Profitability of Chemically Cross-Linked Collagen Scaffold Production Using Bovine Pericardium: Revaluing Waste from the Meat Industry for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bovine Pericardium Composition
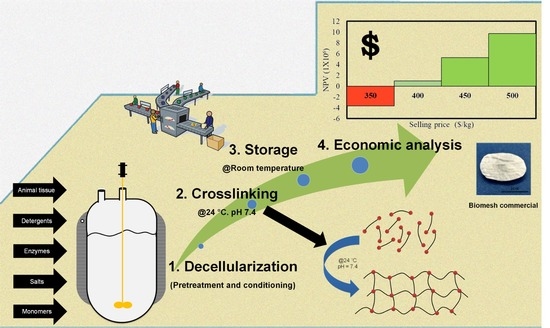
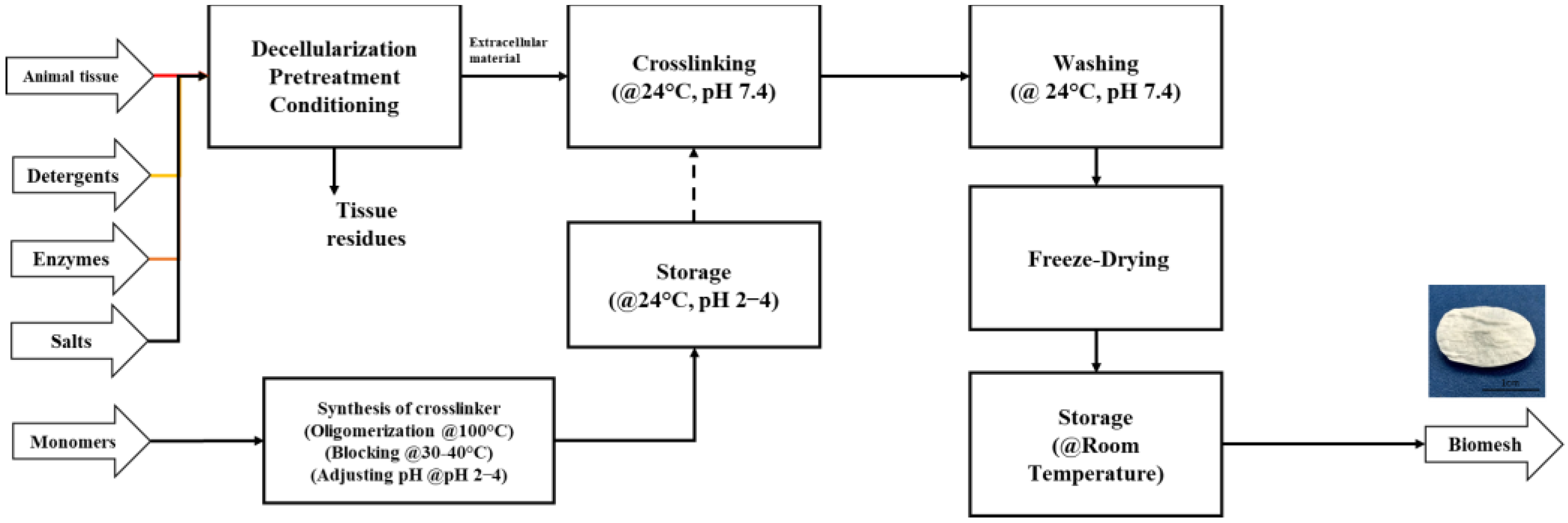
2.2. Process Description
2.2.1. Decellularization Stage
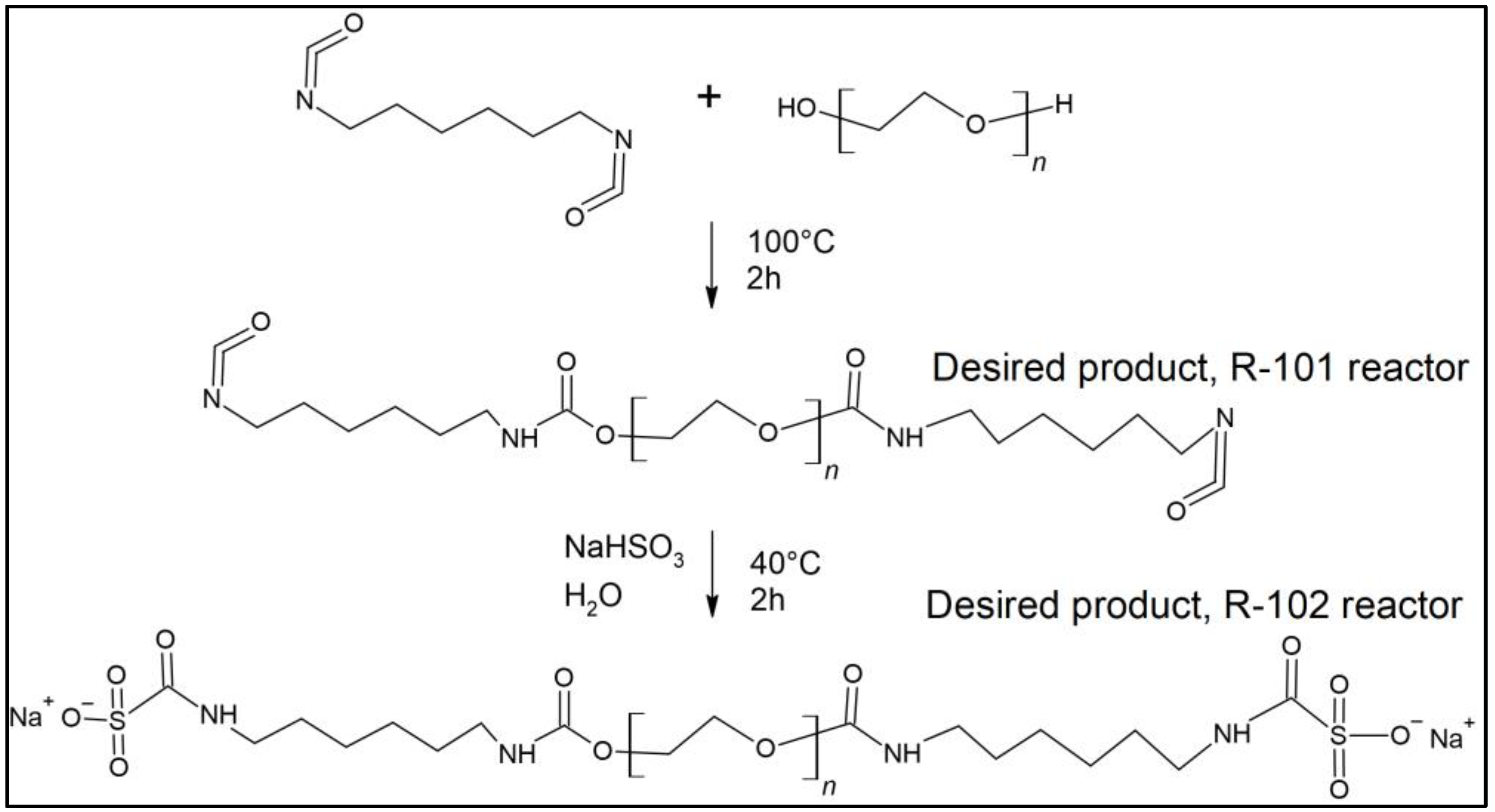
2.2.2. Crosslinker Synthesis
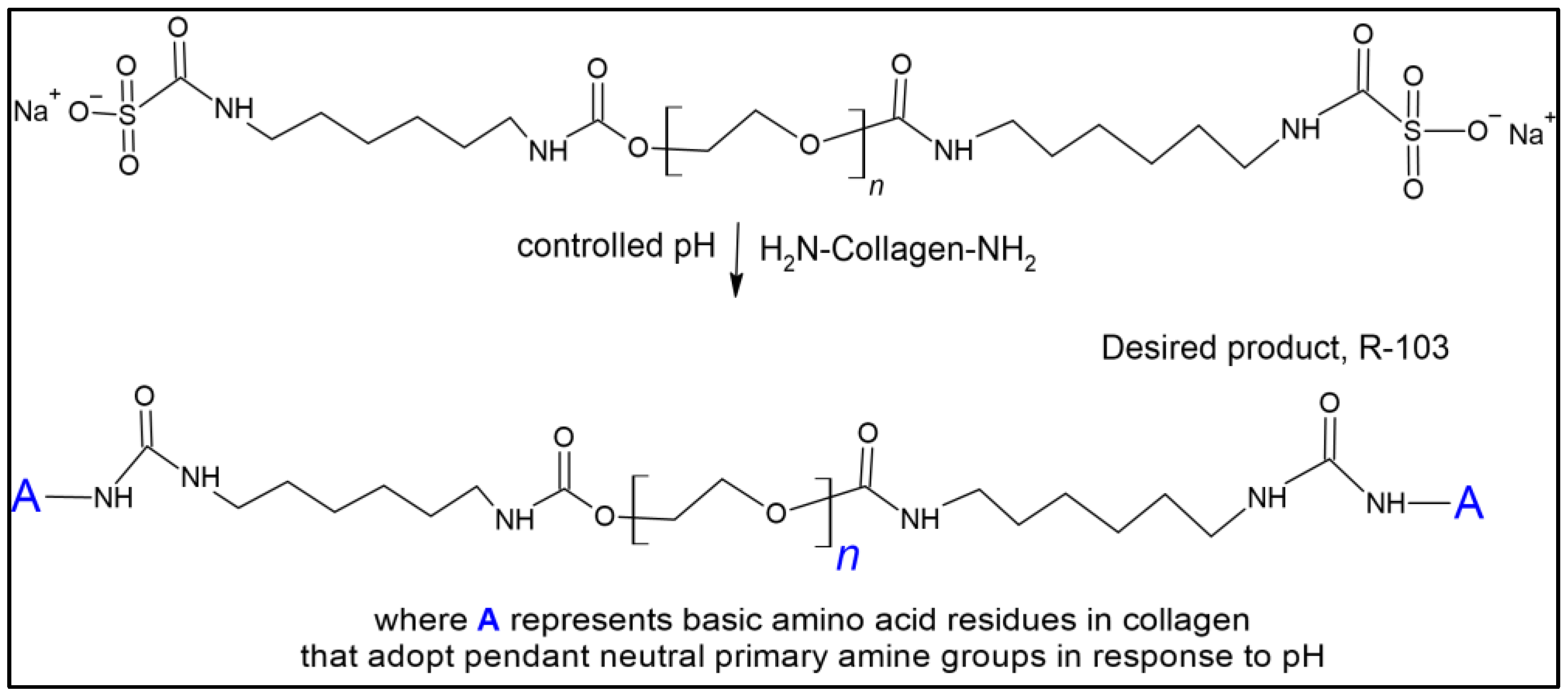
2.2.3. Crosslinking
2.2.4. Washing
2.2.5. Freezing and Drying
2.3. Scenarios
2.4. Financial Investment and Assumptions
2.4.1. Net Present Value Method (NPV)
2.4.2. Economic Considerations
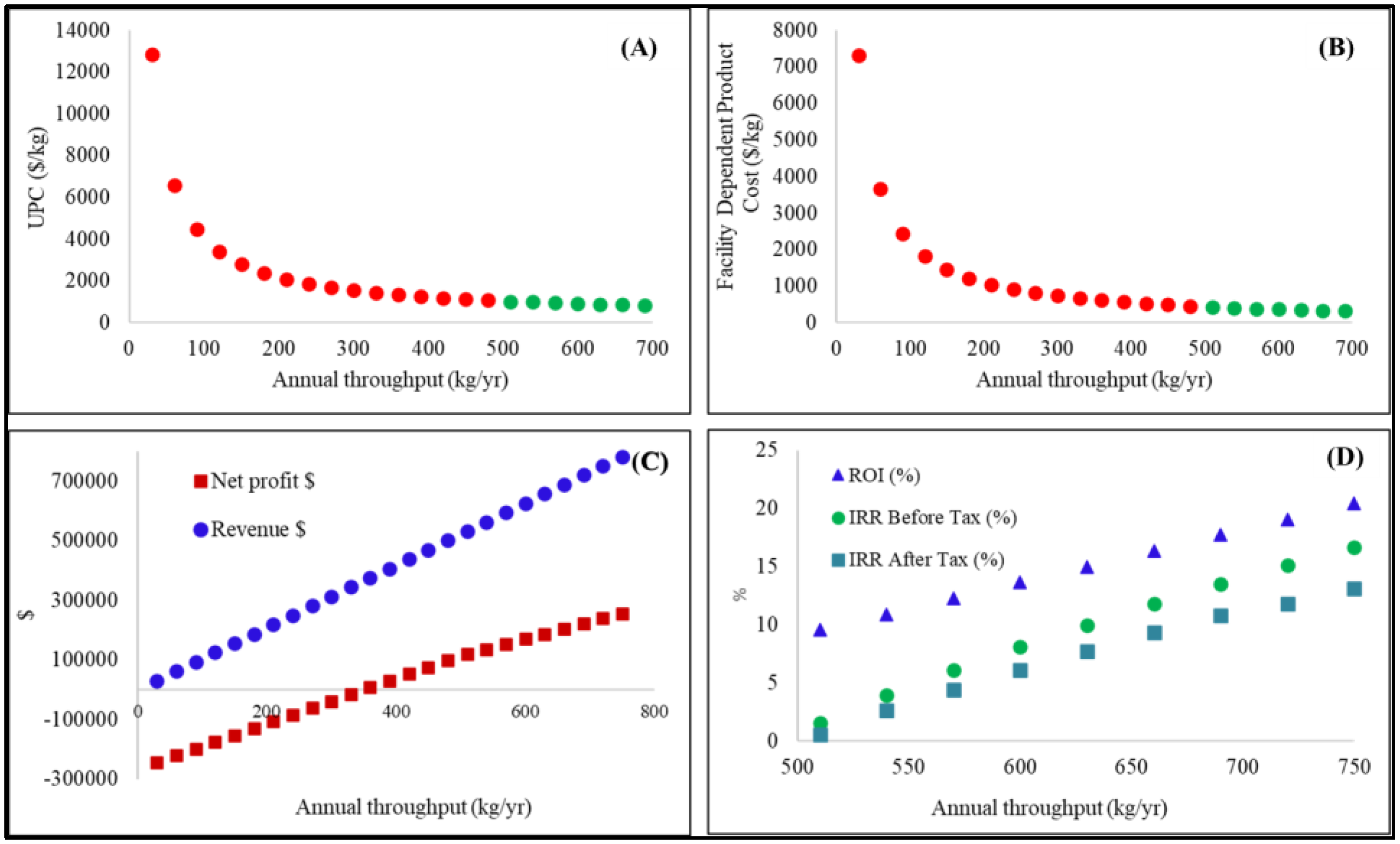
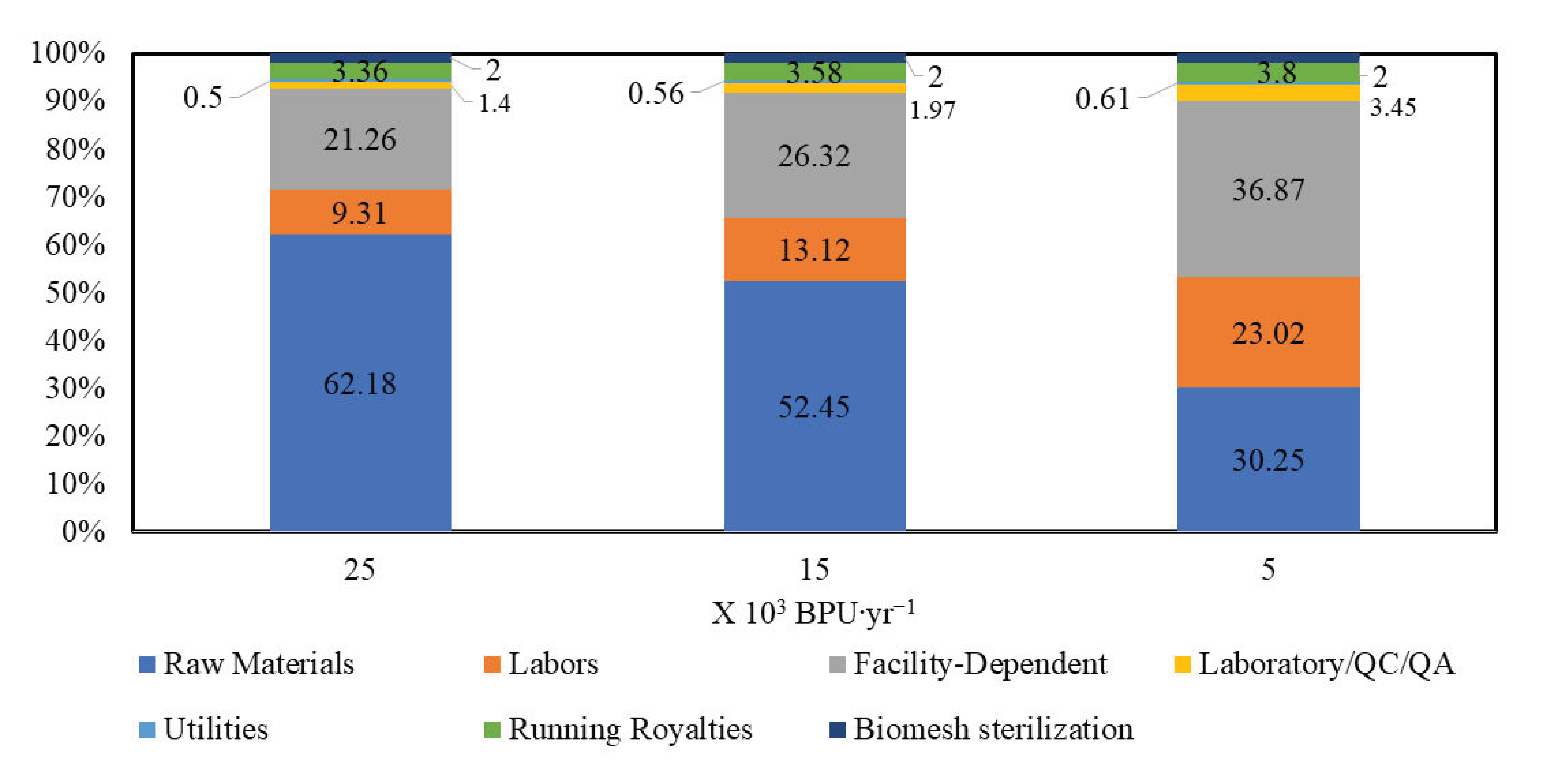
3. Results
Comparison with Actual Commercial Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

| Equipment | Equipment Specifications | ||||||
|---|---|---|---|---|---|---|---|
| Specifications | 5000 BPU.mth−1 | 15,000 BPU.mth−1 | 25,000 BPU.mth−1 | ||||
| P-3/V-101 (Stirring Tank) | Volume | 68.04 | L | 204.13 | L | 340.21 | L |
| Height/Diameter ratio | 3 | n/a | 3 | n/a | 3 | n/a | |
| Height | 0.92 | m | 1.327 | m | 5.164 | m | |
| Diameter | 0.307 | m | 0.442 | m | 1.721 | m | |
| Design pressure | 1.52 | bar | 1.52 | bar | 1.52 | bar | |
| Purchase cost | 38.023 | $ | 54.677 | $ | 65.837 | $ | |
| Material | SS316 | SS316 | SS316 | ||||
| Energy consumption | 26.45 | kWh. yr−1 | 79.37 | kWh. yr−1 | 132.28 | kWh. yr−1 | |
| Operating temperature | 25.3 | °C | 25.3 | °C | 25,3 | °C | |
| Operating pressure | 1.013 | bar | 1.013 | bar | 1.013 | bar | |
| Operating volume | 61.24 | L | 183.72 | L | 306.19 | L | |
| P-1/DS-101 (Centrifuge) | Separation capacity (Factor Sigma Σ) | 108.49 | m2 | 325.46 | m2 | 542.43 | m2 |
| Rated performance | 1.28 | L/h | 3.83 | L/h | 6.39 | L/h | |
| Purchase cost | 4.000 | $ | 4.000 | $ | 4.000 | $ | |
| Material | SS316 | SS316 | SS316 | ||||
| Minimum solid particle diameter | 1 × 10−6 | m | 1 × 10−6 | m | 1 × 10−6 | m | |
| Sedimentation efficiency | 30% | 30% | 30% | ||||
| Operating temperature | 25 | °C | 25 | °C | 25 | °C | |
| Energy consumption | 10.271.50 | kWh. yr−1 | 15939.79 | kWh. yr−1 | 19553.38 | kWh. yr−1 | |
| P-19/V-108 (Stirring Tank) | Volume | 2.52 | L | 7.56 | L | 12.59 | L |
| Height/Diameter ratio | 3 | n/a | 3 | n/a | 3 | n/a | |
| Height | 0.307 | m | 0.442 | m | 0.525 | m | |
| Diameter | 0.102 | m | 0.147 | m | 0.175 | m | |
| Design pressure | 1.52 | bar | 1.52 | bar | 1.52 | bar | |
| Purchase cost | 17.892 | $ | 21.670 | $ | 24.189 | $ | |
| Material | SS316 | SS316 | SS316 | ||||
| Energy consumption | 0 | kWh. yr−1 | 0 | kWh. yr−1 | 0 | kWh. yr−1 | |
| Operating temperature | 25 | °C | 25 | °C | 25 | °C | |
| Operating pressure | 1.013 | bar | 1.013 | bar | 1.013 | bar | |
| Operating volume | 2.27 | L | 6.8 | L | 11.33 | L | |
| P-20/V-105 (Stirring Tank) | Volume | 42.88 | L | 128.63 | L | 214.38 | L |
| Height/Diameter ratio | 3 | n/a | 3 | n/a | 3 | n/a | |
| Height | 0.789 | m | 1.138 | m | 1.349 | m | |
| Diameter | 0.263 | m | 0.379 | m | 0.45 | m | |
| Design pressure | 1.52 | bar | 1.52 | bar | 1.52 | bar | |
| Purchase cost | 33.145 | $ | 46.644 | $ | 55.639 | $ | |
| Material | SS316 | SS316 | SS316 | ||||
| Energy consumption | 0 | kWh. yr−1 | 0 | kWh. yr−1 | 0 | kWh. yr−1 | |
| Operating temperature | 25 | °C | 25 | °C | 25 | °C | |
| Operating pressure | 1.013 | bar | 1.013 | bar | 1.013 | bar | |
| Operating volume | 38.59 | L | 115.76 | L | 192.94 | L | |
| P-4/DS-102 (Centrifuge) | Separation capacity (Factor Sigma Σ) | 136.58 | m−2 | 409.75 | m−2 | 682.91 | m−2 |
| Rated performance | 1.61 | L/h | 4.82 | L/h | 8.04 | L/h | |
| Purchase cost | 4.000 | $ | 4.000 | $ | 4.000 | $ | |
| Material | SS316 | SS316 | SS316 | ||||
| Minimum solid particle diameter | 1 × 10−6 | m | 1 × 10−6 | m | 1 × 10−6 | m | |
| Sedimentation efficiency | 30% | 30% | 30% | ||||
| Operating temperature | 25 | °C | 25 | °C | 25 | °C | |
| Energy consumption | 11262.69 | kWh. yr−1 | 17477.95 | kWh. yr−1 | 21440.26 | kWh. yr−1 | |
| P-5/R-101 (Stirred Reactor) | Volume | 0.13 | L | 0.38 | L | 0.63 | L |
| Height/Diameter ratio | 2.5 | 2.5 | 2.5 | ||||
| Height | 0.102 | m | 0.147 | m | 0.171 | m | |
| Diameter | 0.041 | m | 0.059 | m | 0.068 | m | |
| Design pressure | 1.52 | bar | 1.52 | bar | 1.52 | bar | |
| Purchase cost | 71 | $ | 73 | $ | 98 | $ | |
| Material | Glass | Glass | Glass | ||||
| Energy consumption | 0.04 | kWh. yr−1 | 0.12 | kWh. yr−1 | 0.2 | kWh. yr−1 | |
| Operating temperature | 100 | °C | 100 | °C | 100 | °C | |
| Operating pressure | 1.013 | bar | 1.013 | bar | 1.013 | bar | |
| Operating volume | 0.11 | L | 0.34 | L | 0.56 | L | |
| P-8/R-102 (Stirred Reactor) | Volume | 0.25 | L | 0.61 | L | 1.01 | L |
| Height/Diameter ratio | 2.5 | 2.5 | 2.5 | ||||
| Height | 0.126 | m | 0.169 | m | 0.201 | m | |
| Diameter | 0.05 | m | 0.068 | m | 0.08 | m | |
| Design pressure | 1.52 | bar | 1.52 | bar | 1.52 | bar | |
| Purchase cost | 137 | $ | 112 | $ | 157 | $ | |
| Material | Glass | Glass | Glass | ||||
| Energy consumption | 0.07 | kWh. yr−1 | 0.2 | kWh. yr−1 | 0.33 | kWh. yr−1 | |
| Operating temperature | 40 | °C | 40 | °C | 40 | °C | |
| Operating pressure | 1.013 | bar | 1.013 | bar | 1.013 | bar | |
| Operating volume | 0.18 | L | 0.55 | L | 0.91 | L | |
| P-7/V-103 (Stirred Tank) | Volume | 3.96 | L | 11.89 | L | 19.81 | L |
| Height/Diameter ratio | 3 | n/a | 3 | n/a | 3 | n/a | |
| Height | 0.357 | m | 0.515 | m | 0.61 | m | |
| Diameter | 0.119 | m | 0.172 | m | 0.203 | m | |
| Design pressure | 1.52 | bar | 1.52 | bar | 1.52 | bar | |
| Purchase cost | 2.171 | $ | 2.192 | $ | 3.074 | $ | |
| Material | Glass | Glass | Glass | ||||
| Energy consumption | 0 | kWh. yr−1 | 0 | kWh. yr−1 | 0 | kWh. yr−1 | |
| Operating temperature | 25 | °C | 25 | °C | 25 | °C | |
| Operating pressure | 1.013 | bar | 1.013 | bar | 1.013 | bar | |
| Operating volume | 3.57 | L | 10.7 | L | 17.83 | L | |
| P-13/V-104 (Stirred Tank) | Volume | 0.22 | L | 0.66 | L | 1.1 | L |
| Height/Diameter ratio | 3 | n/a | 3 | n/a | 3 | n/a | |
| Height | 0.136 | m | 0.196 | m | 0.233 | m | |
| Diameter | 0.045 | m | 0.065 | m | 0.078 | m | |
| Design pressure | 1.52 | bar | 1.52 | bar | 1.52 | bar | |
| Purchase cost | 120 | $ | 121 | $ | 171 | $ | |
| Material | Glass | Glass | Glass | ||||
| Energy consumption | 0 | kWh. yr−1 | 0 | kWh. yr−1 | 0 | kWh. yr−1 | |
| Operating temperature | 25 | °C | 25 | °C | 25 | °C | |
| Operating pressure | 1.013 | bar | 1.013 | bar | 1.013 | bar | |
| Operating volume | 0.2 | L | 0.59 | L | 0.99 | L | |
| P-18/R-103 (Stirred Reactor) | Volume | 4.92 | L | 14.76 | L | 24.61 | L |
| Height/Diameter ratio | 2.5 | 2.5 | 2.5 | ||||
| Height | 0.34 | m | 0.49 | m | 0.581 | m | |
| Diameter | 0.136 | m | 0.196 | m | 0.232 | m | |
| Design pressure | 1.52 | bar | 1.52 | bar | 1.52 | bar | |
| Purchase cost | 20.000 | $ | 25.140 | $ | 28.526 | $ | |
| Material | SS316 | SS316 | SS316 | ||||
| Energy consumption | 1.59 | kWh. yr−1 | 4.78 | kWh. yr−1 | 7.97 | kWh. yr−1 | |
| Operating temperature | 25 | °C | 25 | °C | 25 | °C | |
| Operating pressure | 1.013 | bar | 1.013 | bar | 1.013 | bar | |
| Operating volume | 4.43 | L | 13.29 | L | 22.15 | L | |
| P-16/V-107 (Stirred Tank) | Volume | 0.76 | L | 2.27 | L | 3.79 | L |
| Height/Diameter ratio | 3 | n/a | 3 | n/a | 3 | n/a | |
| Height | 0.206 | m | 0.296 | m | 0.351 | m | |
| Diameter | 0.069 | m | 0.099 | m | 0.117 | m | |
| Design pressure | 1.52 | bar | 1.52 | bar | 1.52 | bar | |
| Purchase cost | 15.608 | $ | 17.636 | $ | 19.072 | $ | |
| Material | SS316 | SS316 | SS316 | ||||
| Energy consumption | 0 | kWh. yr−1 | 0 | kWh. yr−1 | 0 | kWh. yr−1 | |
| Operating temperature | 25 | °C | 25 | °C | 25 | °C | |
| Operating pressure | 1.013 | bar | 1.013 | bar | 1.013 | bar | |
| Operating volume | 0.68 | L | 2.05 | L | 3.41 | L | |
| P-17/FDR-101 (Freeze dryer) | Sublimation capacity per cycle | 10.68 | kg | 32.04 | kg | 53.399 | kg |
| Tray area | 0.373 | m−2 | 1.119 | m−2 | 1.864 | m−2 | |
| Purchase cost | 20.000 | $ | 20.000 | $ | 20.000 | $ | |
| Material | SS316 | SS316 | SS316 | ||||
| Final solid temperature | 12 | °C | 12 | °C | 12 | °C | |
| Sublimation rate | 1 | mm/h | 1 | mm/h | 1 | mm/h | |
| Energy consumption | 503.36 | kWh. yr−1 | 1510.09 | kWh yr−1 | 2516.81 | kWh yr−1 | |
Appendix C
| 3A. Total Plant Direct Cost (TPDC) (Physical Cost) | 5000 BPU.mth−1 | 15,000 BPU.mth−1 | 25,000 BPU.mth−1 |
|---|---|---|---|
| Total Plant Direct Cost (TPDC) (physical cost) | |||
| 1. Equipment Purchase Cost | 197,000 | 248,000 | 284,000 |
| 2. Installation | 66,000 | 84,000 | 96,000 |
| 3. Process Piping | 69,000 | 87,000 | 99,000 |
| 4. Instrumentation | 79,000 | 99,000 | 114,000 |
| 5. Insulation | 6,000 | 7,000 | 9,000 |
| 6. Electrical | 20,000 | 25,000 | 28,000 |
| 7. Buildings | 89,000 | 112,000 | 128,000 |
| 8. Yard Improvement | 30,000 | 37,000 | 43,000 |
| 9. Auxiliary Facilities | 79,000 | 99,000 | 114,000 |
| TPDC | 634,000 | 799,000 | 914,000 |
| Total Plant Indirect Cost (TPIC) | |||
| 10. Engineering | 158,000 | 200,000 | 228,000 |
| 11. Construction | 222,000 | 280,000 | 320,000 |
| TPIC | 380,000 | 479,000 | 548,000 |
| Total Plant Cost (TPC = TPDC + TPIC) | |||
| TPC | 1,014,000 | 1,279,000 | 1,462,000 |
| Contractor’s Fee & Contingency (CFC) | |||
| 12. Contractor’s Fee | 51,000 | 64,000 | 73,000 |
| 13. Contingency | 101,000 | 128,000 | 146,000 |
| CFC = 12 + 13 | 152,000 | 192,000 | 219,000 |
| Direct Fixed Capital Cost (DFC = TPC + CFC) | |||
| DFC | 1,166,000 | 1,470,000 | 1,681,000 |
| Bulk Material | Unit Cost ($/kg) | Source |
|---|---|---|
| Sodium bisulphite | 24.85 | Sigma-Aldrich ® |
| EDTA Disodium | 193.80 | Sigma-Aldrich ® |
| Ethyl alcohol | 0.75 | Sigma-Aldrich ® |
| HDI | 155.40 | Sigma-Aldrich ® |
| MgO | 895.00 | Sigma-Aldrich ® |
| Phosphate-buffer | 4.00 | Sigma-Aldrich ® |
| Polyethyleneglycol | 72.00 | Sigma-Aldrich ® |
| TEOS | 83.00 (L STP) | Sigma-Aldrich ® |
| Triton-X-100 | 1.50 | Sigma-Aldrich ® |
| Tris-HCl | 125 | Sigma-Aldrich ® |
| Unit cost ($/mg) | ||
| RNase | 0.34 | Sigma-Aldrich ® |
| DNase | 1.34 | Sigma-Aldrich ® |
References
- Steffens, D.; Braghirolli, D.I.; Maurmann, N.; Pranke, P. Update on the main use of biomaterials and techniques associated with tissue engineering. Drug Discov. Today 2018, 23, 1474–1488. [Google Scholar] [CrossRef]
- Campion, G.; Hershberger, K.; Whelan, A.; Conroy, J.; Lally, C.; Murphy, B. A Biomechanical and Microstructural Analysis of Bovine and Porcine Pericardium for Use in Bioprosthetic Heart Valves. Struct. Hear. 2021, 5, 486–496. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Materials & Design Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, E.; Wang, Y. Biomedicine & Pharmacotherapy Advances in the applications of polymer biomaterials for in vitro follicle culture. Biomed. Pharmacother. 2021, 140, 111422. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Seifalian, A.; Moiemen, N.S.; Butler, P.E.; Seifalian, A.M. The Use of Skin Substitutes in the Treatment of Burns; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Wahyu, R.; Nugroho, N.; Harjumäki, R.; Zhang, X.; Lou, Y. Colloids and Surfaces B : Biointerfaces Quantifying the interactions between biomimetic biomaterials—Collagen I, collagen IV, laminin 521 and cellulose nano fibrils—By colloidal probe microscopy. Colloids Surf. B Biointerfaces 2019, 173, 571–580. [Google Scholar] [CrossRef]
- Connolly, J.M.; Alferiev, I.; Clark-Gruel, J.N.; Eidelman, N.; Sacks, M.; Palmatory, E.; Kronsteiner, A.; DeFelice, S.; Xu, J.; Ohri, R.; et al. Triglycidylamine crosslinking of porcine aortic valve cusps or bovine pericardium results in improved biocompatibility, biomechanics, and calcification resistance: Chemical and biological mechanisms. Am. J. Pathol. 2005, 166, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, K.; Zhang, W.; Qiang, M.; Luo, Y. A bilaminated decellularized scaffold for islet transplantation: Structure, properties and functions in diabetic mice. Biomaterials 2017, 138, 80–90. [Google Scholar] [CrossRef] [PubMed]
- INEGI Estadística de Sacrificio de Ganado en Rastros Municipales por Entidad Federativa 2009–2014; Aguascalientes, Mexico. 2014. Available online: https://www.inegi.org.mx/app/biblioteca/ficha.html?upc=702825070625 (accessed on 18 June 2023).
- Senado de la Republica Nota legislativa: Ley General de Economía Circular. 2021. Available online: http://bibliodigitalibd.senado.gob.mx/bitstream/handle/123456789/5431/125.NL_Economia_circular.pdf?sequence=1&isAllowed=y (accessed on 18 June 2023).
- Arias González, J.; Baena-Moreno, F.M.; Gonzalez-Castaño, M.; Arellano-García, H.; Lichtfouse, E.; Zhang, Z. Unprofitability of small biogas plants without subsidies in the Brandenburg region. Environ. Chem. Lett. 2021, 19, 1823–1829. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. Technoeconomic analysis of the recovery of phenols from olive mill wastewater through membrane filtration and resin adsorption/desorption. Sustainability 2021, 13, 2376. [Google Scholar] [CrossRef]
- Grisales Díaz, V.H.; Olivar Tost, G. Techno-economic analysis of extraction-based separation systems for acetone, butanol, and ethanol recovery and purification. Bioresour. Bioprocess. 2017, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glivin, G.; Kalaiselvan, N.; Mariappan, V.; Premalatha, M.; Murugan, P.C.; Sekhar, J. Conversion of biowaste to biogas : A review of current status on techno-economic challenges, policies, technologies and mitigation to environmental impacts Internal Rate of Return. Fuel 2021, 302, 121153. [Google Scholar] [CrossRef]
- Jang, M.O.; Choi, G. Techno-economic analysis of butanol production from lignocellulosic biomass by concentrated acid pretreatment and hydrolysis plus continuous fermentation. Biochem. Eng. J. 2018, 134, 30–43. [Google Scholar] [CrossRef]
- Rémi, E.; Khelil, N.; Di Centa, I.; Roques, C.; Ba, M.; Medjahed-Hamidi, F.; Chaubet, F.; Letourneur, D.; Lansac, E.; Meddahi-Pellé, A. Pericardial Processing: Challenges, Outcomes and Future Prospects. In Biomaterials Science and Engineering; IntechOpen: London, UK, 2011. [Google Scholar]
- Courtman, D.; Lee, J.M. Development of a pericardial acellular matrix biomaterial : Biochemical and mechanical effects of cell extraction. J. Biomed. Mater. Res. 1994, 28, 655–666. [Google Scholar] [CrossRef]
- Mendoza-Novelo, B.; Alvarado-Castro, D.I.; Mata-Mata, J.L.; Cauich-Rodríguez, J.V.; Vega-González, A.; Jorge-Herrero, E.; Rojo, F.J. Stability and mechanical evaluation of bovine pericardium cross-linked with polyurethane prepolymer in aqueous medium. Mater. Sci. Eng. C 2013, 33, 2392–2398. [Google Scholar] [CrossRef] [Green Version]
- de Jesus Palacios-Rodriguez, A.; Flores-Moreno, M.; Castellano, L.E.; Murguia-Pérez, M.; Guillermo, C.; Vargas-Mancilla, J.; Vega-gonza, A.; Mendoza-Novelo, B. Effect of varying the crosslinking degree of a pericardial implant with oligourethane on the repair of a rat full-thickness abdominal wall defect. J. Biomater. Appl. 2019, 33, 903–914. [Google Scholar] [CrossRef]
- Mendoza-Novelo, B.; Mata-Mata, J.L.; Vega-González, A.; Cauich-Rodríguez, J.V.; Marcos-Fernández, Á. Synthesis and characterization of protected oligourethanes as crosslinkers of collagen-based scaffolds. J. Mater. Chem. B 2014, 2, 2874–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claudio-Rizo, J.A.; Rangel-Argote, M.; Muñoz-Gonzalez, P.U.; Castellano, L.E.; Delgado, J.; Gonzalez-garcı, G.; Mata-Mata, J.L.; Mendoza-Novelo, B. Improved properties of composite collagen hydrogels: Protected oligourethanes and silica particles as modulators. J. Mater. Chem. B 2016, 4, 6497–6509. [Google Scholar] [CrossRef] [PubMed]
- Comisión Federal de Electricidad. 2020. Available online: https://app.cfe.mx/Aplicaciones/CCFE/Tarifas/TarifasCREIndustria/Tarifas/DemandaIndustrialTran.aspx (accessed on 21 June 2023).
- Corporation, E.L. A Legacy of Tissue Innovation Edwards Bovine Pericardial Patch. 2017. Available online: https://edwardsprod.blob.core.windows.net/media/Default/devices/duravess/duravessbrochure_final.pdf (accessed on 21 June 2023).

| Equation | Oligomer Structure | Yield (%) | Equation # |
|---|---|---|---|
| Reactions involved in the synthesis of the crosslinker (oligomerization) | |||
| Trimer | 70 | (1) | |
| Pentamer | 80 | (2) | |
| Heptamer | 99 | (3) | |
| Reactions used to block oligourethane | |||
| 90 | (4) | ||
| 90 | (5) | ||
| 90 | (6) | ||
| 90 | (7) | ||
| Crosslinking reactions. | |||
| 90 | (8) | ||
| 90 | (9) | ||
| 90 | (10) | ||
| 90 | (11) | ||
| 5000 BPU.mth−1 | 15,000 BPU.mth−1 | 25,000 BPU.mth−1 | Units | |
|---|---|---|---|---|
| Total capital investment | 1,258,000 | 1,613,000 | 1,872,000 | USD |
| Operating cost | 612,560 | 1,074,320 | 1,515,280 | USD/yr |
| Revenues | 783,000 | 1,305,000 | 1,740,000 | USD/yr |
| Cost basis annual rate | 750.00 | 2,250 | 3,750 | kg MP/yr |
| Net unit production cost | 784.57 | 458.94 | 388.26 | USD/kg MP |
| Unit production revenue | 1,043.75 | 579.86 | 463.89 | USD/kg MP |
| Unit production revenue | 0.16 ± 0.078 | 0.086 ± 0.043 | 0.069 ± 0.035 | USD/cm2 MP |
| Gross margin | 24.79 | 20.85 | 16.30 | % |
| Return on investment | 20.38 | 21.31 | 19.90 | % |
| Payback time | 4.91 | 4.69 | 5.03 | years |
| IRR (After taxes) | 13.06 | 14.34 | 13.25 | % |
| NPV (at 7.0% interest) | 523,000 | 817,000 | 787,000 | $ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Bosques, J.A.d.l.; Ibarra Sánchez, J.d.J.; Mendoza-Novelo, B.; Segovia-Hernandez, J.G.; Molina-Guerrero, C.E. Profitability of Chemically Cross-Linked Collagen Scaffold Production Using Bovine Pericardium: Revaluing Waste from the Meat Industry for Biomedical Applications. Polymers 2023, 15, 2797. https://doi.org/10.3390/polym15132797
Cruz Bosques JAdl, Ibarra Sánchez JdJ, Mendoza-Novelo B, Segovia-Hernandez JG, Molina-Guerrero CE. Profitability of Chemically Cross-Linked Collagen Scaffold Production Using Bovine Pericardium: Revaluing Waste from the Meat Industry for Biomedical Applications. Polymers. 2023; 15(13):2797. https://doi.org/10.3390/polym15132797
Chicago/Turabian StyleCruz Bosques, José Arturo de la, José de Jesús Ibarra Sánchez, Birzabith Mendoza-Novelo, Juan Gabriel Segovia-Hernandez, and Carlos Eduardo Molina-Guerrero. 2023. "Profitability of Chemically Cross-Linked Collagen Scaffold Production Using Bovine Pericardium: Revaluing Waste from the Meat Industry for Biomedical Applications" Polymers 15, no. 13: 2797. https://doi.org/10.3390/polym15132797
APA StyleCruz Bosques, J. A. d. l., Ibarra Sánchez, J. d. J., Mendoza-Novelo, B., Segovia-Hernandez, J. G., & Molina-Guerrero, C. E. (2023). Profitability of Chemically Cross-Linked Collagen Scaffold Production Using Bovine Pericardium: Revaluing Waste from the Meat Industry for Biomedical Applications. Polymers, 15(13), 2797. https://doi.org/10.3390/polym15132797