Sorafenib-Based Drug Delivery Systems: Applications and Perspectives
Abstract
:1. Introduction
SF Inhibition Mechanism in HCC and Other Cancers
2. SF-Based Nanomedicine
2.1. Biomacromolecule as Nanocarrier
2.2. Synthetic Polymer as Nanocarriers
2.3. Inorganic and Metal Nanocarriers
2.4. Co-Delivery of SF and Other Drugs
2.5. Imaging-Guided SF Nanodrugs
2.6. Actively Targeted Nanosystems
2.7. Combination of Ferroptosis and Others
3. Challenges and Opportunities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Yang, X.R.; Huang, X.W.; Wang, W.M.; Shi, R.Y.; Xu, Y.; Wang, Z.; Qiu, S.J.; Fan, J.Z. Sorafenib in treatment of patients with advanced hepatocellular carcinoma: A systematic review. J. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.F.; Tai, W.T.; Hsu, C.Y.; Huang, J.W.; Liu, C.Y.; Chen, P.J. Blockade of STAT3 Activation by sorafenib derivatives through enhancing SHP-1 phosphatase activity. Eur. J. Med. Chem. 2012, 55, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.T.; Cheng, A.L.; Shiau, C.W.; Huang, H.P.; Huang, J.W.; Chen, P.J. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J. Hepatol. 2011, 55, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Luo, C.; Li, Y.; Lu, C.; Zhou, W.; Huang, H. The study of a novel sorafenib derivative HLC-080 as an antitumor agent. PLoS ONE 2014, 9, e101889. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, B.; Gong, X.; Wang, T.; Liu, Y.; Zhang, N. In vivo biodistribution, biocompatibility, and efficacy of sorafenib-loaded lipid-based nanosuspensions evaluated experimentally in cancer. Int. J. Nanomed. 2016, 11, 2329–2343. [Google Scholar]
- Patel, K.C.; Bothiraja, A.; Mali, R.; Kamble. Investigation of sorafenib tosylate loaded liposomal dry powder inhaler for the treatment of non-small cell lung cancer. Part. Sci. Technol. 2021, 39, 990–999. [Google Scholar] [CrossRef]
- Pethe, A.M.; Yadav, K.S. Polymers, responsiveness and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 395–405. [Google Scholar] [CrossRef]
- Valencia-Lazcano, A.A.; Hassan, D.; Pourmadadi, M.; Shamsabadipour, A.; Behzadmehr, R.; Rahdar, A.; Medina, D.I.; Díez-Pascual, A.M. 5-Fluorouracil nano-delivery systems as a cutting-edge for cancer therapy. Eur. J. Med. Chem. 2023, 246, 114995. [Google Scholar] [CrossRef]
- Caro, C.; Pourmadadi, M.; Eshaghi, M.M.; Rahmani, E.; Shojaei, S.; Paiva-Santos, A.C.; Rahdar, A.; Behzadmehr, R.; García-Martín, M.L.; Díez-Pascual, A.M. Nanomaterials loaded with quercetin as an advanced tool for cancer treatment. J. Drug Deliv. Sci. Tech. 2022, 78, 103938. [Google Scholar] [CrossRef]
- Thapa, R.K.; Choi, J.Y.; Poudel, B.K.; Hiep, T.T.; Pathak, S.; Gupta, B. Multilayer-coated liquid crystalline nanoparticles for effective sorafenib delivery to hepatocellulr carcinoma. ACS Appl. Mater. Interfaces 2015, 7, 20360–20368. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sheu, A.Y.; Li, W.; Zhang, Z.; Kim, D.H.; Lewandowski, R.J. Poly(lactide-co-glycolide) Microspheres for MRI-monitored Transcatheter Delivery of Sorafenib to Liver Tumors. J. Control Release. 2014, 184, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.K.; Ye, Q.F.; Miao, X.Y.; Liu, X.; Huang, S.Q.; Xiong, L.; Yu, W.; Zhang, Z.J. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics 2021, 11, 5464–5490. [Google Scholar] [CrossRef]
- Chen, F.M.; Fang, Y.F.; Chen, X.; Deng, R.; Zhang, Y.J.; Shao, J.W. Recent advances of sorafenib nanoformulations for cancer therapy: Smart nanosystem and combination therapy. Asian J. Pharm. Sci. 2021, 16, 318–336. [Google Scholar] [CrossRef] [PubMed]
- Hrushikesh, R.; Chetana, J.; Karishma, S.; Neha, L.; Harsh, P.; Nijhawan, H.P.; Rao, G.K.; Rajasekhar, R.A.; Garima, J.C.; Patro, N.J.; et al. Sorafenib tosylate novel drug delivery systems: Implications of nanotechnology in both approved and unapproved indications. OpenNano 2022, 8, 100103. [Google Scholar]
- Shukla, S.K. Sorafenib loaded inhalable polymeric nanocarriers against non-small cell lung cancer. Pharm. Res. 2020, 37, 67. [Google Scholar] [CrossRef]
- Angela Gauthier, A.; Ho, M. Role of sorafenib in the treatment of advanced hepatocellular carcinoma: An update. Hepatol. Res. 2013, 43, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Su, Y.; Zhang, F.G.; Chen, K.R.; Xu, X.T.; Xu, L.; Zhou, J.P.; Wang, W. A dual-targeting reconstituted high density lipoprotein leveraging the synergy of sorafenib and anti-miRNA21 for enhanced hepatocellular carcinoma therapy. Acta Biomater. 2018, 75, 413–426. [Google Scholar] [CrossRef]
- Muller, R.H.; Runge, S.A.; Ravelli, V.; Thunemann, A.F.; Mehnert, W.; Souto, E.B. Cyclosporine-loaded solid lipid nanoparticles (SLN): Drug-lipid physicochemical interactions and characterization of drug incorporation. Eur. J. Pharm. Biopharm. 2008, 68, 535–544. [Google Scholar] [CrossRef]
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of active targeting lipid nanoparticles: Challenges and perspectives. Mater. Today Adv. 2022, 16, 100299. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Alavi, S.M.; Yahyazadeh, A. Formulation and therapeutic efficacy of PEG-liposomes of sorafenib for the production of NL-PEG-SOR FUM and NL-PEG-SOR TOS. Res. Chem. Intermed. 2022, 48, 3915–3935. [Google Scholar] [CrossRef]
- Bondì, M.L.; Botto, C.; Amore, E.; Emma, M.R.; Augello, G.; Craparo, E.F.; Cervello, C. Lipid nanocarriers containing sorafenib inhibit colonies formation inhuman hepatocarcinoma cells. Int. J. Pharm. 2015, 493, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.Y.; Yang, W.L.; Yu, M.J.; Sun, S.L.; Xie, B.G. Improved oral bioavailability and liver targeting of sorafenib solid lipid nanoparticles in rats. AAPS PharmSciTech. 2018, 19, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Sebastien, B.; Ferey, L.; Bruno, A.; Mebarek, N.; Gaelle, V.; Ananda, A.; Cathy, S.; Karen, G.; Philippe, B. Nucleoside-lipid-based nanocarriers for sorafenib delivery. Nanoscale Res. Lett. 2018, 13, 17–24. [Google Scholar]
- Ahiwale, R.J.; Chellampillai, B.; Pawar, A.P. Investigation of novel sorafenib tosylate loaded biomaterial-based nano-cochleates dispersion system for treatment of hepatocellular carcinoma. J. Dispers. Sci. Technol. 2022, 43, 1568–1586. [Google Scholar] [CrossRef]
- Bartos, A.; Iancu, I.; Ciobanu, L.; Onaciu, A.; Moldovan, C.; Moldovan, A.; Moldovan, R.C.; Tigu, A.B.; Stiufiuc, G.F.; Toma, V.; et al. Hybrid lipid nanoformulationsfor hepatoma therapy: Sorafenib loaded nanoliposomes—A preliminary study. Nanomaterials 2022, 12, 2833. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, L.; Jin, H.; Chen, Y.; Cheng, D.; Jiang, Y. Sorafenib-loaded long-circulating nanoliposomes for liver cancer therapy. BioMed Res. Inter. 2020, 2020, 1351046. [Google Scholar] [CrossRef]
- Liu, R.; Meng, Y.; Zhu, M.; Zhai, H.; Lv, W.; Wen, T.; Jin, N. Study on novel PtNP–sorafenib and its interaction with VEGFR2. J. Biochem. 2021, 170, 411–417. [Google Scholar] [CrossRef]
- Yang, Y.C.; Cai, J.; Yin, J.; Zhang, J.; Wang, K.L.; Zhang, Z.T. Heparin-functionalized pluronic nanoparticles to enhance the antitumor efficacy of sorafenib in gastric cancers. Carbohydr. Polym. 2016, 136, 782–790. [Google Scholar] [CrossRef]
- Jaleh, V.; Fatemeh, R.; Mahboubeh, R.; Ali, J. PEGylated trimethylchitosan emulsomes conjugated to octreotide for targeted delivery of sorafenib to hepatocellular carcinoma cells of HepG2. J. Liposome Res. 2019, 31, 64–78. [Google Scholar]
- Yao, Y.; Su, Z.; Liang, Y.; Zhang, N. pH-Sensitive carboxymethyl chitosan-modified cationic liposomes for sorafenib and siRNA co-delivery. Int. J. Nanomed. 2015, 10, 6185–6197. [Google Scholar]
- Dayani, L.; Dehghani, M.; Aghaei, M.; Taymouri, S.; Taheri, A. Preparation and evaluation of targeted albumin lipid nanoparticles with lactobionic acid for targeted drug delivery of sorafenib in hepatocellular carcinoma. J. Drug Deliv. Sci. Tech. 2022, 69, 103142. [Google Scholar] [CrossRef]
- Gopakumar, L.; Sreeranganathan, M.; Chappan, S. Enhanced oral bioavailability and antitumor therapeutic efficacy of sorafenib administered in core–shell protein nanoparticle. Drug Deliv. Transl. Res. 2022, 12, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef]
- Bondì, M.L.; Scala, A.; Sortino, G.; Amore, E.; Botto, C.; Azzolina, A.; Balasus, D.; Cervello, M.; Mazzaglia, A. Nanoassemblies based on supramolecular complexes of nonionic amphiphilic cyclodextrin and sorafenib as effective weapons to kill human HCC cells. Biomacromolecules 2015, 16, 3784–3791. [Google Scholar] [CrossRef]
- Mo, L.X.; Song, J.G.; Lee, H.K.; Zhao, M.J.; Kim, H.Y.; Lee, Y.J.; Ko, H.W.; Han, H.Y. PEGylated hyaluronic acid-coated liposome for enhanced in vivo efficacy of sorafenib via active tumor cell targeting and prolonged systemic exposure. Nanomedicine 2018, 14, 557–567. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, J.; Wu, B.; Shen, Z. pH-responsive hyaluronic acid nanoparticles coloaded with sorafenib and cisplatin for treatment of hepatocellular carcinoma. J. Biomater. Appl. 2019, 34, 219–228. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, M.D.; Choi, C.W.; Chung, C.W.; Ha, S.H.; Kim, C.H.; Shim, Y.H.; Jeong, Y.; Kang, D.H. Antitumor activity of sorafenib-incorporated nanoparticles of dextran/poly(dl-lactide-co-glycolide) block copolymer. Nanoscale. Res Lett. 2012, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.T.; Gao, D.Y.; Liu, Y.C.; Sung, Y.C.; Wan, D.H.; Liu, J.Y.; Chiang, T.; Wang, L.Y.; Chen, Y.C. Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J. Control. Release 2016, 221, 62–70. [Google Scholar] [CrossRef]
- Monajati, M.; Tavakoli, S.; Abolmaali, S.S.; Yousefi, G.; Tamaddon, A. Effect of PEGylation on assembly morphology and cellular uptake of poly ethyleneimine-cholesterol conjugates for delivery of sorafenib tosylate in hepatocellular carcinoma. BioImpacts 2018, 8, 1–252. [Google Scholar] [CrossRef] [Green Version]
- Melchiorre, C.; Giovanna, P.; Antonella, B.V.; Barbara, P.; Daniele, B.; Maria, R.E.; Antonina, A.; Roberto, P.; Guido, R.L.; Stefano, P.; et al. Nanoparticles of a polyaspartamide-based brush copolymer for modified release of sorafenib: In vitro and in vivo evaluation. J. Control. Release 2017, 266, 47–56. [Google Scholar]
- Yang, D.; Armitage, B.; Marder, S.R. Cubic Liquid-crystalline nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 4402–4409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Wang, X.; Liu, J.; Wang, Z.; Liu, H. In vitro and in vivo evaluation of redox-responsive sorafenib carrier nanomicelles synthesized from poly(acryic acid)-cystamine hydrochloride-D-alpha-tocopherol succinate. J. Biomater. Sci. Polym. Ed. 2016, 27, 1729–1747. [Google Scholar] [CrossRef]
- Känkänen, V.; Fernandes, M.; Liu, Z.; Seitsonen, J.; Hirvonen, S.; Ruokolainen, J.; Pinto, J.F.; Hirvonen, J.; Balasubramanian, V.; Santos, H.A. Microfluidic preparation and optimization of sorafenib-loaded poly(ethylene glycol-block-caprolactone) nanoparticles for cancer therapy applications. J. Colloid Interface Sci. 2023, 633, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hu, Y.; Lin, A.; Ma, C.; Zhang, C.; Su, Y. Matrix metalloproteinase responsive nanoparticles for synergistic treatment of colorectal cancer via simultaneous anti-angiogenesis and chemotherapy. Bioconjug. Chem. 2016, 27, 2943–2953. [Google Scholar] [CrossRef]
- Fan, W.; Shen, B.; Bu, W.; Chen, F.; He, Q.; Zhao, K.; Zhang, S.; Zhou, L.; Xiao, Q.; Ni, D.; et al. A smart upconversion-based mesoporous silica nanotheranostic system for synergetic chemo-/radio-/photodynamic therapy and simultaneous MR/UCL imaging. Biomaterials 2014, 35, 8992–9002. [Google Scholar] [CrossRef]
- Barkat, A.; Beg, S.; Panda, S.K.; Alharbi, S.K.; Rahman, M.; Ahmed, F.J. Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin Cancer Biol. 2021, 69, 365–375. [Google Scholar] [CrossRef]
- Zheng, G.; Zhao, R.; Xu, A.; Shen, Z.; Chen, X.; Shao, J. Co-delivery of sorafenib and siVEGF based on mesoporous silica nanoparticles for ASGPR mediated targeted HCC therapy. Eur. J. Pharmac. Sci. 2018, 111, 492–502. [Google Scholar] [CrossRef]
- Zhang, B.C.; Luo, B.Y.; Zou, J.J.; Wu, P.Y.; Jiang, J.L.; Le, J.L.; Zhao, R.R.; Chen, L.; Shao, J.W. Co-delivery of sorafenib and CRISPR/Cas9 based on targeted core–Shell hollow mesoporous organosilica nanoparticles for synergistic HCC therapy. ACS Appl. Mater. Interfaces. 2020, 12, 57362–57372. [Google Scholar] [CrossRef]
- Tang, H.; Chen, D.; Li, C.; Zheng, C.; Wu, X.; Zhang, Y. Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells. Int. J. Pharm. 2019, 572, 118782. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wu, Z.; Chu, T.; Yang, Z.; Xu, S.; Wu, S.; Qie, Y.; Lu, Z.; Qi, F.; et al. Reducing postoperative recurrence of early-stage hepatocellular carcinoma by a wound-targeted nanodrug. Adv. Sci. 2022, 9, 2200477. [Google Scholar] [CrossRef]
- Dave, V.; Sharma, R.; Gupta, C.; Sur, C. Folic acid modified gold nanoparticle for targeted delivery of Sorafenib tosylate towards the treatment of diabetic retinopathy. Colloids Surf. B Biointerfaces 2020, 194, 111151. [Google Scholar] [CrossRef]
- Cai, H.; Yang, Y.; Peng, Y.; Liu, Y.; Fu, X.; Ji, B. Gold nanoparticles-loaded anti-mir221 enhances antitumor effect of sorafenib in hepatocellular carcinoma cells. Int. J. Med. Sci. 2019, 16, 1541–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukkar, F.; Shafaa, M.; Nagdy, M.; Darwish, W.; Korraa, S. Alginate/CaCO3 hybrid loaded with sorafenib tosylate and gold hexagons: A model for efficient dual (Chemo-Radio) treatment of HepG2 cells. Egypt. J. Chem. 2021, 16, 8309–8321. [Google Scholar] [CrossRef]
- Moni, S.S.; Alam, M.F.; Safhi, M.M.; Sultan, M.H.; Makeen, H.A.; Elmobark, M.E. Development of formulation methods and physical characterization of injectable sodium selenite nanoparticles for the delivery of sorafenib tosylate. Curr. Pharm. Biotechnol. 2020, 21, 659–666. [Google Scholar] [CrossRef]
- Zheng, L.; Li, C.; Huang, X.; Lin, X.; Lin, W.; Yang, F.; Chen, T. Thermosensitive hydrogels for sustained release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials 2019, 216, 119220. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Jin, H.E. Synthesis, characterization, and three-dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef] [Green Version]
- Nabil, A. Zinc oxide nanoparticle synergizes sorafenib anticancer efficacy with minimizing its cytotoxicity. Oxid. Med. Cell. Longev. 2020, 2020, 1362104. [Google Scholar] [CrossRef]
- György, B.; Biró, E.; Mónika, M.; Tivadar, F. Dual drug delivery of sorafenib and doxorubicin from PLGA and PEG-PLGA polymeric nanoparticles. Polymers 2018, 10, 895. [Google Scholar]
- Duan, D.W.; Liu, Y. Targeted and synergistic therapy for hepatocellular carcinoma: Monosaccharide modified lipid nanoparticles for the co-delivery of doxorubicin and sorafenib. Drug Des. Devel. Ther. 2018, 12, 2149–2161. [Google Scholar] [CrossRef] [Green Version]
- Mato, E.; Puras, G.; Bell, O.; Agirre, M.; Hernández, R.M.; Igartua, M.; Moreno, R.; Gonzalez, G.; Leiva, A.; Pedraz, J.L. Selective antitumoral effect of sorafenib loaded PLGA nanoparticles conjugated with cetuximab on undifferentiated/anaplastic thyroid carcinoma cells. J. Nanomed. Nanotechnol. 2015, 6, 1000281. [Google Scholar]
- Li, S.; Dong, S.; Xu, W.; Jiang, Y.; Li, Z. Polymer nanoformulation of sorafenib and all-trans retinoic acid for synergistic inhibition of thyroid cancer. Front. Pharmacol. 2020, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Yu, H.Y.; Zhang, D.W.; Wang, G.Y.; Song, W.T.; Liu, Y.M.; Ma, S.; Tang, Z.H.; Liu, Z.L.; Sakurai, K.; et al. Co-administration of combretastatin A4 nanoparticles and sorafenib before systemic therapy of hepatocellular carcinoma. Acta Biomater. 2019, 92, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Pawde, D.; Dumpala, R.L. Preparation of Sorafenib tosylate self-emulsified drug delivery system and the effect on combination therapy with Bosutinib against HCT116/SW1417 cells. Results Chem. 2022, 4, 100385. [Google Scholar] [CrossRef]
- Yin, X.; Xiao, Y.; Han, L.; Zhang, B.; Wang, T.; Su, Z.; Zhang, N. Ceramide-fabricated co-loaded liposomes for the synergistic treatment of hepatocellular carcinoma. AAPS PharmSciTech. 2018, 19, 2133–2143. [Google Scholar] [CrossRef]
- Sukkar, F.; Shafaa, M.; El-Nagdy, M.; Darwish, W. Polymeric nanocarriers for effective synergistic action of sorafenib tosylate and gold-sensitized gamma radiation against HepG2 Cells. Inter. J. Nanomed. 2021, 16, 8309–8321. [Google Scholar] [CrossRef]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef]
- Rao, Y.F.; Chen, W.; Liang, X.G.; Huang, Y.Z.; Miao, J.; Liu, Y.; Lou, Y.; Zhang, X.G.; Wang, B.; Tang, R.K. Epirubicin-loaded superparamagnetic iron-oxide nanoparticles for transdermal delivery: Cancer therapy by circumventing the skin barrier. Small 2015, 11, 239–247. [Google Scholar] [CrossRef]
- Taratula, O.; Garbuzenko, O.; Savla, R.; Wang, Y.A.; Minko, H.T. Multifunctional nanomedicine platform for cancer-specific delivery of siRNA by superparamagnetic iron oxide nanoparticles-dendrimer complexes. Curr. Drug Deliv. 2011, 8, 59–69. [Google Scholar] [CrossRef]
- Grillone, A.; Riva, E.R.; Mondini, A.; Forte, C.; Calucci, L.; Innocenti, C.; de Julian Fernandez, C.; Cappello, V.; Gemmi, M.; Moscato, S.; et al. Active targeting of sorafenib: Preparation, characterization, and in vitro testing of drug-loaded magnetic solid lipid nanoparticles. Adv. Healthc. Mater. 2015, 4, 1681–1690. [Google Scholar] [CrossRef]
- Greeshma, T.; Sheena, P.; Rimal, I.; Praseetha, P.K.; Jiji, S.G.; Asha, V.V. Preparation of an efficient and safe polymeric-magnetic nanoparticle delivery system for sorafenib in hepatocellular carcinoma. Life Sci. 2018, 206, 10–21. [Google Scholar]
- Cai, M.; Li, B.; Lin, L.; Huang, J.; An, Y.; Huang, W.; Zhou, Z.; Wang, Y.; Shuai, X.; Zhu, K. A reduction and pH dual-sensitive nanodrug for targeted theranostics in hepatocellular carcinoma. Biomater. Sci. 2020, 8, 3485–3499. [Google Scholar] [CrossRef]
- Amir, H.; Homayon, A.P.; Mehdi, F.; Fatemeh, F. Synthesis of thermosensitive magnetic nanocarrier for controlled sorafenib delivery. Mater. Sci. Eng. C. 2016, 67, 42–50. [Google Scholar]
- Dahiya, M.; Awasthi, R.; Dua, K.; Dureja, H. Sorafenib tosylate loaded superparamagnetic nanoparticles: Development, optimization and cytotoxicity analysis on HepG2 human hepatocellular carcinoma cell line. J. Drug Deliv. Sci. Tech. 2023, 79, 104044. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.J.; Yang, S.; Zhang, B.; Wang, T.Q.; Jiang, D.D.; Zhang, J.; Yu, D.X.; Zhang, N. Sorafenib and gadolinium co-loaded liposomes for drug delivery and MRI-guided HCC treatment. Colloids Surf. B. 2016, 141, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qing, X.; Liu, S.; Yang, D.; Dong, X.; Zhang, Y. Hollow mesoporous manganese oxides: Application in cancer diagnosis and therapy. Small 2022, 18, e2106511. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duan, J.; Liu, Y.; Kuang, Y.; Duan, Y.; Liao, J.; Xu, T.; Jiang, Z.; Li, B. Multi-stimuli responsive hollow MnO2-based drug delivery system for magnetic resonance imaging and combined chemo-chemodynamic cancer therapy. Acta Biomater. 2021, 126, 445–462. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, C.; Liu, J.; Hu, S.; Yu, J.; Yin, Q.; Tian, H.; Ding, Z.; Qi, G.; Wang, L.; et al. Aptamer-mediated hollow MnO2 for targeting the delivery of sorafenib. Drug Deliv. 2023, 30, 28–39. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Gui, J.; Liu, P.; Huang, Y.; Shao, B.; Ping, Y.; Li, B. A biomimetic nanodrug self-assembled from small molecules for enhanced ferroptosis therapy. Biomater. Sci. 2022, 10, 770–780. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Dai, Y.; Zhao, Q.; Zhu, L.; Guan, X.; Li, G.; Yang, S.; Yuan, Z. NIR-II dual-modal optical coherence tomography and photoacoustic imaging-guided dose-control cancer chemotherapy. ACS Appl. Polym. Mater. 2020, 2, 1964–1973. [Google Scholar] [CrossRef]
- Park, J.Y.; You, S.J.; Park, K.; Song, Y.J.; Park, J.; Yang, D.H. Cyclic RGDfK- and Sulfo-Cy5.5-functionalized mPEG-PCL theranostic nanosystems for hepatocellular carcinoma. J. Indus. Eng. Chem. 2021, 99, 204–213. [Google Scholar] [CrossRef]
- Feng, S.; Zhou, J.; Li, Z.; Appelman, H.D.; Zhao, L.L.; Zhu, J.Y.; Thomas, D. Sorafenib encapsulated in nanocarrier functionalized with glypican-3 specific peptide for targeted therapy of hepatocellular carcinoma. Colloids Surf. B. 2019, 184, 10. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, T.K.; Hitesh, K.; Lakshma, N.V.; Madhusudana, M.; Suresh, B.; Deep, P.; Ramakrishna, S. Modulating the site-specific oral delivery of sorafenib using sugar-grafted nanoparticles for hepatocellular carcinoma treatment. Eur. J. Pharm. Sci. 2019, 137, 104978. [Google Scholar]
- Li, Y.W.; Wei, J.J.; Wei, Y.H.; Cheng, L.; Guo, B.B.; Meng, F.H.; Li, F.; Zhong, Z.Y. Apolipoprotein E peptide-guided disulfide-cross-linked micelles for targeted delivery of sorafenib to hepatocellular carcinoma. Biomacromolecules 2020, 21, 716–724. [Google Scholar] [CrossRef]
- Gao, D.Y.; Lin, T.T.; Sung, Y.C.; Liu, Y.C.; Chiang, W.H.; Chang, C.H.; Liu, J.Y.; Chen, Y.C. CXCR4-tareted lipid-coated PLGA nanoparticles deliver sorafenib and overcome acquired drug resistance in liver cancer. Biomaterials 2015, 67, 194–203. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, F.; Zhang, F. Targeted therapy for humanhepatic carcinoma cells using folate-functionalized polymeric micelles loaded with superparamagnetic iron oxide and sorafenib in vitro. Int. J. Nanomed. 2013, 8, 1517–1524. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Dong, M.; Kong, F.M. Folate-decorated anticancer drug and magnetic nanoparticles encapsulated polymeric carrier for liver cancer therapeutics. Int. J. Pharm. 2015, 489, 83–90. [Google Scholar] [CrossRef]
- Wang, H.P.; Sun, S.L.; Zhang, Y.; Wang, J.Y.; Zhang, S.H.; Yao, X.B.; Chen, L.; Gao, Z.; Xie, B.G. Improved drug targeting to liver tumor by sorafenib-loaded folate-decorated bovine serum albumin nanoparticles. Drug Deliv. 2019, 26, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.; Li, Y.; Tian, Y.; Ma, M.; Shi, K.; Shang, X.; Yuan, H.; Hu, F. A Hypoxia-sensitive drug delivery system constructed by nitroimidazole and its application in the treatment of hepatocellular carcinoma. AAPS PharmSciTech. 2022, 23, 167. [Google Scholar] [CrossRef]
- Shen, J.; Cai, W.; Ma, Y.; Xu, R.; Huo, Z.; Song, L. hGC33-Modified and sorafenib-loaded nanoparticles have a synergistic anti-hepatoma effect by inhibiting Wnt signaling pathway. Nanoscale Res. Lett. 2020, 15, 220. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, J.; Wang, K.; Guo, S.; Yang, W. Zwitterionic polymer coated sorafenib-loaded Fe3O4 composite nanoparticles induced ferroptosis for cancer therapy. J. Mater. Chem. B 2022, 10, 5784–5795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Xu, W.; Zhang, X.; Wang, S.; Li, Y.; Yang, L.; Cai, R.; Xu, Q.; Li, G.; Guo, X. Complex of nanocarriers based on the metal polyphenol network: Multi-modal synergistic inhibition of tumor cell proliferation by inducing ferroptosis and photodynamic effect. New J. Chem. 2022, 46, 21962–21967. [Google Scholar] [CrossRef]
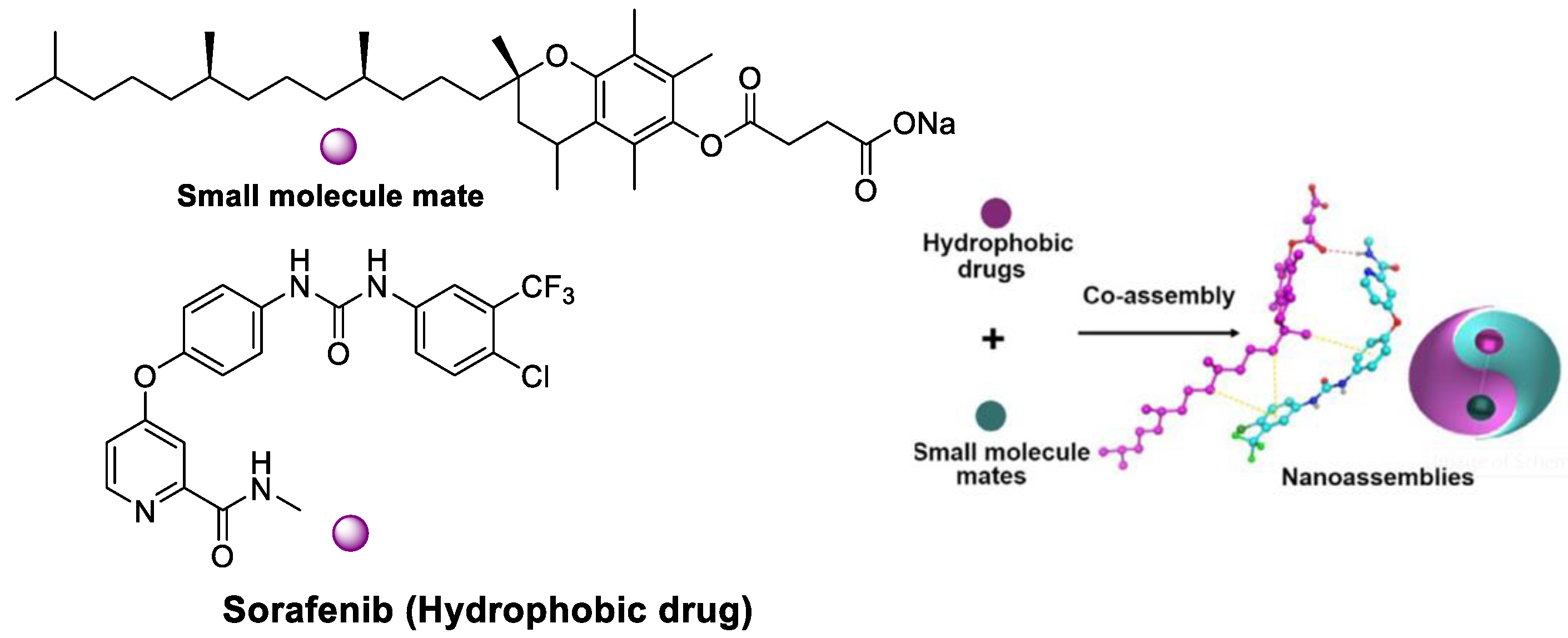
- Han, L.; Liang, S.; Mu, W.; Zhang, Z.; Wang, L.; Ouyang, S.; Yao, B.; Liu, Y.; Zhang, N. Amphiphilic small molecular mates match hydrophobic drugs to form nanoassemblies based on drug-mate strategy. Asian J. Pharm. Sci. 2022, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]











| NPs | Average Particle Size (nm) | Zeta Potential (mV) | Entrapment Efficiency (EE%) | Drug Loading (DL%) | Application | Ref |
|---|---|---|---|---|---|---|
| Cyclodextrin @SF | 200 | −11 | 100 | 17 | HCC cell line | [35] |
| PEI-cholesterol @SF | 30 | 12.4 | - | 13.1 | HepG2 cells | [40] |
| PLGA@ cetuximab @SF | 277 | −11.1 | - | - | CAL-62 and Nthyori 3-1 cell | [61] |
| PEG-PLGA @SF | 230 | - | - | 15% | IC50: 2.6 μM (HSC); IC50: 1.6 μM (HUVEC); liver fibrosis of C3H mice | [39] |
| Glypican-3@SF | 114 | −20.9 | >80% | [SF]: 69.5 mg/L, T1/2 = 22.7 h | HCC cells in vitro, growth inhibition of HCC tumors | [82] |
| SPIONs @SF | 5–15 | - | 76.37% | - | HCC cells, Wistar rats | [71] |
| PEGylated galactose @SF | 111 | −19.8 | 95 | - | HepG2 cells and BALB/c mice | [83] |
| lipid@SF | 221 | −37 | 100 | 18.46 | Four different HCC cell lines | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chen, M.; Ran, X.; Tang, H.; Cao, D. Sorafenib-Based Drug Delivery Systems: Applications and Perspectives. Polymers 2023, 15, 2638. https://doi.org/10.3390/polym15122638
Wang L, Chen M, Ran X, Tang H, Cao D. Sorafenib-Based Drug Delivery Systems: Applications and Perspectives. Polymers. 2023; 15(12):2638. https://doi.org/10.3390/polym15122638
Chicago/Turabian StyleWang, Lingyun, Meihuan Chen, Xueguang Ran, Hao Tang, and Derong Cao. 2023. "Sorafenib-Based Drug Delivery Systems: Applications and Perspectives" Polymers 15, no. 12: 2638. https://doi.org/10.3390/polym15122638
APA StyleWang, L., Chen, M., Ran, X., Tang, H., & Cao, D. (2023). Sorafenib-Based Drug Delivery Systems: Applications and Perspectives. Polymers, 15(12), 2638. https://doi.org/10.3390/polym15122638







