Tensile and Surface Wettability Properties of the Solvent Cast Cellulose Fatty Acid Ester Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cellulose Fatty Acid Esters
2.1.1. Cellulose Dissolution and Esterification Procedure
2.1.2. Determination of Degree of Substitution (DS)
2.2. Film Casting
| Nr | Cellulose Ester Full Name | Ester | Cellulose Concentration (%) | DS | pH |
|---|---|---|---|---|---|
| 1 | Cellulose acetate—commercial (C2) | Acetate_COM | 15 | 2.48 | 4.67 |
| 2 | Cellulose laurate (C12) | Laurate | 7 | 1.5 | 5.50 |
| 3 | Cellulose myristate (C14) | Myristate | 7 | 1.7 | 4.86 |
| 4 | Cellulose palmitate (C16) | Palmitate | 7.1 | 0.8 | 4.51 |
| 5 | Cellulose stearate (C18) * | Stearate | 5.8 | 0.8 | 4.83 |
2.3. Characterization
3. Results
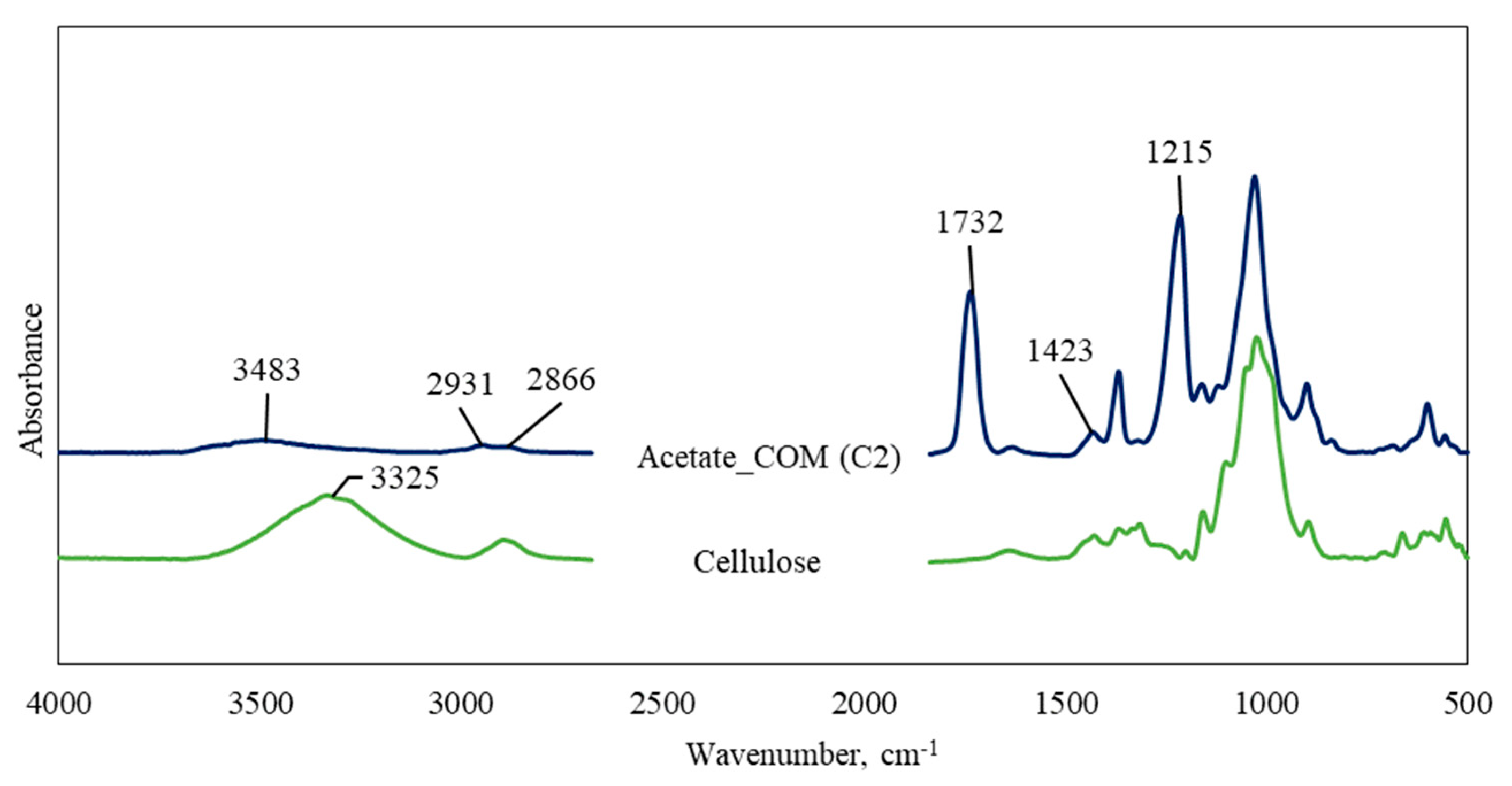
3.1. FTIR Characterisation
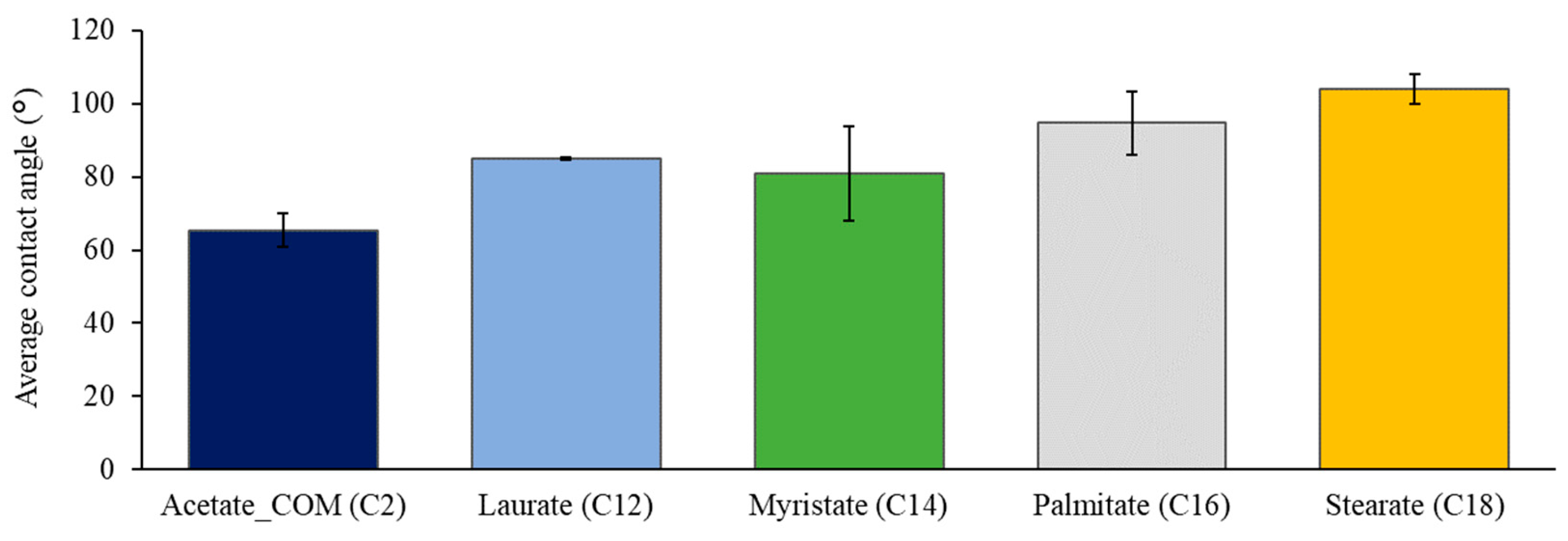
3.2. Surface Wettability
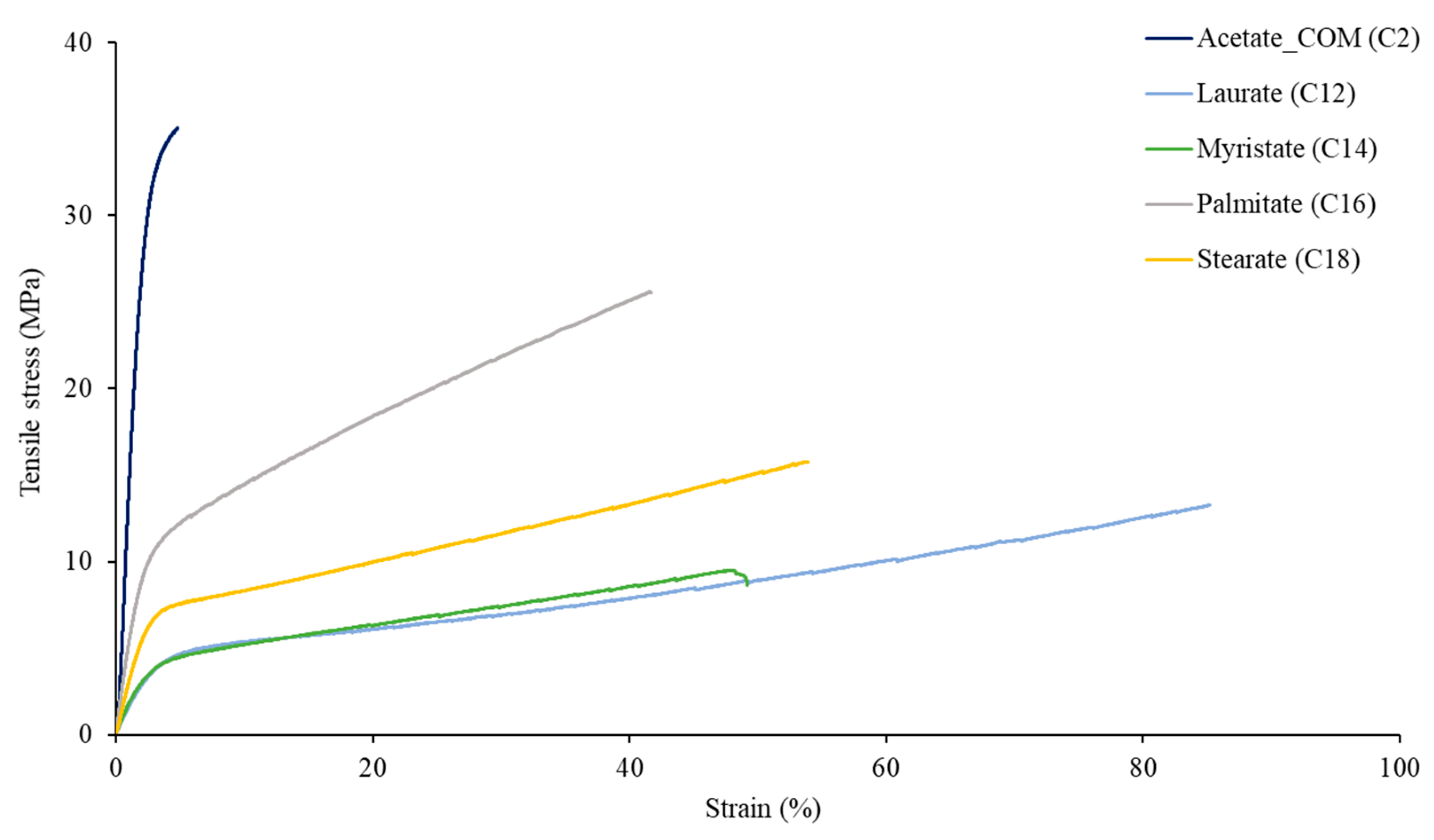
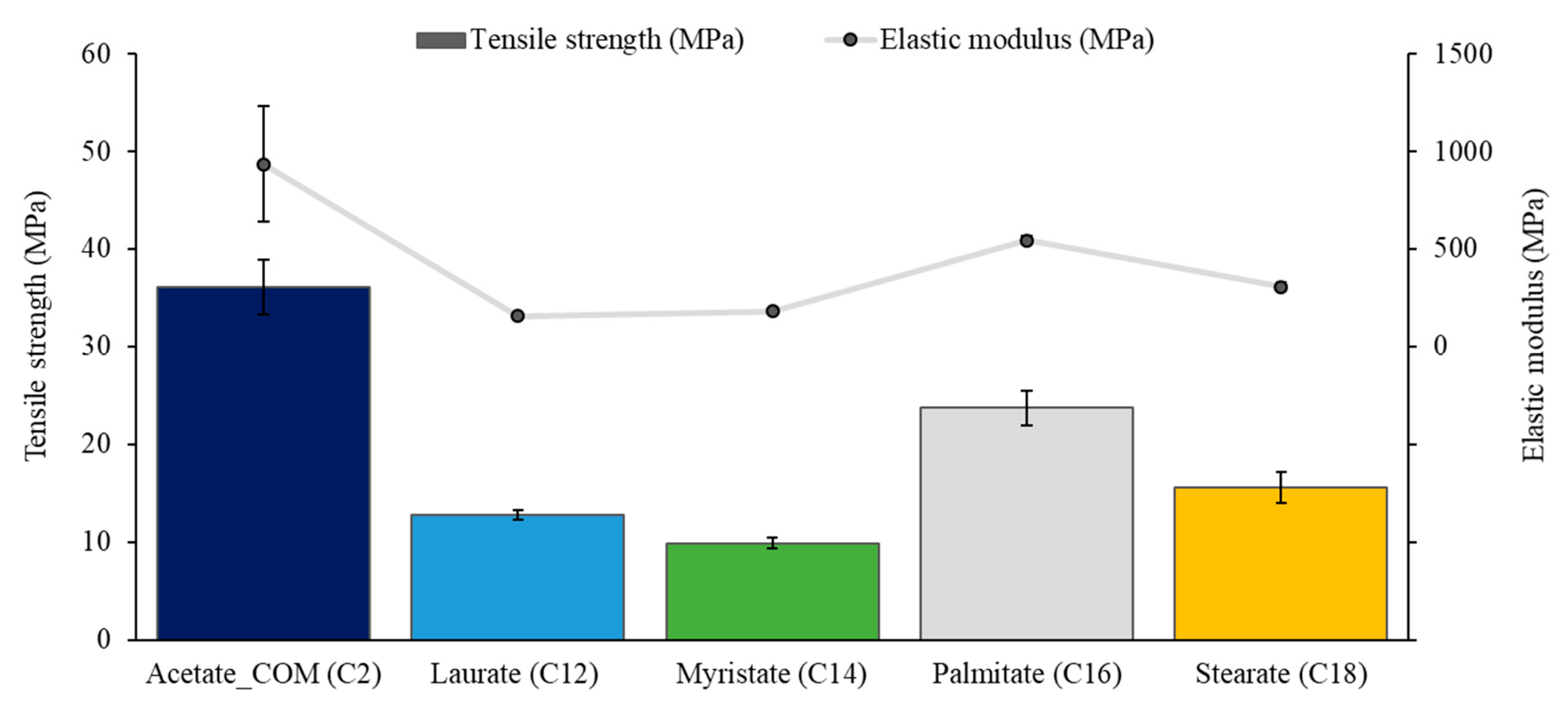
3.3. Tensile Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willberg-Keyriläinen, P.; Orelma, H.; Ropponen, J. Injection molding of thermoplastic cellulose esters and their compatibility with poly(lactic acid) and polyethylene. Materials 2018, 11, 2358. [Google Scholar] [CrossRef]
- Gustavsson, L.H.; Adolfsson, K.H.; Hakkarainen, M. Thermoplastic ‘all-Cellulose’ Composites with Covalently Attached Carbonized Cellulose. Biomacromolecules 2020, 21, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Onwukamike, K.N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Sustainable Transesterification of Cellulose with High Oleic Sunflower Oil in a DBU-CO2 Switchable Solvent. ACS Sustain. Chem. Eng. 2018, 6, 8826–8835. [Google Scholar] [CrossRef]
- Shibata, T. Renewable Resources for Functional Polymers and Biomaterials; Williams, P., Ed.; Polymer Chemistry Series; RSC Publishing: Cambridge, UK, 2011; Volume 3, pp. 48–87. ISBN 978-1-78262-584-1. [Google Scholar]
- Willberg-Keyriläinen, P.; Vartiainen, J.; Harlin, A.; Ropponen, J. The effect of side-chain length of cellulose fatty acid esters on their thermal, barrier and mechanical properties. Cellulose 2017, 24, 505–517. [Google Scholar] [CrossRef]
- Matsumura, H.; Sugiyama, J.; Glasser, W.G. Cellulosic Nanocomposites. I. Thermally Deformable Cellulose Hexanoates from Heterogeneous Reaction. J. Appl. Polym. Sci. 2000, 78, 2242–2253. [Google Scholar] [CrossRef]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Luan, Y.; Wu, J.; Zhan, M.; Zhang, J.; Zhang, J.; He, J. “One pot” homogeneous synthesis of thermoplastic cellulose acetate-graft-poly(l-lactide) copolymers from unmodified cellulose. Cellulose 2013, 20, 327–337. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Marson, G.A.; Ciacco, G.T.; Frollini, E. An efficient, one-pot acylation of cellulose under homogeneous reaction conditions. Macromol. Chem. Phys. 2000, 201, 882–889. [Google Scholar] [CrossRef]
- Virtanen, S.; Talja, R.; Vuoti, S. Synthesis and melt processing of cellulose esters for preparation of thermoforming materials and extended drug release tablets. Carbohydr. Polym. 2017, 177, 105–115. [Google Scholar] [CrossRef]
- Hernandez, S.C.; Milotskyi, R.; Takagi, S.; Ito, E.R.D.; Suzuki, S.; Wada, N.; Takahashi, K. Continuous production of cellulose mixed esters via homogeneous reactive twin-screw extrusion catalyzed by ionic liquid. Cellulose 2023, 30, 2873–2882. [Google Scholar] [CrossRef]
- Kusuma, S.B.W.; Hirose, D.; Yoshizawa, A.; Szabo, L.; Ina, D.; Wada, N.; Takahashi, K. Direct Synthesis of Full-Biobased Cellulose Esters from Essential Oil Component α,β-Unsaturated Aldehydes. ACS Sustain. Chem. Eng. 2021, 9, 8450–8457. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, M.S.; Nam, J.D.; Lee, Y. Melting processing of biodegradable cellulose diacetate/starch composites. Macromol. Symp. 2006, 242, 126–130. [Google Scholar] [CrossRef]
- Immonen, K.; Willberg-Keyriläinen, P.; Ropponen, J.; Nurmela, A.; Metsä-Kortelainen, S.; Kaukoniemi, O.-V.; Kangas, H. Thermoplastic Cellulose-Based Compound for Additive Manufacturing. Molecules 2021, 26, 1701. [Google Scholar] [CrossRef]
- Crépy, L.; Chaveriat, L.; Banoub, J.; Martin, P.; Joly, N. Synthesis of cellulose fatty esters as plastics-influence of the degree of substitution and the fatty chain length on mechanical properties. ChemSusChem 2009, 2, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, H.; Wei, Y.; Wang, X.; Liu, C. Preparation and characterization of cellulose laurate ester by catalyzed transesterification. Carbohydr. Polym. 2017, 168, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Duchatel-Crépy, L.; Joly, N.; Martin, P.; Marin, A.; Tahon, J.-F.; Lefebvre, J.-M.; Gaucher, V. Substitution degree and fatty chain length influence on structure and properties of fatty acid cellulose esters. Carbohydr. Polym. 2020, 234, 115912. [Google Scholar] [CrossRef] [PubMed]
- Willberg-Keyriläinen, P. The Properties and Applications of Long Chain Cellulose Esters. Ph.D. Dissertation, Aalto University, Espoo, Finland, 2020. Available online: http://urn.fi/URN:ISBN:978-952-64-0084-6 (accessed on 6 April 2023).
- Lowman, D.W. Characterization of Cellulose Esters by Solution-State and Solid-State NMR Spectroscopy. ACS Symp. Ser. 1998, 688, 131–162. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 2003. [Google Scholar]
- ISO 527-3:2018; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheet. ISO Standards: Geneva, Switzerland, 2018.
- Jandura, P.; Kokta, B.V.; Riedl, B. Fibrous Long-Chain Organic Acid Cellulose Esters and Their Characterization by Diffuse Reflectance FTIR Spectroscopy, Solid-State CP/MAS 13 C-NMR, and X-ray Diffraction. J. Appl. Polym. Sci. 2000, 78, 1354–1365. [Google Scholar] [CrossRef]
- Schilling, M.; Bouchard, M.; Khanjian, H.; Learner, T.; Phenix, A.; Rivenc, R. Application of chemical and thermal analysis methods for studying cellulose ester plastics. Accounts Chem. Res. 2010, 43, 888–896. [Google Scholar] [CrossRef]
- Chen, H.; Yang, F.; Du, J.; Xie, H.; Zhang, L.; Guo, Y.; Xu, Q.; Zheng, Q.; Li, N.; Liu, Y. Efficient transesterification reaction of cellulose with vinyl esters in DBU/DMSO/CO2 solvent system at low temperature. Cellulose 2018, 25, 6935–6945. [Google Scholar] [CrossRef]
- Sun, J.X.; Xu, F.; Geng, Z.C.; Sun, X.F.; Sun, R.C. Comparative Study of Cellulose Isolated by Totally Chlorine-Free Method from Wood and Cereal Straw. J. Appl. Polym. Sci. 2005, 97, 322–335. [Google Scholar] [CrossRef]
- Kramar, A.; Rodríguez Ortega, I.; González-Gaitano, G.; González-Benito, J. Solution casting of cellulose acetate films: Influence of surface substrate and humidity on wettability, morphology and optical properties. Cellulose 2023, 30, 2037–2052. [Google Scholar] [CrossRef]
- Wu, S.; Qin, X.; Li, M. The structure and properties of cellulose acetate materials: A comparative study on electrospun membranes and casted films. J. Ind. Text. 2014, 44, 85–98. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Mohammad, M. Recent advances in cellulose-based hydrophobic food packaging. Emergent Mater. 2021, 5, 703–718. [Google Scholar] [CrossRef]
- Joly, N.; Martin, P.; Liénard, L.; Rutot, D.; Stassin, F.; Granet, R.; Krausz, P.; Cavrot, J.-P. Effect of degree of substitution on the mechanical and thermomechanical properties of lauroyl cellulose ester films. E-Polymers 2006, 6, 895–903. [Google Scholar] [CrossRef]

| Parameter | Condition |
|---|---|
| MCC content (%) | 3.5 |
| Solvent | 1:1 DMSO:IL |
| Molar ratio of AGU:Vinyl ester | 01:03 |
| Temperature (°C) | 70 |
| Reaction time (h) | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallakas, H.; Kattamanchi, T.; Kilumets, C.; Tarasova, E.; Krasnou, I.; Savest, N.; Ahmadian, I.; Kers, J.; Krumme, A. Tensile and Surface Wettability Properties of the Solvent Cast Cellulose Fatty Acid Ester Films. Polymers 2023, 15, 2677. https://doi.org/10.3390/polym15122677
Kallakas H, Kattamanchi T, Kilumets C, Tarasova E, Krasnou I, Savest N, Ahmadian I, Kers J, Krumme A. Tensile and Surface Wettability Properties of the Solvent Cast Cellulose Fatty Acid Ester Films. Polymers. 2023; 15(12):2677. https://doi.org/10.3390/polym15122677
Chicago/Turabian StyleKallakas, Heikko, Tanuj Kattamanchi, Catherine Kilumets, Elvira Tarasova, Illia Krasnou, Natalja Savest, Iman Ahmadian, Jaan Kers, and Andres Krumme. 2023. "Tensile and Surface Wettability Properties of the Solvent Cast Cellulose Fatty Acid Ester Films" Polymers 15, no. 12: 2677. https://doi.org/10.3390/polym15122677
APA StyleKallakas, H., Kattamanchi, T., Kilumets, C., Tarasova, E., Krasnou, I., Savest, N., Ahmadian, I., Kers, J., & Krumme, A. (2023). Tensile and Surface Wettability Properties of the Solvent Cast Cellulose Fatty Acid Ester Films. Polymers, 15(12), 2677. https://doi.org/10.3390/polym15122677











