Toughening Weak Polyampholyte Hydrogels with Weak Chain Entanglements via a Secondary Equilibrium Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyampholyte (PA) Hydrogels
2.3. Preparation of PA-M Hydrogels
2.4. Volume Swelling Ratio of Hydrogels
2.5. Scanning Electron Microscopy (SEM)
2.6. Contact Angle Measurements
2.7. Tensile Tests
3. Results and Discussion
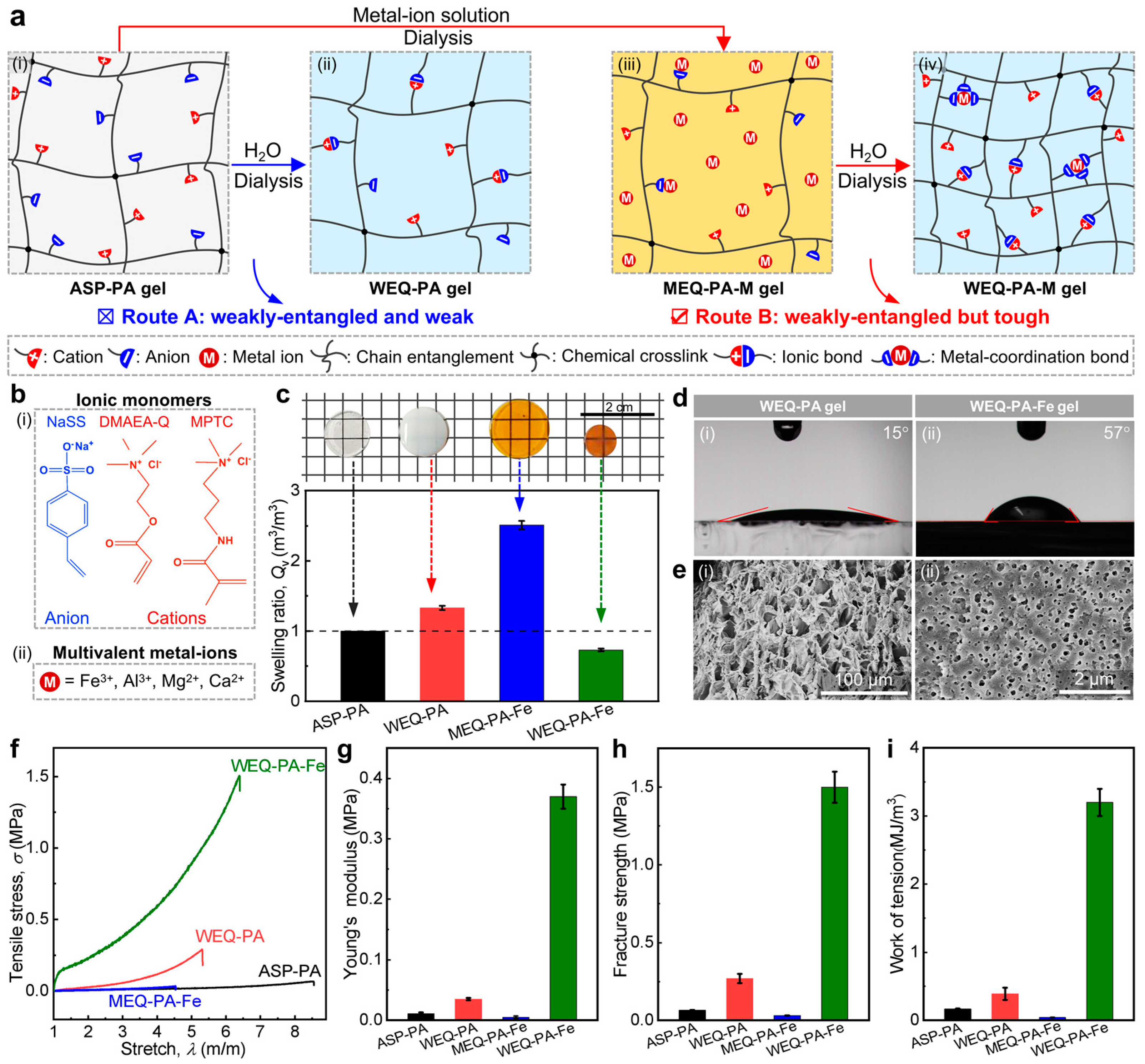
3.1. Design, Preparation, and Characterizations
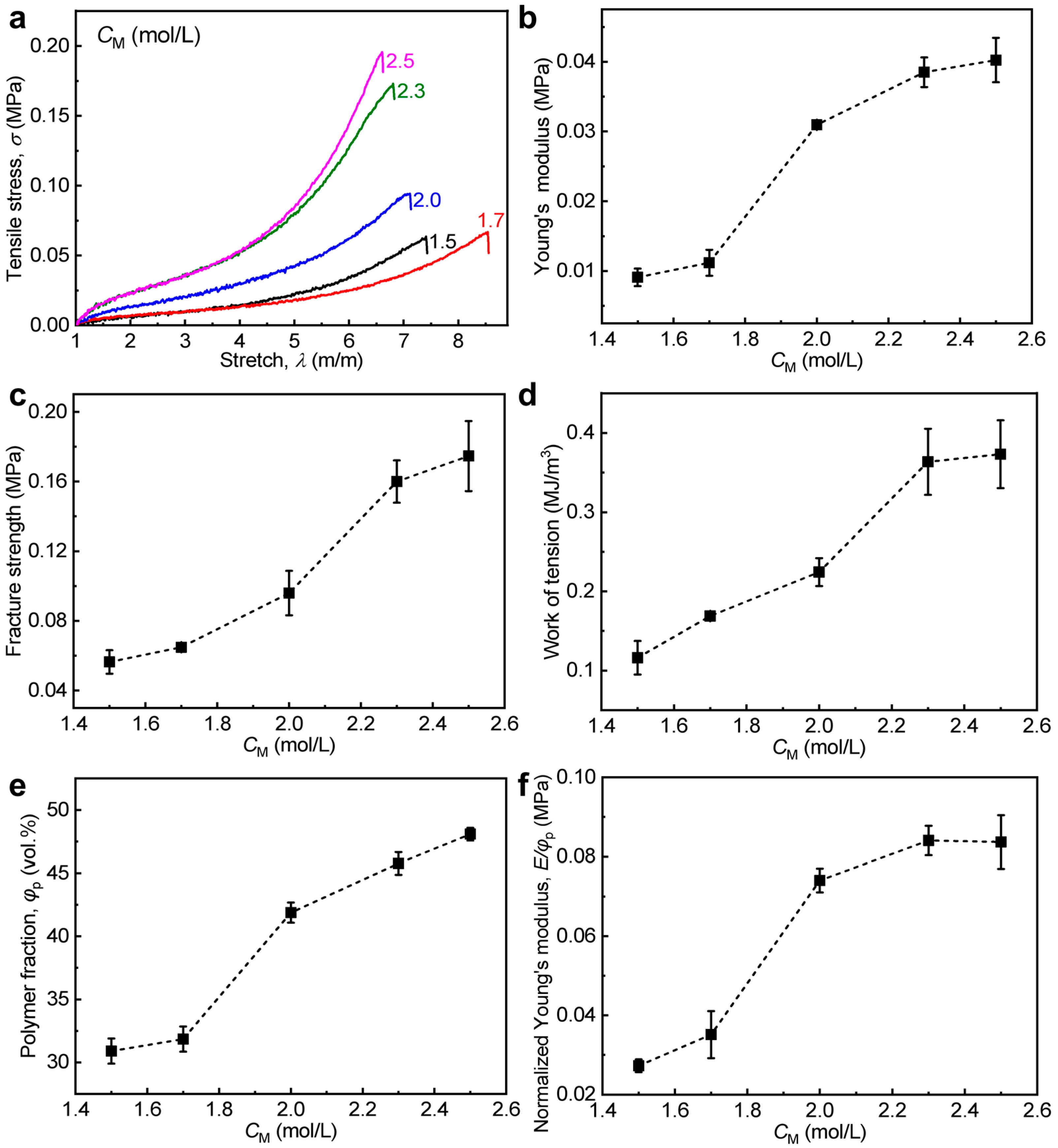
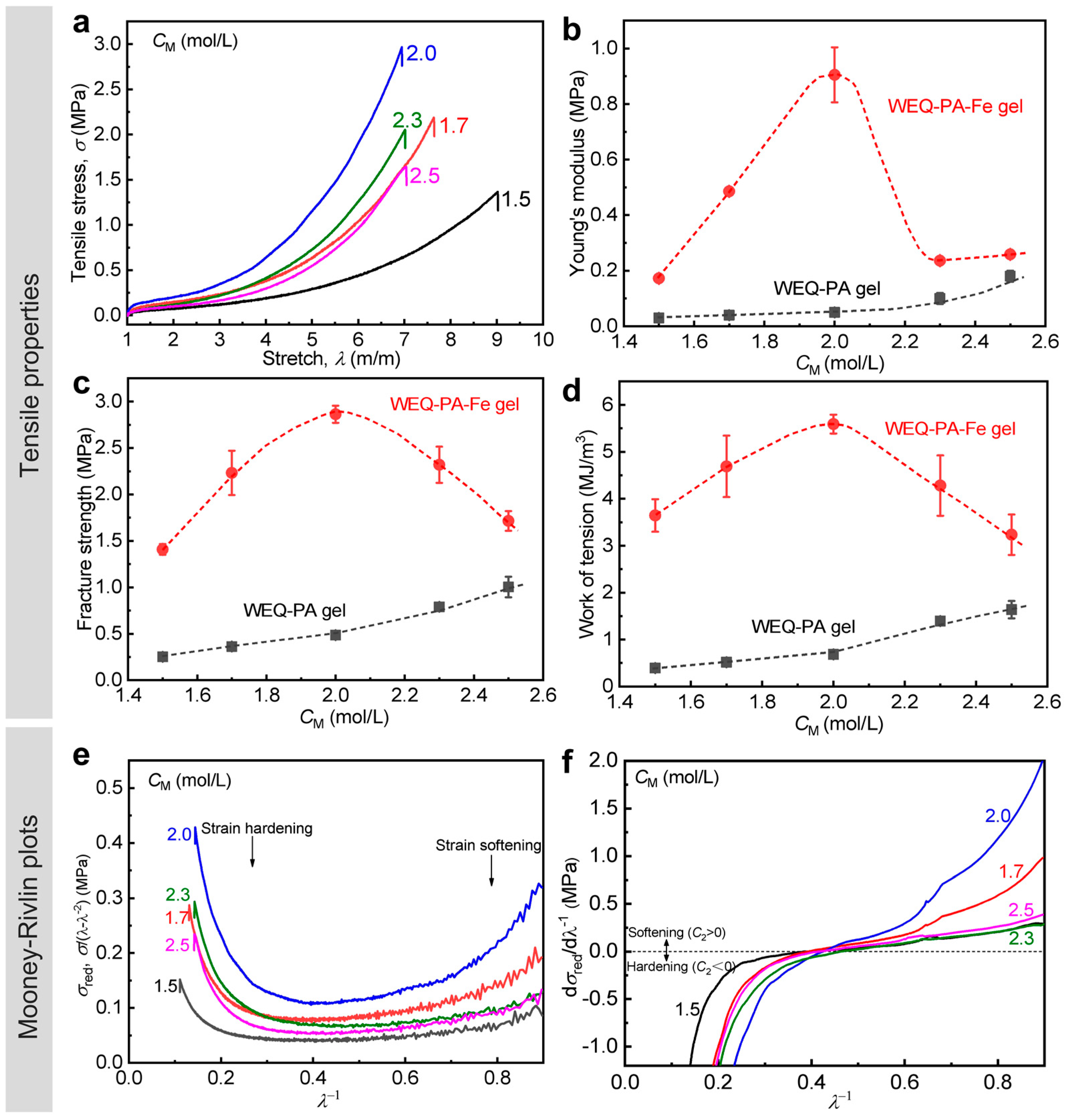
3.2. Effect of Monomer Concentration in Pre-Gel Solutions
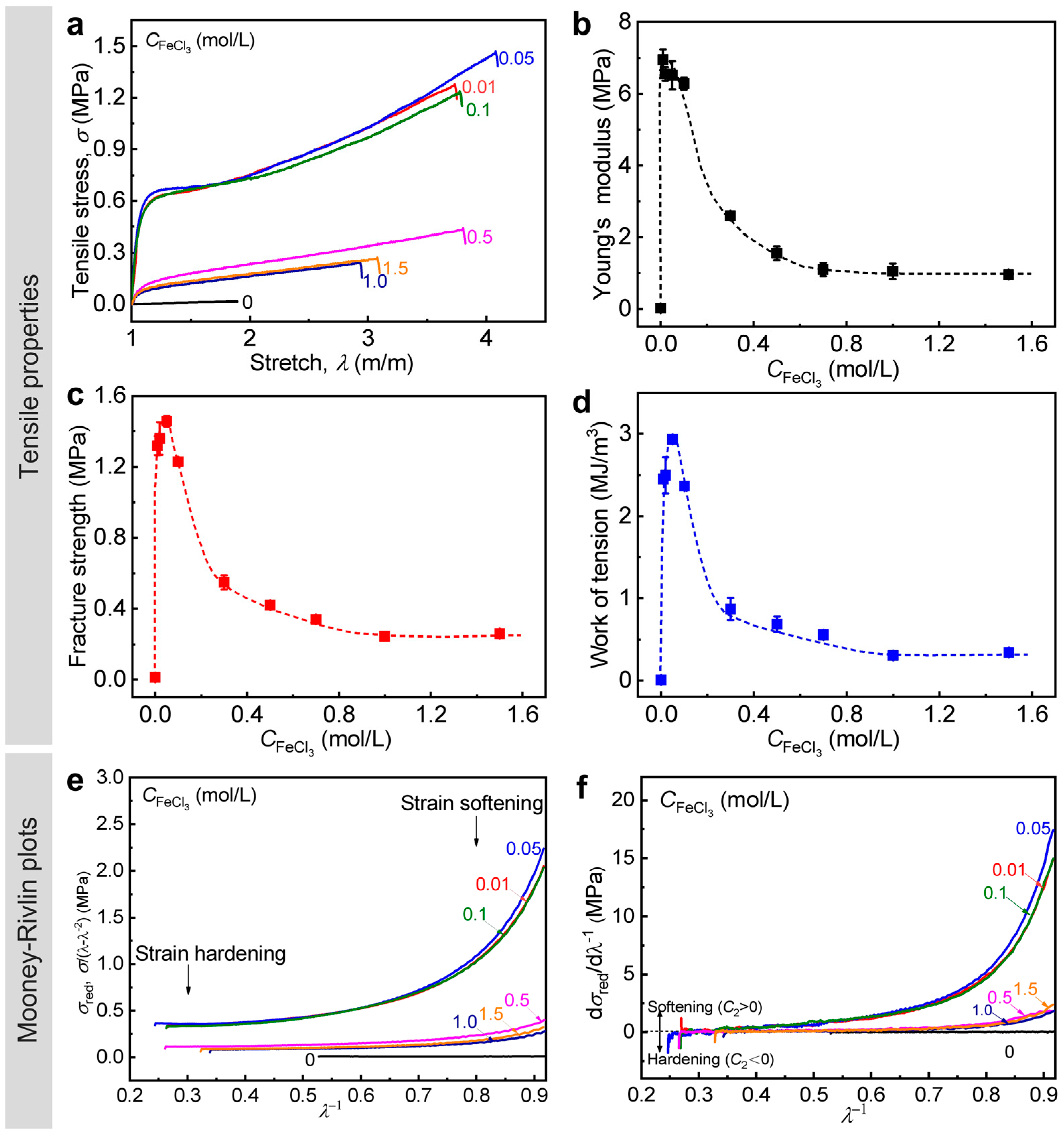
3.3. Effect of Metal ion Concentration of Dialysis Solution
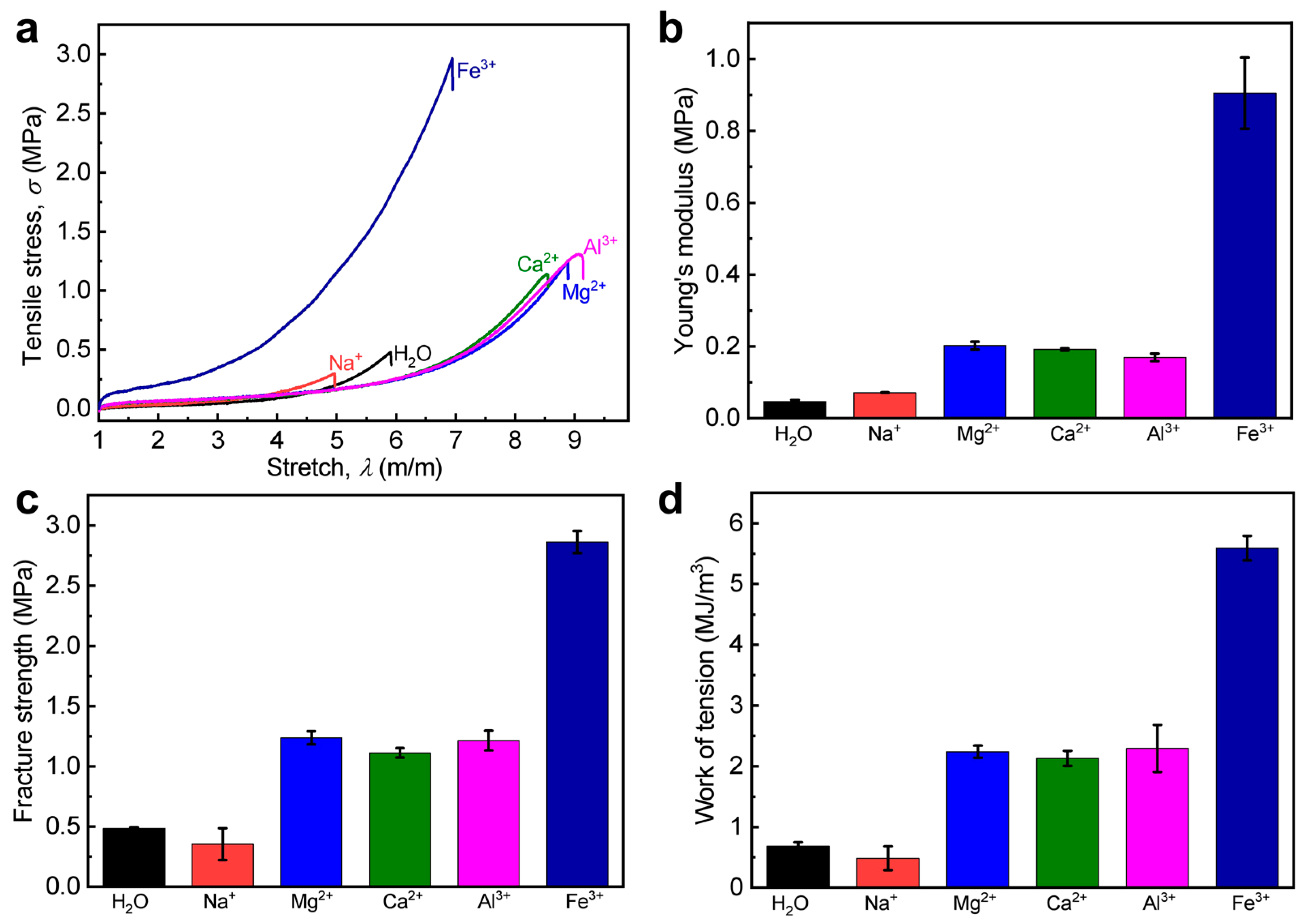
3.4. Generality of the Proposed Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and Swelling Properties of Graphene Oxide/Poly(Acrylic Acid-Co-Acrylamide) Super-Absorbent Hydrogel Nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Wei, H.; Lei, M.; Zhang, P.; Leng, J.; Zheng, Z.; Yu, Y. Orthogonal Photochemistry-Assisted Printing of 3D Tough and Stretchable Conductive Hydrogels. Nat. Commun. 2021, 12, 2082. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wu, H.; Xie, W.; Jia, H.; Xia, Z.; Wang, H. Metal Cation-Responsive and Excitation-Dependent Nontraditional Multicolor Fluorescent Hydrogels for Multidimensional Information Encryption. ACS Appl. Mater. Interfaces 2021, 13, 39967–39975. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Wang, L.; Makihata, M.; Liu, H.-C.; Zhou, T.; Zhao, X. Bioadhesive Ultrasound for Long-Term Continuous Imaging of Diverse Organs. Science 2022, 377, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bourquard, C.; Gao, Q.; Jiang, S.; De Iure-Grimmel, T.; Huo, R.; Li, X.; He, Z.; Yang, Z.; Yang, G.; et al. Controlled Tough Bioadhesion Mediated by Ultrasound. Science 2022, 377, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Na, H.; Kang, Y.-W.; Park, C.S.; Jung, S.; Kim, H.-Y.; Sun, J.-Y. Hydrogel-Based Strong and Fast Actuators by Electroosmotic Turgor Pressure. Science 2022, 376, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zheng, S.Y.; Hao, X.P.; Hong, W.; Zheng, Q.; Wu, Z.L. Spontaneous and Rapid Electro-Actuated Snapping of Constrained Polyelectrolyte Hydrogels. Sci. Adv. 2022, 8, eabm9608. [Google Scholar] [CrossRef]
- Sun, Y.; Le, X.; Zhou, S.; Chen, T. Recent Progress in Smart Polymeric Gel-Based Information Storage for Anti-Counterfeiting. Adv. Mater. 2022, 34, 2201262. [Google Scholar] [CrossRef]
- Liang, X.; Chen, G.; Lin, S.; Zhang, J.; Wang, L.; Zhang, P.; Lan, Y.; Liu, J. Bioinspired 2D Isotropically Fatigue-Resistant Hydrogels. Adv. Mater. 2022, 34, 2107106. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Zhang, W.; Wang, M.; Yan, L.; Wang, K.; Han, L.; Lu, X. Infant Skin Friendly Adhesive Hydrogel Patch Activated at Body Temperature for Bioelectronics Securing and Diabetic Wound Healing. ACS Nano 2022, 16, 8662–8676. [Google Scholar] [CrossRef]
- Bian, X.; Cui, C.; Qi, Y.; Sun, Y.; Zhang, Z.; Liu, W. Amino Acid Surfactant-Induced Superfast Gelation of Silk Fibroin for Treating Noncompressible Hemorrhage. Adv. Funct. Mater. 2022, 32, 2207349. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas Aeruginosa -Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Xu, H.; Cheng, Z.; Yan, J.; Xie, X.-M. Biomimetic Gradient Hydrogel Actuators with Ultrafast Thermo-Responsiveness and High Strength. ACS Appl. Mater. Interfaces 2022, 14, 32541–32550. [Google Scholar] [CrossRef]
- Chen, G.; Wang, K.; Yang, J.; Huang, J.; Chen, Z.; Zheng, J.; Wang, J.; Yang, H.; Li, S.; Miao, Y.; et al. Printable Thermochromic Hydrogel-Based Smart Window for All-Weather Building Temperature Regulation in Diverse Climates. Adv. Mater. 2023, 35, 2211716. [Google Scholar] [CrossRef]
- Zhong, R.; Talebian, S.; Mendes, B.B.; Wallace, G.; Langer, R.; Conde, J.; Shi, J. Hydrogels for RNA Delivery. Nat. Mater. 2023. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Yuan, Y.; Liu, C.; Zhang, M.; Zhang, L.; Wan, P. Flexible Antiswelling Photothermal-Therapy MXene Hydrogel-Based Epidermal Sensor for Intelligent Human–Machine Interfacing. Adv. Funct. Mater. 2023, 33, 2300299. [Google Scholar] [CrossRef]
- He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid Assembly of Polymeric Nanofiber Network for Robust and Electronically Conductive Hydrogels. Nat. Commun. 2023, 14, 759. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Chen, Y.; Wang, Z.; Liu, H.; Le, M.; Lin, C.; Wu, G.; Wang, L.; Shi, X.; Jia, Y.-G.; et al. Polymerizable Rotaxane Hydrogels for Three-Dimensional Printing Fabrication of Wearable Sensors. Nat. Commun. 2023, 14, 1331. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Hou, L.; Wu, P. Synergetic Lithium and Hydrogen Bonds Endow Liquid-Free Photonic Ionic Elastomer with Mechanical Robustness and Electrical/Optical Dual-Output. Adv. Mater. 2023, 35, 2211342. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Huang, J.; Xiong, J.; Liu, C.; Chen, F.; Shen, J.; Huang, Y.; Li, X. Designing Multistimuli-Responsive Anisotropic Bilayer Hydrogel Actuators by Integrating LCST Phase Transition and Photochromic Isomerization. Polymers 2023, 15, 786. [Google Scholar] [CrossRef]
- Gong, J.P. Why Are Double Network Hydrogels so Tough? Soft Matter 2010, 6, 2583. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U. Il Design and Fabrication of a High-Strength Hydrogel with Ideally Homogeneous Network Structure from Tetrahedron-like Macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]
- Liu, C.; Morimoto, N.; Jiang, L.; Kawahara, S.; Noritomi, T.; Yokoyama, H.; Mayumi, K.; Ito, K. Tough Hydrogels with Rapid Self-Reinforcement. Science 2021, 372, 1078–1081. [Google Scholar] [CrossRef]
- Matsuda, T.; Kawakami, R.; Namba, R.; Nakajima, T.; Gong, J.P. Mechanoresponsive Self-Growing Hydrogels Inspired by Muscle Training. Science 2019, 363, 504–508. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Zhou, J.; Li, X.; Liu, J.; Zeng, M. Mechanical Enhancement of Graphene Oxide-Filled Chitosan-Based Composite Hydrogels by Multiple Mechanisms. J. Mater. Sci. 2020, 55, 14690–14701. [Google Scholar] [CrossRef]
- Yan, Y.; Xiao, L.; Teng, Q.; Jiang, Y.; Deng, Q.; Li, X.; Huang, Y. Strong, Tough, and Adhesive Polyampholyte/Natural Fiber Composite Hydrogels. Polymers 2022, 14, 4984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, Y.; Li, M.; Shang, Q.; Xie, R.; Yu, J.; Shen, K.; Zhang, Y.; Cheng, Y. Toughening Double Network Hydrogels by Polyelectrolytes. Adv. Mater. 2023, 2301551. [Google Scholar] [CrossRef]
- Huang, Y.; King, D.R.; Sun, T.L.; Nonoyama, T.; Kurokawa, T.; Nakajima, T.; Gong, J.P. Energy-Dissipative Matrices Enable Synergistic Toughening in Fiber Reinforced Soft Composites. Adv. Funct. Mater. 2017, 27, 1605350. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wu, B.; Sun, S.; Wu, P. Aqueous Spinning of Robust, Self-Healable, and Crack-Resistant Hydrogel Microfibers Enabled by Hydrogen Bond Nanoconfinement. Nat. Commun. 2023, 14, 1370. [Google Scholar] [CrossRef]
- Hou, L.X.; Ju, H.; Hao, X.P.; Zhang, H.; Zhang, L.; He, Z.; Wang, J.; Zheng, Q.; Wu, Z.L. Intrinsic Anti-Freezing and Unique Phosphorescence of Glassy Hydrogels with Ultrahigh Stiffness and Toughness at Low Temperatures. Adv. Mater. 2023, 35, 2300244. [Google Scholar] [CrossRef]
- Xu, L.; Qiao, Y.; Qiu, D. Coordinatively Stiffen and Toughen Hydrogels with Adaptable Crystal-Domain Cross-Linking. Adv. Mater. 2023, 35, 2209913. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, Superstiff, and Conductive Alginate Hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Lian, W.Z.; Fan, Z.W.; Cui, K.; Yin, P.; Yang, J.; Jiang, H.; Tang, L.; Sun, T. Tough Hydrogels with Dynamic H-Bonds: Structural Heterogeneities and Mechanical Performances. Macromolecules 2021, 54, 8996–9006. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.; Chen, Y.; Zhang, L.; Zhou, J. Dual Physical Crosslinking Strategy to Construct Moldable Hydrogels with Ultrahigh Strength and Toughness. Adv. Funct. Mater. 2018, 28, 1800739. [Google Scholar] [CrossRef]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong Tough Hydrogels via the Synergy of Freeze-Casting and Salting Out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhang, X.N.; Sun, T.L.; Du, M.; Zheng, Q.; Wu, Z.L. Hydrogen-Bond Association-Mediated Dynamics and Viscoelastic Properties of Tough Supramolecular Hydrogels. Macromolecules 2021, 54, 4313–4325. [Google Scholar] [CrossRef]
- Cui, W.; Zheng, Y.; Zhu, R.; Mu, Q.; Wang, X.; Wang, Z.; Liu, S.; Li, M.; Ran, R. Strong Tough Conductive Hydrogels via the Synergy of Ion-Induced Cross-Linking and Salting-Out. Adv. Funct. Mater. 2022, 32, 2204823. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhao, Z.; Wang, M.; Dong, L.; Gao, H.; Liu, C.; Zhou, P.; Chen, L.; Shi, C.; et al. Anti-Swelling, Robust, and Adhesive Extracellular Matrix-Mimicking Hydrogel Used as Intraoral Dressing. Adv. Mater. 2022, 34, 2200115. [Google Scholar] [CrossRef]
- Xu, J.; Jin, R.; Ren, X.; Gao, G. Cartilage-Inspired Hydrogel Strain Sensors with Ultrahigh Toughness, Good Self-Recovery and Stable Anti-Swelling Properties. J. Mater. Chem. A 2019, 7, 25441–25448. [Google Scholar] [CrossRef]
- Zhang, X.N.; Du, C.; Wang, Y.J.; Hou, L.X.; Du, M.; Zheng, Q.; Wu, Z.L. Influence of the α-Methyl Group on Elastic-To-Glassy Transition of Supramolecular Hydrogels with Hydrogen-Bond Associations. Macromolecules 2022, 55, 7512–7525. [Google Scholar] [CrossRef]
- Huang, G.; Tang, Z.; Peng, S.; Zhang, P.; Sun, T.; Wei, W.; Zeng, L.; Guo, H.; Guo, H.; Meng, G. Modification of Hydrophobic Hydrogels into a Strongly Adhesive and Tough Hydrogel by Electrostatic Interaction. Macromolecules 2022, 55, 156–165. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, Y.; Qian, S.; Zeng, P.; Long, S.; Li, X. Strengthening and Stiffening in Swollen Polyampholyte Hydrogels. Mater. Lett. 2022, 324, 132582. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Zhou, J.; Liu, T.; Yan, Y.; Long, S.; Li, X. Strong Tough Polyampholyte Hydrogels via the Synergistic Effect of Ionic and Metal–Ligand Bonds. Adv. Funct. Mater. 2021, 31, 2103917. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly Stretchable and Tough Hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical Hydrogels Composed of Polyampholytes Demonstrate High Toughness and Viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihsan, A.B.; Sun, T.L.; Kuroda, S.; Haque, M.A.; Kurokawa, T.; Nakajima, T.; Gong, J.P. A Phase Diagram of Neutral Polyampholyte—From Solution to Tough Hydrogel. J. Mater. Chem. B 2013, 1, 4555. [Google Scholar] [CrossRef]
- Cui, K.; Sun, T.L.; Liang, X.; Nakajima, K.; Ye, Y.N.; Chen, L.; Kurokawa, T.; Gong, J.P. Multiscale Energy Dissipation Mechanism in Tough and Self-Healing Hydrogels. Phys. Rev. Lett. 2018, 121, 185501. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, K.; Kurokawa, T.; Ye, Y.N.; Sun, T.L.; Yu, C.; Creton, C.; Gong, J.P. Effect of Mesoscale Phase Contrast on Fatigue-Delaying Behavior of Self-Healing Hydrogels. Sci. Adv. 2021, 7, eabe8210. [Google Scholar] [CrossRef]
- Huang, Y.; King, D.R.; Cui, W.; Sun, T.L.; Guo, H.; Kurokawa, T.; Brown, H.R.; Hui, C.-Y.; Gong, J.P. Superior Fracture Resistance of Fiber Reinforced Polyampholyte Hydrogels Achieved by Extraordinarily Large Energy-Dissipative Process Zones. J. Mater. Chem. A 2019, 7, 13431–13440. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, S.; Zhou, J.; Chen, W.; Liu, T.; Yang, S.; Long, S.; Li, X. Achieving Swollen yet Strengthened Hydrogels by Reorganizing Multiphase Network Structure. Adv. Funct. Mater. 2023, 33, 2213549. [Google Scholar] [CrossRef]
- Obukhov, S.P.; Rubinstein, M.; Colby, R.H. Network Modulus and Superelasticity. Macromolecules 1994, 27, 3191–3198. [Google Scholar] [CrossRef]
- Sun, T.L.; Luo, F.; Kurokawa, T.; Karobi, S.N.; Nakajima, T.; Gong, J.P. Molecular Structure of Self-Healing Polyampholyte Hydrogels Analyzed from Tensile Behaviors. Soft Matter 2015, 11, 9355–9366. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y.; Shen, M.; Chen, F.; Fan, P.; Zhong, M.; Ren, B.; Yang, J.; Zheng, J. Structural Dependence of Salt-Responsive Polyzwitterionic Brushes with an Anti-Polyelectrolyte Effect. Langmuir 2018, 34, 97–105. [Google Scholar] [CrossRef]
- Lin, W.C.; Fan, W.; Marcellan, A.; Hourdet, D.; Creton, C. Large Strain and Fracture Properties of Poly(Dimethylacrylamide)/Silica Hybrid Hydrogels. Macromolecules 2010, 43, 2554–2563. [Google Scholar] [CrossRef]
- Wang, T.; Liu, D.; Lian, C.; Zheng, S.; Liu, X.; Tong, Z. Large Deformation Behavior and Effective Network Chain Density of Swollen Poly(N-Isopropylacrylamide)–Laponite Nanocomposite Hydrogels. Soft Matter 2012, 8, 774–783. [Google Scholar] [CrossRef]
- Nian, G.; Kim, J.; Bao, X.; Suo, Z. Making Highly Elastic and Tough Hydrogels from Doughs. Adv. Mater. 2022, 34, 2206577. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.C.; Li, C.Y.; Du, M.; Song, Y.; Wu, Z.L.; Zheng, Q. Improved Toughness and Stability of κ-Carrageenan/Polyacrylamide Double-Network Hydrogels by Dual Cross-Linking of the First Network. Macromolecules 2019, 52, 629–638. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A Found. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Li, Y.; Wen, J.; Qin, M.; Cao, Y.; Ma, H.; Wang, W. Single-Molecule Mechanics of Catechol-Iron Coordination Bonds. ACS Biomater. Sci. Eng. 2017, 3, 979–989. [Google Scholar] [CrossRef] [PubMed]


| ) (a) | E (MPa) | εb (m m−1) | σb (MPa) | Wb (MJ m−3) | Qv (m3 m−3) |
|---|---|---|---|---|---|
| WEQ-PA-1.5-Fe-0.3 | 0.17 ± 0.01 | 8.0 ± 0.1 | 1.4 ± 0.1 | 3.6 ± 0.3 | 0.52 ± 0.01 |
| WEQ-PA-1.7-Fe-0.3 | 0.48 ± 0.01 | 6.7 ± 0.1 | 2.2 ± 0.2 | 4.7 ± 0.6 | 0.62 ± 0.02 |
| WEQ-PA-2.0-Fe-0.3 | 0.90 ± 0.09 | 6.1 ± 0.1 | 2.9 ± 0.1 | 5.6 ± 0.2 | 0.57 ± 0.01 |
| WEQ-PA-2.3-Fe-0.3 | 0.23 ± 0.01 | 6.0 ± 0.1 | 2.3 ± 0.2 | 4.3 ± 0.6 | 0.70 ± 0.01 |
| WEQ-PA-2.5-Fe-0.3 | 0.26 ± 0.01 | 6.0 ± 0.1 | 1.7 ± 0.1 | 3.2 ± 0.4 | 0.70 ± 0.01 |
| WEQ-PA-2.0 | 0.05 ± 0.01 | 5.1 ± 0.1 | 0.48 ± 0.01 | 0.68 ± 0.06 | 0.94 ± 0.06 |
| WEQ-PA-2.0-Fe-0.01 | 0.53 ± 0.04 | 6.0 ± 0.1 | 2.3 ± 0.1 | 4.1 ± 0.1 | 0.59 ± 0.01 |
| WEQ-PA-2.0-Fe-0.02 | 0.52 ± 0.03 | 5.9 ± 0.1 | 2.4 ± 0.1 | 4.0 ± 0.2 | 0.61 ± 0.01 |
| WEQ-PA-2.0-Fe-0.05 | 0.59 ± 0.01 | 6.0 ± 0.1 | 2.4 ± 0.3 | 4.6 ± 0.7 | 0.58 ± 0.01 |
| WEQ-PA-2.0-Fe-0.1 | 0.80 ± 0.05 | 6.1 ± 0.1 | 2.7 ± 0.1 | 5.2 ± 0.3 | 0.58 ± 0.01 |
| WEQ-PA-2.0-Fe-0.5 | 0.72 ± 0.05 | 6.0 ± 0.1 | 2.5 ± 0.1 | 4.9 ± 0.6 | 0.60 ± 0.02 |
| WEQ-PA-2.0-Fe-0.7 | 0.58 ± 0.01 | 6.0 ± 0.1 | 2.2 ± 0.1 | 4.5 ± 0.1 | 0.60 ± 0.01 |
| WEQ-PA-2.0-Fe-1.0 | 0.51 ± 0.03 | 6.1 ± 0.1 | 2.2 ± 0.2 | 4.3 ± 0.4 | 0.61 ± 0.02 |
| WEQ-PA-2.0-Fe-1.5 | 0.52 ± 0.03 | 6.0 ± 0.1 | 2.2 ± 0.1 | 4.4 ± 0.2 | 0.61 ± 0.02 |
| WEQ-PA-2.0-Fe-2.0 | 0.52 ± 0.02 | 6.2 ± 0.1 | 2.4 ± 0.1 | 4.9 ± 0.4 | 0.60 ± 0.03 |
| ) (a) | E (MPa) | εb (m m−1) | σb (MPa) | Wb (MJ m−3) | Qv (m3 m−3) |
|---|---|---|---|---|---|
| WEQ-PA-1.5 | 0.02 ± 0.01 | 0.82 ± 0.02 | 0.01 ± 0.01 | 0.007 ± 0.001 | 4.8 ± 0.2 |
| WEQ-PA-1.5-Fe-0.01 | 7.0 ± 0.3 | 2.7 ± 0.1 | 1.3 ± 0.1 | 2.5 ± 0.1 | 0.68 ± 0.01 |
| WEQ-PA-1.5-Fe-0.02 | 6.6 ± 0.2 | 2.7 ± 0.1 | 1.4 ± 0.1 | 2.5 ± 0.2 | 0.67 ± 0.01 |
| WEQ-PA-1.5-Fe-0.05 | 6.5 ± 0.4 | 3.1 ± 0.1 | 1.5 ± 0.1 | 2.9 ± 0.1 | 0.66 ± 0.01 |
| WEQ-PA-1.5-Fe-0.1 | 6.3 ± 0.2 | 2.8 ± 0.1 | 1.2 ± 0.1 | 2.4 ± 0.1 | 0.67 ± 0.01 |
| WEQ-PA-1.5-Fe-0.3 | 2.6 ± 0.1 | 2.6 ± 0.1 | 0.55 ± 0.04 | 0.87 ± 0.13 | 1.1 ± 0.1 |
| WEQ-PA-1.5-Fe-0.5 | 1.6 ± 0.2 | 2.6 ± 0.1 | 0.42 ± 0.02 | 0.68 ± 0.09 | 1.3 ± 0.1 |
| WEQ-PA-1.5-Fe-0.7 | 1.1 ± 0.2 | 2.6 ± 0.1 | 0.34 ± 0.01 | 0.55 ± 0.05 | 1.4 ± 0.1 |
| WEQ-PA-1.5-Fe-1.0 | 1.0 ± 0.2 | 2.0 ± 0.1 | 0.24 ± 0.01 | 0.30 ± 0.01 | 1.6 ± 0.1 |
| WEQ-PA-1.5-Fe-1.5 | 1.0 ± 0.1 | 2.0 ± 0.1 | 0.26 ± 0.01 | 0.34 ± 0.03 | 1.5 ± 0.1 |
| ) (a) | E (MPa) | εb (m m−1) | σb (MPa) | Wb (MJ m−3) | Qv (m3 m−3) |
|---|---|---|---|---|---|
| WEQ-PA-2.0 | 0.05 ± 0.01 | 5.1 ± 0.1 | 0.48 ± 0.01 | 0.68 ± 0.06 | 0.94 ± 0.06 |
| WEQ-PA-2.0-Fe-0.3 | 0.91 ± 0.09 | 6.1 ± 0.1 | 2.9 ± 0.1 | 5.6 ± 0.2 | 0.57 ± 0.01 |
| WEQ-PA-2.0-Al-0.3 | 0.17 ± 0.01 | 7.9 ± 0.2 | 1.2 ± 0.1 | 2.3 ± 0.4 | 0.69 ± 0.01 |
| WEQ-PA-2.0-Mg-0.3 | 0.20 ± 0.01 | 7.7 ± 0.2 | 1.2 ± 0.1 | 2.2 ± 0.1 | 0.74 ± 0.01 |
| WEQ-PA-2.0-Ca-0.3 | 0.19 ± 0.01 | 7.5 ± 0.2 | 1.1 ± 0.1 | 2.1 ± 0.1 | 0.77 ± 0.02 |
| WEQ-PA-2.0-Na-0.3 | 0.071 ± 0.002 | 4.0 ± 0.1 | 0.35 ± 0.13 | 0.48 ± 0.02 | 0.96 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Chen, W.; Li, K.; Long, S.; Li, X.; Huang, Y. Toughening Weak Polyampholyte Hydrogels with Weak Chain Entanglements via a Secondary Equilibrium Approach. Polymers 2023, 15, 2644. https://doi.org/10.3390/polym15122644
Liu T, Chen W, Li K, Long S, Li X, Huang Y. Toughening Weak Polyampholyte Hydrogels with Weak Chain Entanglements via a Secondary Equilibrium Approach. Polymers. 2023; 15(12):2644. https://doi.org/10.3390/polym15122644
Chicago/Turabian StyleLiu, Tao, Wenjun Chen, Kai Li, Shijun Long, Xuefeng Li, and Yiwan Huang. 2023. "Toughening Weak Polyampholyte Hydrogels with Weak Chain Entanglements via a Secondary Equilibrium Approach" Polymers 15, no. 12: 2644. https://doi.org/10.3390/polym15122644





