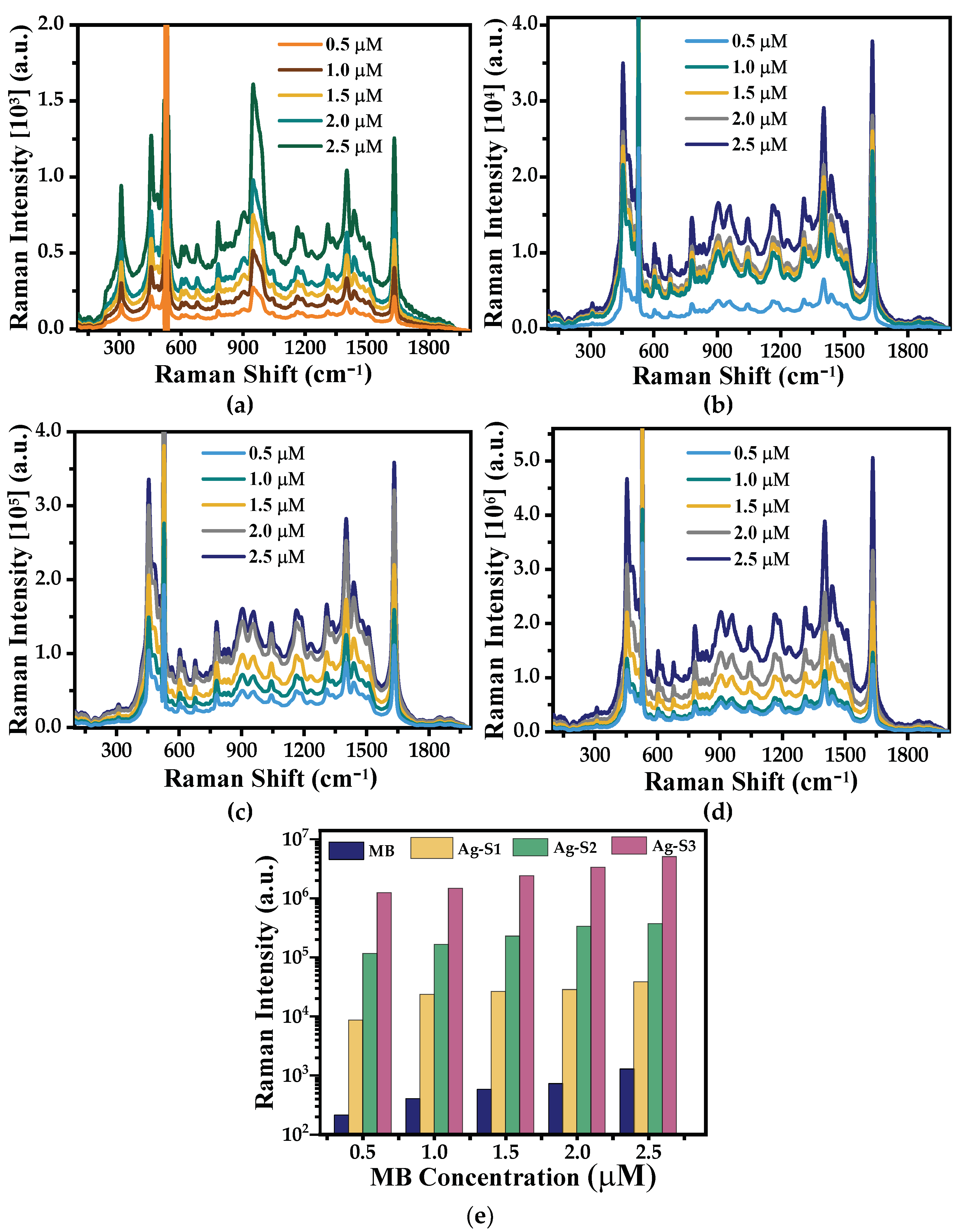
3.2. SERS Performance
3.2.1. Ag-NP SERS Substrates
3.2.2. Ag/PMMA Composite SERS Substrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman Spectroscopy for Chemical Biology Research. J. Am. Chem. Soc. 2022, 144, 19651–19667. [Google Scholar] [CrossRef]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtulus, O.; Lee, S.H.; Lindquist, N.C.; Oh, S.H.; Haynes, C.L. Recent progress in SERS biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef] [PubMed]
- Rycenga, M.; Camargo, P.H.C.; Li, W.; Moran, C.H.; Xia, Y. Understanding the SERS Effects of Single Silver Nanoparticles and Their Dimers, One at a Time. J. Phys. Chem. Lett. 2010, 4, 696–703. [Google Scholar] [CrossRef]
- Han, X.X.; Rodriguez, R.S.; Haynes, C.L.; Ozaki, Y.; Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Prim. 2021, 1, 87. [Google Scholar] [CrossRef]
- Jeon, T.Y.; Kim, D.J.; Park, S.G.; Kim, S.H.; Kim, D.H. Nanostructured plasmonic substrates for use as SERS sensors. Nano Converg. 2016, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Kuang, C.; Liu, X.; Tang, L. Single-Molecule Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 4889. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Garcia-Leis, A.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Silver nanostars with high SERS performance. J. Phys. Chem. C 2013, 117, 7791–7795. [Google Scholar] [CrossRef]
- Awiaz, G.; Lin, J.; Wu, A. Recent advances of Au@Ag core–shell SERS-based biosensors. Exploration 2023, 3, 20220072. [Google Scholar] [CrossRef]
- Izquierdo-Lorenzo, I.; Alda, I.; Sanchez-Cortes, S.; Garcia-Ramos, J.V. Adsorption and detection of sport doping drugs on metallic plasmonic nanoparticles of different morphology. Langmuir 2012, 28, 8891–8901. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Kasálková, N.S.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of gold and silver nanoparticles preparation. Materials 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 1–23. [Google Scholar] [CrossRef]
- Pinheiro, P.C.; Fateixa, S.; Nogueira, H.I.S.; Trindade, T. SERS study on adenine using a Ag/poly(t-butylacrylate) nanocomposite. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2013, 101, 36–39. [Google Scholar] [CrossRef]
- Pinheiro, P.C.; Fateixa, S.; Nogueira, H.I.S.; Trindade, T. SERS studies of DNA nucleobases using new silver poly(methyl methacrylate) nanocomposites as analytical platforms. J. Raman Spectrosc. 2015, 46, 47–53. [Google Scholar] [CrossRef]
- Fateixa, S.; Pinheiro, P.C.; Nogueira, H.I.S.; Trindade, T. Composite blends of gold nanorods and poly(t-butylacrylate) beads as new substrates for SERS. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2013, 113, 100–106. [Google Scholar] [CrossRef]
- Gushiken, N.K.; Paganoto, G.T.; Temperini, M.L.A.; Teixeira, F.S.; Salvadori, M.C. Substrate for Surface-Enhanced Raman Spectroscopy Formed by Gold Nanoparticles Buried in Poly(methyl methacrylate). ACS Omega 2020, 5, 10366–10373. [Google Scholar] [CrossRef]
- Wang, T.-J.; Barveen, N.R.; Liu, Z.-Y.; Chen, C.-H.; Chou, M.-H. Transparent, Flexible Plasmonic Ag NP/PMMA Substrates Using Chemically Patterned Ferroelectric Crystals for Detecting Pesticides on Curved Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 34910–34922. [Google Scholar] [CrossRef]
- Tang, S.; Liu, H.; Tian, Y.; Chen, D.; Gu, C.; Wei, G.; Jiang, T.; Zhou, J. Surface-enhanced Raman scattering-based lateral flow immunoassay mediated by hydrophilic-hydrophobic Ag-modified PMMA substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120092. [Google Scholar] [CrossRef]
- Chang, K.-J.; Chen, H.-R.; Hung, C.-H.; Hung, P.-S.; Tseng, H.-F.; Lin, Y.-L.; Hsu, H.-H.; Kao, T.-H.; Wu, P.-W.; Liau, I.; et al. Highly Ordered Polymer Nanostructures via Solvent On-Film Annealing for Surface-Enhanced Raman Scattering. Langmuir 2022, 38, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Ge, M.; Pan, H.; Wang, J.; Nie, X.; Zhang, Q.; Zhao, W.; Liu, X.; Yu, Y. In situ synthesis of low-cost and large-scale flexible metal nanoparticle-polymer composite films as highly sensitive SERS substrates for surface trace analysis. RSC Adv. 2019, 9, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Matamoros-Ambrocio, M.; Sánchez-Mora, E.; Gómez-Barojas, E.; Luna-López, J.A. Synthesis and Study of the Optical Properties of PMMA Microspheres and Opals. Polymers 2021, 13, 2171. [Google Scholar] [CrossRef]
- Lin, X.M.; Cui, Y.; Xu, Y.H.; Ren, B.; Tian, Z.Q. Surface-enhanced Raman spectroscopy: Substrate-related issues. Anal. Bioanal. Chem. 2009, 394, 1729–1745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Langille, M.R.; Mirkin, C.A. Photomediated Synthesis of Silver Triangular Bipyramids and Prisms: The Effect of pH and BSPP. J. Am. Chem. Soc. 2010, 132, 12502–12510. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Mirkin, C.A. pH-Switchable Silver Nanoprism Growth Pathways. Angew. Chem. 2007, 119, 2082–2084. [Google Scholar] [CrossRef]
- Radziuk, D.; Moehwald, H. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Waterhouse, G.I.N.; Waterland, M.R. Opal and inverse opal photonic crystals: Fabrication and characterization. Polyhedron 2007, 26, 356–368. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Maurin, G.; Llewellyn, P. Introduction. In Adsorption by Powders and Porous Solids; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–24. [Google Scholar]
- Yu, B.; Xue, T.; Pang, L.; Zhang, X.; Shen, Y.; Cong, H. The Effect of Different Porogens on Porous PMMA Microspheres by Seed Swelling Polymerization and Its Application in High-Performance Liquid Chromatography. Materials 2018, 11, 705. [Google Scholar] [CrossRef]
- Dutta Roy, S.; Ghosh, M.; Chowdhury, J. Adsorptive parameters and influence of hot geometries on the SER(R) S spectra of methylene blue molecules adsorbed on gold nanocolloidal particles. J. Raman Spectrosc. 2015, 46, 451–461. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Lai, K.; Rasco, B.A.; Fan, Y. Analysis of trace methylene blue in fish muscles using ultra-sensitive surface-enhanced Raman spectroscopy. Food Control 2016, 65, 99–105. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; De Souza, M.L.; Souza, K.S.; Dos Santos, D.P.; Andrade, G.F.S.; Temperini, M.L.A. Critical assessment of enhancement factor measurements in surface-enhanced Raman scattering on different substrates. Phys. Chem. Chem. Phys. 2015, 17, 21294–21301. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Removal of Methylene Blue from Aqueous Solution by Bone Char. Appl. Sci. 2018, 8, 1903. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Jiao, Y.; Liu, A.; Wang, S.; Zhang, C.; Yang, C.; Xu, Y.; Li, C.; Man, B. Different number of silver nanoparticles layers for surface enhanced raman spectroscopy analysis. Sens. Actuators B Chem. 2018, 255, 374–383. [Google Scholar] [CrossRef]
- De León Portilla, P.; Sánchez-Mora, E.; González, A.L. Influence on SERS enhancement factor of the components of an artificial opal loaded with metal NPs: A systematic study. Curr. Appl. Phys. 2022, 39, 248–257. [Google Scholar] [CrossRef]
- Fränzl, M.; Moras, S.; Gordan, O.D.; Zahn, D.R.T. Interaction of One-Dimensional Photonic Crystals and Metal Nanoparticle Arrays and Its Application for Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2018, 122, 10153–10158. [Google Scholar] [CrossRef]




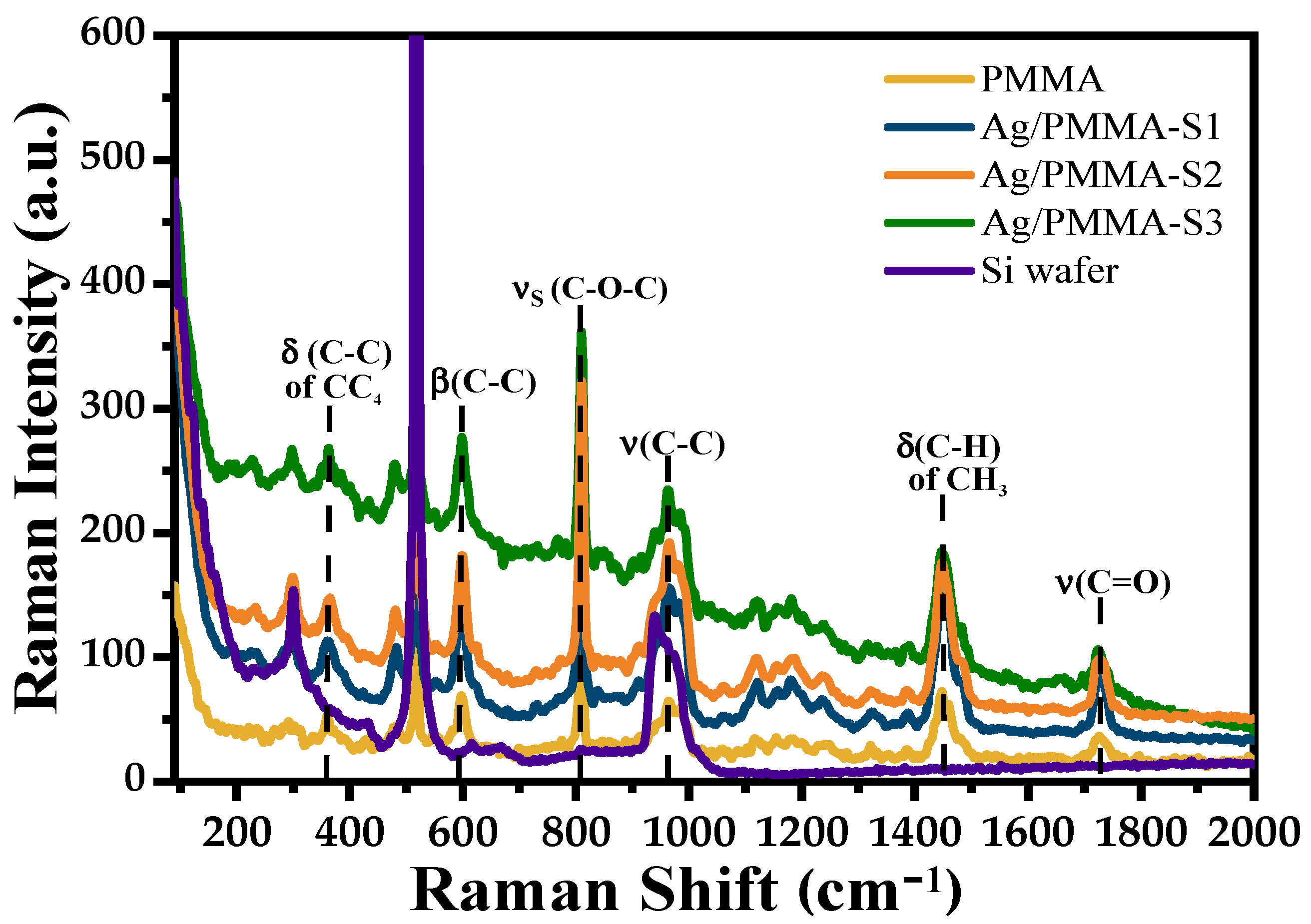





| PMMA | Ag/PMMA | Assignment |
|---|---|---|
| 365 | 359 | (C-C) of CH4 |
| 601 | 597 | (C-C) |
| 813 | 810 | (C-O-C) |
| 969 | 965 | (C-C) |
| 990 | 985 | (C-C) |
| 1066 | 1060 | (C-C) |
| 1123 | 1120 | (C-O), (CH3) |
| 1159 | 1155 | (C-O-C) |
| 1185 | 1180 | (C-O-C) |
| 1240 | 1235 | (C-O) |
| 1326 | 1320 | (CH2) |
| 1390 | 1386 | (C-H) of CH3 |
| 1452 | 1447 | (C-H) of CH3 |
| 1481 | 1475 | (C-H) of CH2 |
| 1728 | 1725 | (C=O) |
| MB Concentration | SERS EF | ||
|---|---|---|---|
| (×106 M) | Ag-S1 (×104) | Ag-S2 (×105) | Ag-S3 (×106) |
| 0.5 | 0.62 0.03 | 2.18 0.07 | 1.14 0.12 |
| 1.0 | 1.35 0.04 | 3.30 0.08 | 1.44 0.13 |
| 1.5 | 1.56 0.05 | 4.75 0.07 | 1.62 0.15 |
| 2.0 | 1.79 0.04 | 4.92 0.08 | 3.25 0.11 |
| 2.5 | 1.81 0.05 | 5.34 0.09 | 5.18 0.09 |
| MB Concentration | SERS EF | ||
|---|---|---|---|
| (×106 M) | Ag/PMMA-S1 (×103) | Ag/PMMA-S2 (×104) | Ag/PMMA-S3 (×105) |
| 0.5 | 0.80 0.11 | 0.41 0.05 | 0.47 0.08 |
| 1.0 | 2.35 0.07 | 0.80 0.07 | 0.85 0.09 |
| 1.5 | 2.70 0.09 | 1.38 0.09 | 1.48 0.12 |
| 2.0 | 3.12 0.14 | 2.21 0.09 | 2.04 0.10 |
| 2.5 | 3.91 0.12 | 2.30 0.08 | 3.69 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matamoros-Ambrocio, M.; Sánchez-Mora, E.; Gómez-Barojas, E. Surface-Enhanced Raman Scattering (SERS) Substrates Based on Ag-Nanoparticles and Ag-Nanoparticles/Poly (methyl methacrylate) Composites. Polymers 2023, 15, 2624. https://doi.org/10.3390/polym15122624
Matamoros-Ambrocio M, Sánchez-Mora E, Gómez-Barojas E. Surface-Enhanced Raman Scattering (SERS) Substrates Based on Ag-Nanoparticles and Ag-Nanoparticles/Poly (methyl methacrylate) Composites. Polymers. 2023; 15(12):2624. https://doi.org/10.3390/polym15122624
Chicago/Turabian StyleMatamoros-Ambrocio, Mayra, Enrique Sánchez-Mora, and Estela Gómez-Barojas. 2023. "Surface-Enhanced Raman Scattering (SERS) Substrates Based on Ag-Nanoparticles and Ag-Nanoparticles/Poly (methyl methacrylate) Composites" Polymers 15, no. 12: 2624. https://doi.org/10.3390/polym15122624
APA StyleMatamoros-Ambrocio, M., Sánchez-Mora, E., & Gómez-Barojas, E. (2023). Surface-Enhanced Raman Scattering (SERS) Substrates Based on Ag-Nanoparticles and Ag-Nanoparticles/Poly (methyl methacrylate) Composites. Polymers, 15(12), 2624. https://doi.org/10.3390/polym15122624








