Chemical Recycling of Fully Recyclable Bio-Epoxy Matrices and Reuse Strategies: A Cradle-to-Cradle Approach
Abstract
:1. Introduction
2. Materials and Methods
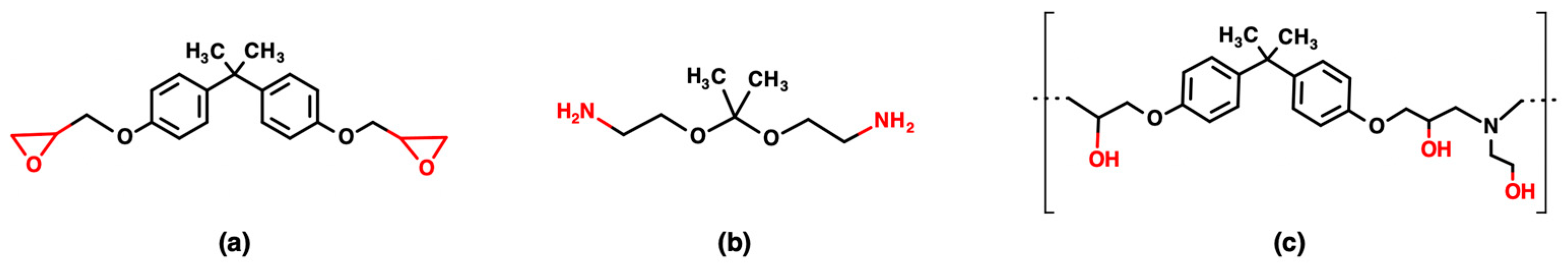
2.1. Materials
2.2. Virgin Epoxy Resin Formulation
2.3. Chemical Recycling Process
2.4. Epoxy Resin Formulation by Reusing the Recycled Polymer
2.5. Thermo-Mechanical Characterization
2.5.1. Thermal Analyses: Dynamic Mechanical Analysis (DMA) and Differential Scanning Calorimetry (DSC)
2.5.2. Mechanical Characterization: Flexural Test
2.6. Chemical Characterization
Fourier Transform Infrared Spectroscopy
2.7. Morphological Characterization: Scanning Electron Microscopy
3. Results and Discussion
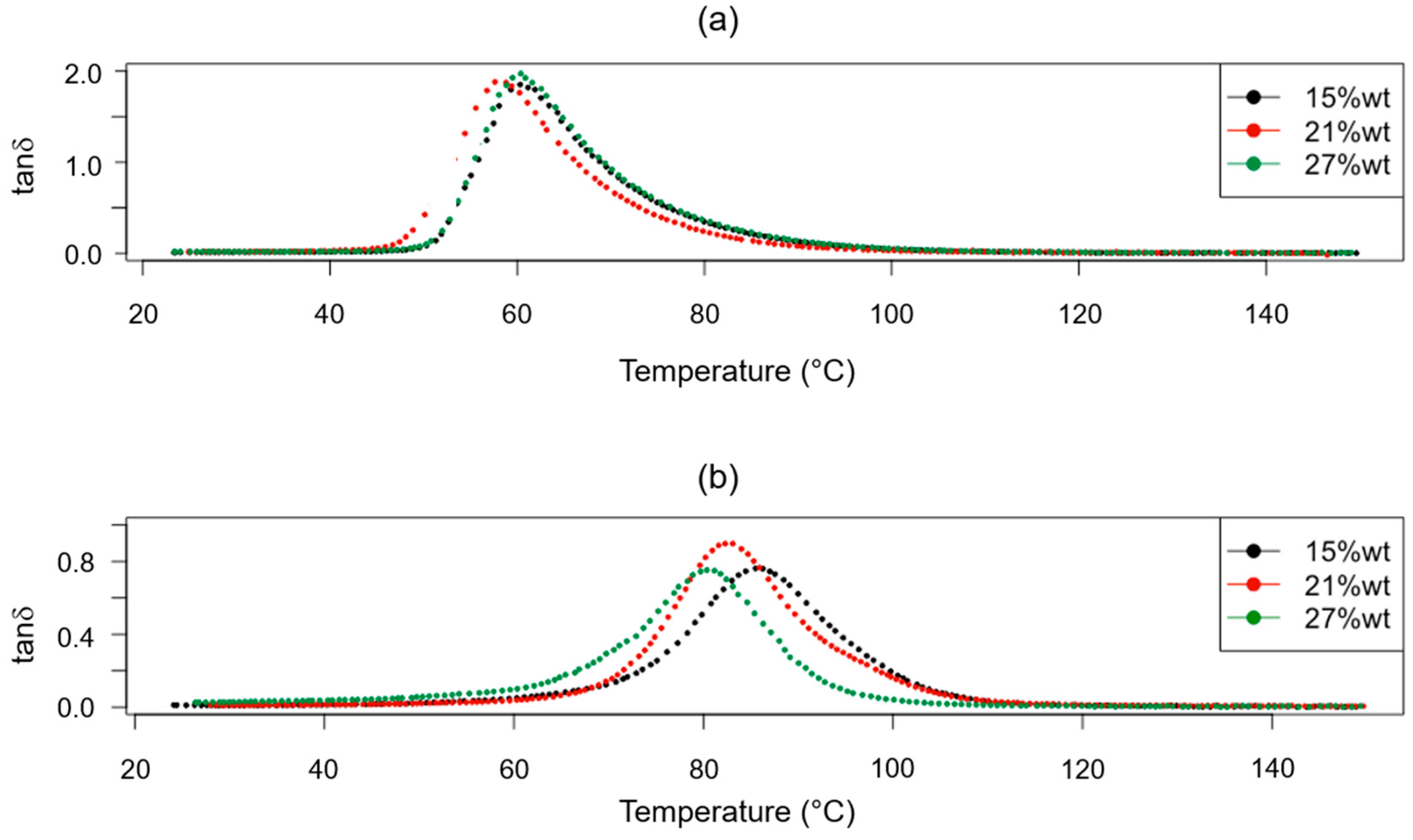
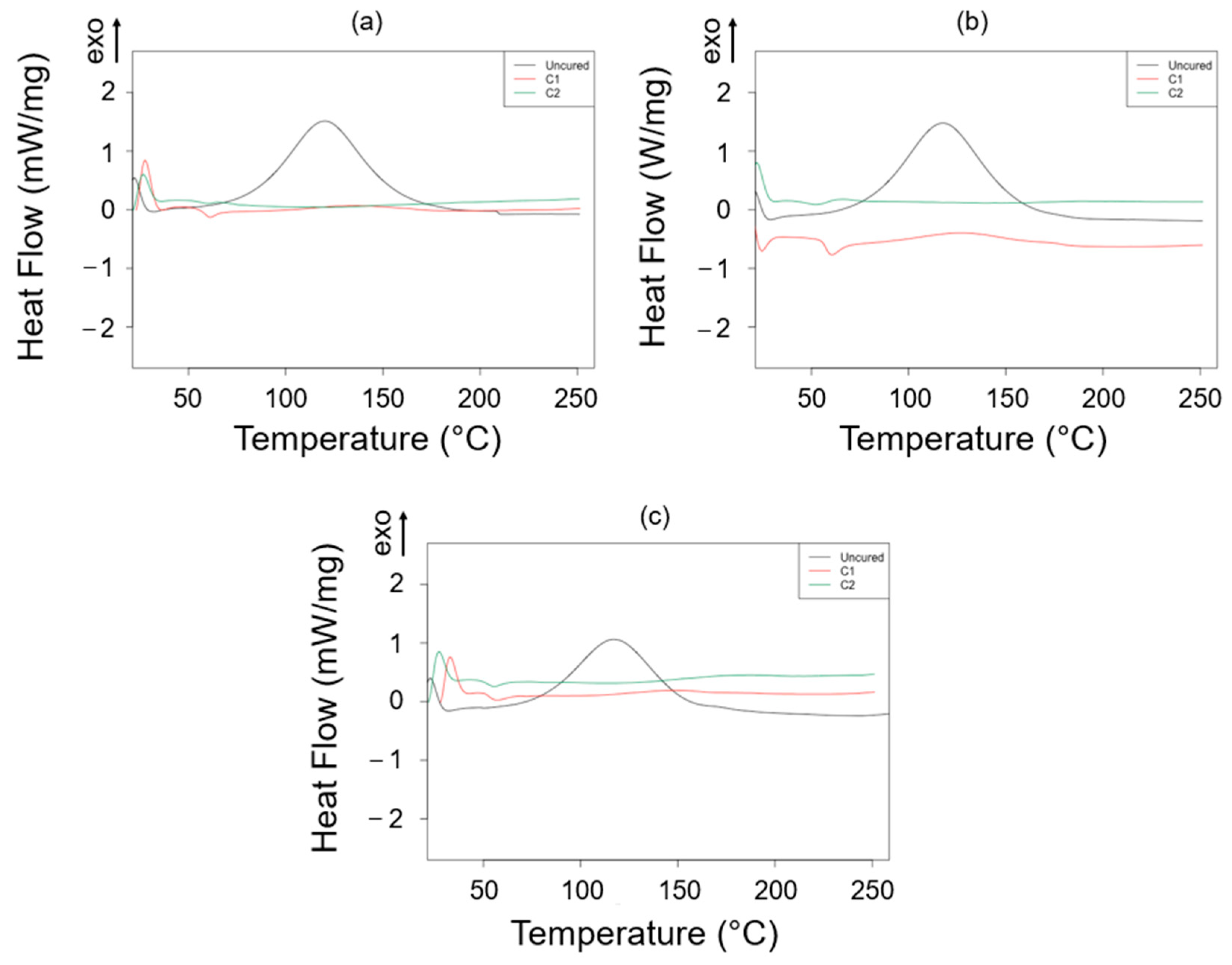
3.1. Thermal Characterization
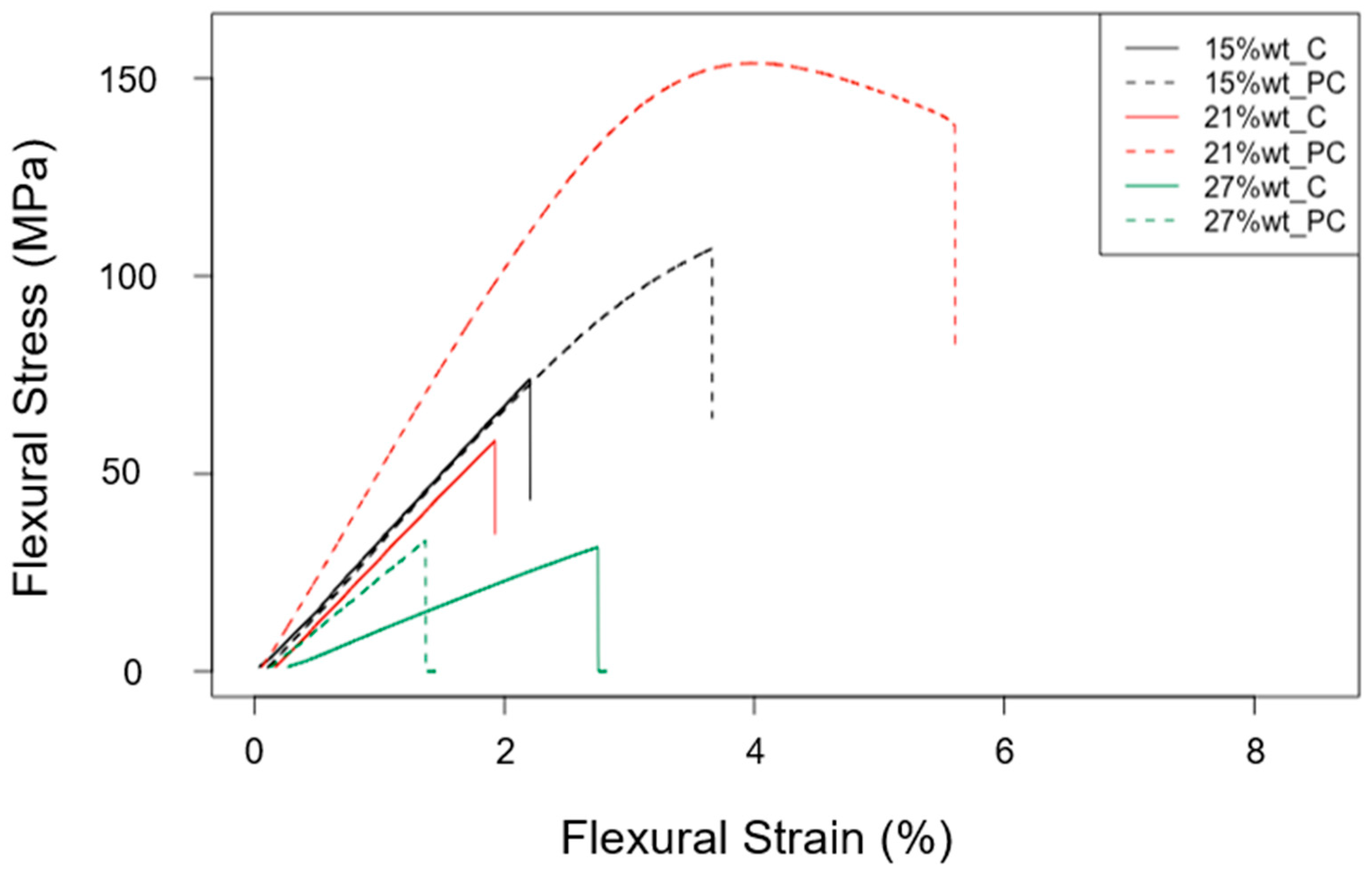
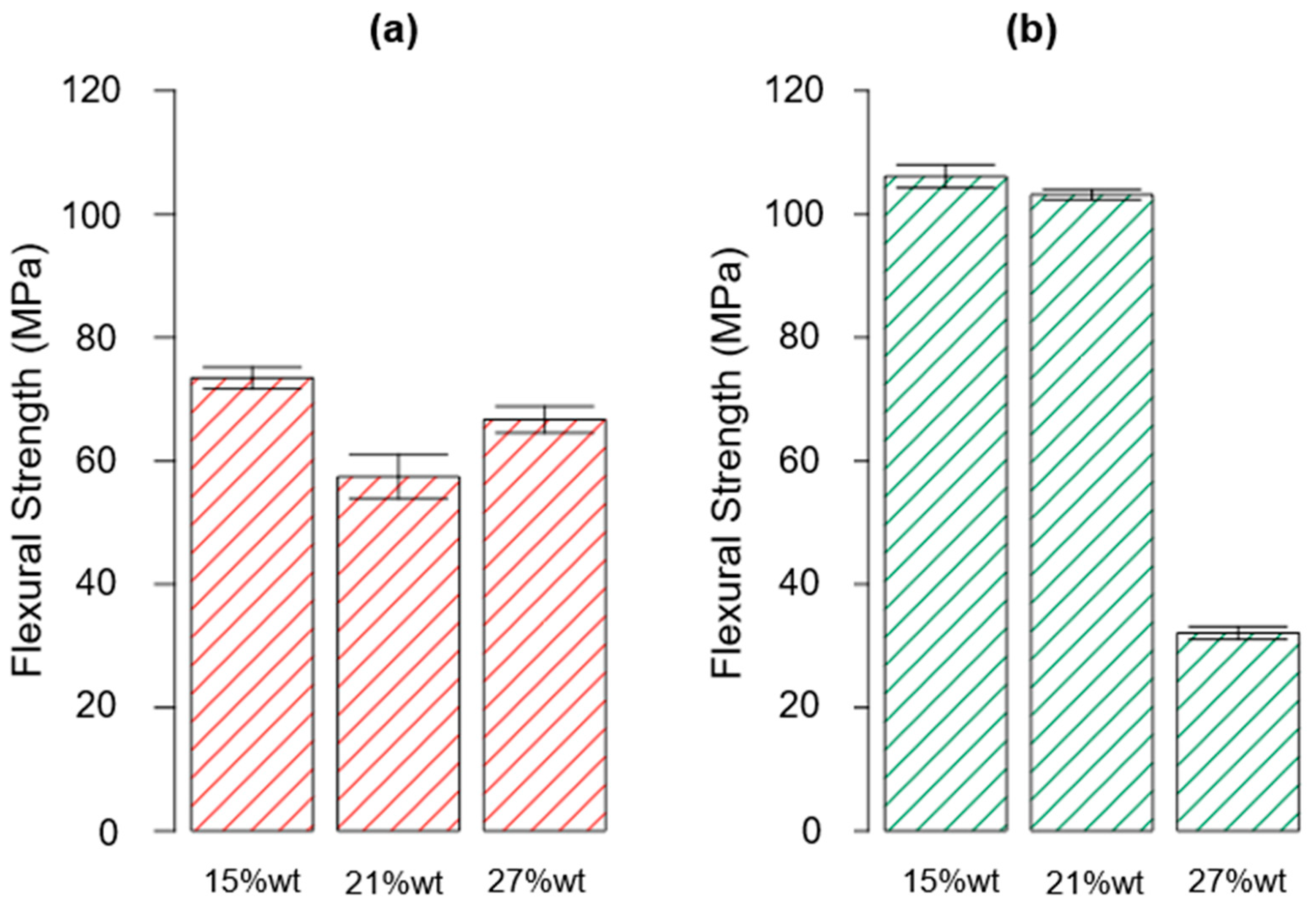
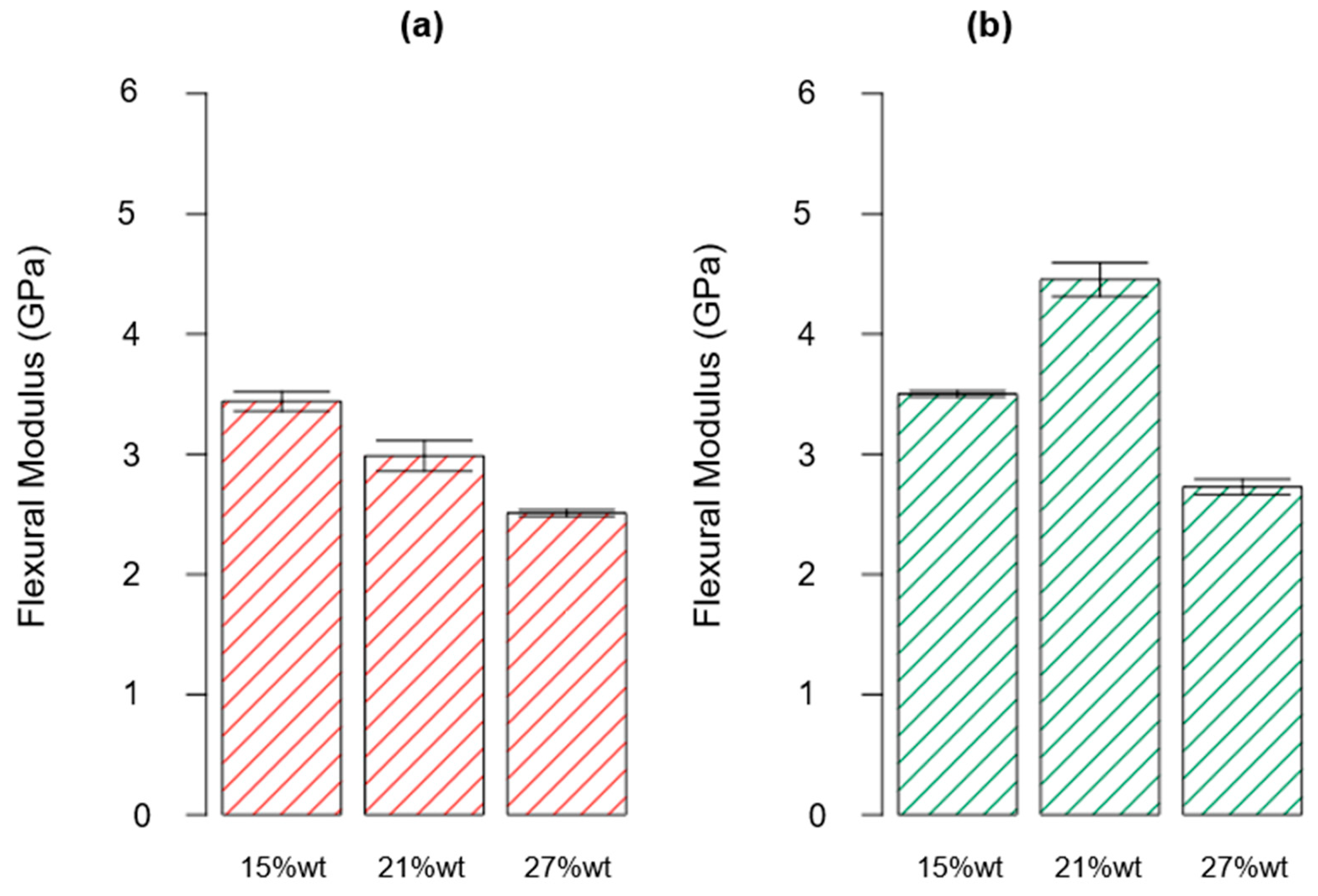
3.2. Mechanical Characterization
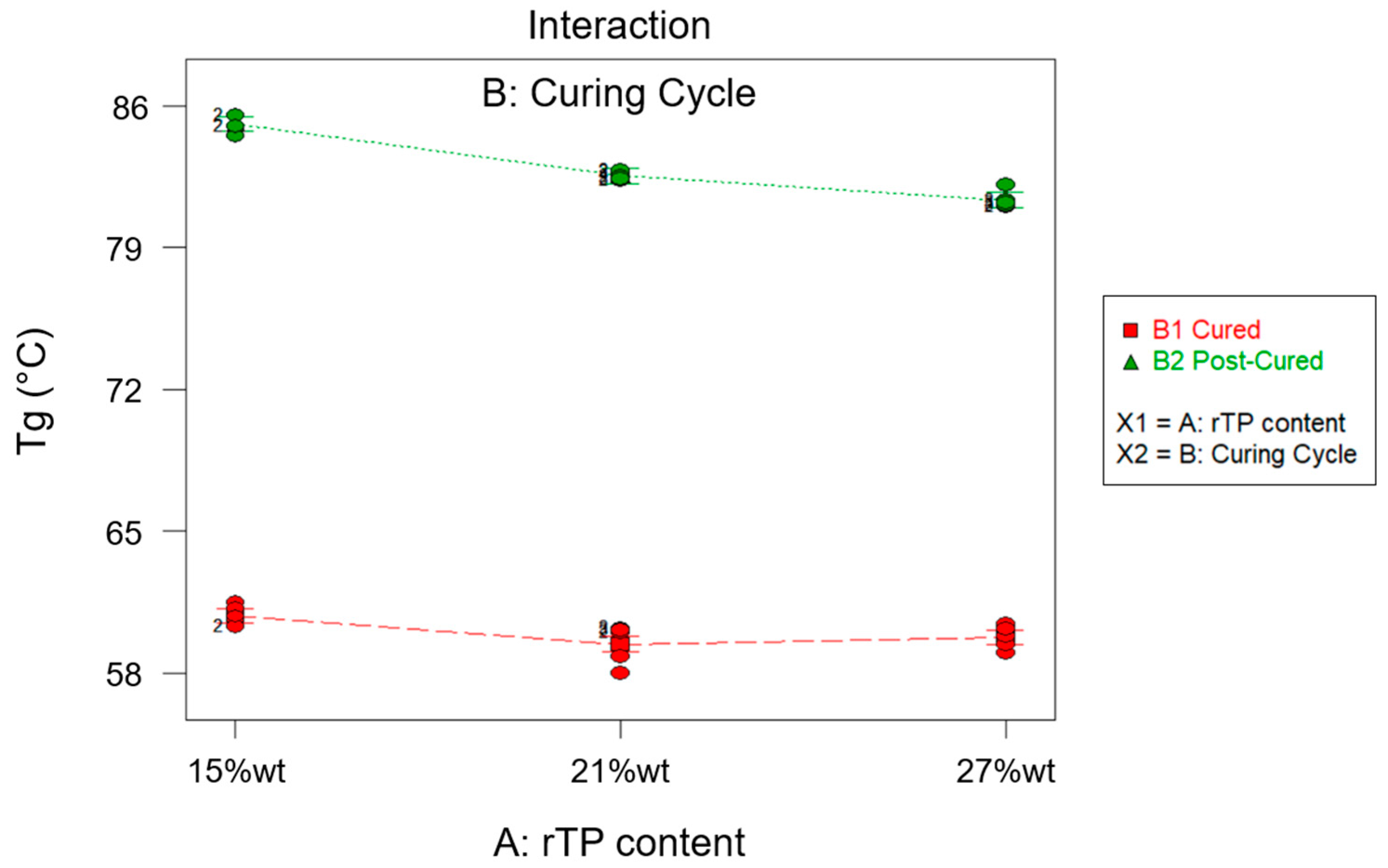
3.3. Optimization Process
4. Conclusions
- homogeneous epoxy/thermoplastic blends with good thermo-mechanical properties were obtained for an rTP content not exceeding a threshold value of 27%wt;
- by implementing an additional post-curing process at 100 °C for 3 h, it was possible to enhance the thermo-mechanical properties;
- the best-performing epoxy system in terms of thermo-mechanical properties, as determined by an optimization process relying on the approach of desirability functions, was the one containing 21%wt rTP which was cured exploiting the C2 curing cycles.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Epoxy Composite Market Size, Share & Trends Analysis Report by Fiber Type (Glass, Carbon), by End-Use (Automotive & Transportation, Wind Energy), by Region (APAC, North America), and Segment Forecasts, 2021–2028; Rep ID GVR-4-68039-095-9; 2021; p. 133. Available online: https://www.researchandmarkets.com/reports/5440519/epoxy-composite-market-size-share-and-trends (accessed on 19 May 2023).
- Pompidou, S.; Prinçaud, M.; Perry, N.; Leray, D. Recycling of carbon fiber. Identification of bases for a synergy between recyclers and designers. In Proceedings of the ASME 2012 11th Biennial Conference on Engineering Systems Design and Analysis, Nantes, France, 2–4 July 2012; Volume 3, pp. 551–560. [Google Scholar] [CrossRef] [Green Version]
- Mohan, P. A Critical Review: The Modification, Properties, and Applications of Epoxy Resins. Polym.-Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Epoxy Resin Market Size, Share & Trends Analysis Report by Application (Adhesives, Electrical & Electronics, Paints & Coatings, Wind Turbines, Composites, Construction), by Region, and Segment Forecasts, 2022–2030; Report ID: 978-1-68038-171-9, Marke Analysis Report, Grand View Research; Research and Markets: Dublin, Ireland, 2022.
- Rani, M.; Choudhary, P.; Krishnan, V.; Zafar, S. A Review on Recycling and Reuse Methods for Carbon Fiber/Glass Fiber Composites Waste from Wind Turbine Blades. Compos. B Eng. 2021, 215, 108768. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent Trends in Recycling and Reusing Techniques of Different Plastic Polymers and Their Composite Materials. Sustain. Mater. Technol. 2022, 31, e00382. [Google Scholar] [CrossRef]
- Mishnaevsky, L. Sustainable end-of-life management of wind turbine blades: Overview of current and coming solutions. Materials 2021, 14, 1124. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef] [Green Version]
- Krauklis, A.E.; Karl, C.W.; Gagani, A.I.; Jørgensen, J.K. Composite Material Recycling Technology—State-of-the-Art and Sustainable Development for the 2020s. J. Compos. Sci. 2021, 5, 28. [Google Scholar] [CrossRef]
- Rani, M.; Choudhary, P.; Krishnan, V.; Zafar, S. Development of Sustainable Microwave-Based Approach to Recover Glass Fibers for Wind Turbine Blades Composite Waste. Resour. Conserv. Recycl. 2022, 179, 106107. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Hossain, M.; Umer, R. Recycling of Wind Turbine Blades through Modern Recycling Technologies: A Road to Zero Waste. Renew. Energy Focus 2023, 44, 373–389. [Google Scholar] [CrossRef]
- Liang, B. Data RUSA. Patent Application Publication Pub No. US 2013/0245204 A1, 17 October 2013. [Google Scholar]
- La Rosa, A.D.; Blanco, I.; Banatao, D.R.; Pastine, S.J.; Björklund, A.; Cicala, G. Innovative chemical process for recycling thermosets cured with recyclamines® by converting bio-epoxy composites in reusable thermoplastic-an LCA study. Materials 2018, 11, 353. [Google Scholar] [CrossRef] [Green Version]
- Dattilo, S.; Cicala, G.; Riccobene, P.M.; Puglisi, C.; Saitta, L. Full Recycling and Re-Use of Bio-Based Epoxy Thermosets: Chemical and Thermomechanical Characterization of the Recycled Matrices. Polymers 2022, 14, 4828. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Ahmed, W.; Arshad, H.; Ullah, S. Recycling of the glass/carbon fibre reinforced polymer composites: A step towards the circular economy. Polym.-Plast. Technol. Mater. 2022, 61, 761–788. [Google Scholar] [CrossRef]
- McDonough, W.; Braungart, M. Cradle to Cradle: Remaking the Way We Make Things, 1st ed.; North Point Press: New York, NY, USA, 2002. [Google Scholar]
- Lieder, M.; Rashid, A. Towards circular economy implementation: A comprehensive review in context of manufacturing industry. J. Clean. Prod. 2016, 115, 36–51. [Google Scholar] [CrossRef]
- Brennan, G.; Tennant, M.; Blomsma, F. Business and Production Solutions: Closing the Loop, 1st ed.; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Sun, B.; Zhang, J.; Wang, M.; Yu, S.; Xu, Y.; Tian, S.; Gao, Z.; Xiao, D.; Liu, G.; Zhou, W.; et al. Valorization of Waste Biodegradable Polyester for Methyl Methacrylate Production. Nat. Sustain. 2023, 6, 712–719. [Google Scholar] [CrossRef]
- Sultan, J.N.; Mcgarry, F.J. Effect of Rubber Particle Size on Deformation Mechanisms in Glassy Epoxy. Polym. Eng. Sci. 1973, 13, 29–34. [Google Scholar] [CrossRef]
- Manzione, L.T.; Gillham, J.K. Rubber-Modified Epoxies. II. Morphology and Mechanical Properties. J. Appl. Polym. Sci. 1981, 26, 907–919. [Google Scholar] [CrossRef]
- Yee, A.F.; Pearson, R.A. Toughening mechanisms in elastomer-modified epoxies. J. Mater. Sci. 1986, 21, 2462–2474. [Google Scholar] [CrossRef] [Green Version]
- Kinloch, A.J.; Shaw, S.J.; Tod, D.A.; Hunston, D.L. Deformation and fracture behaviour of a and fracture studies. Polymer 1983, 24, 1341–1354. [Google Scholar] [CrossRef]
- Maloney, A.C.; Kausch, H.H.; Kaiser, T.; Beer, H.R. Parameters determining the strength and toughness of particulate filled epoxide resins. J. Mater. Sci. 1987, 22, 381–393. [Google Scholar] [CrossRef]
- Hsia, H.C.; Ma, C.C.M.; Li, M.S.; Li, Y.S.; DSChen, J. Glycidyl-Terminated Polyurethane Modified Epoxy Resins: Mechanical Properties, Adhesion Properties, and morphology. Appl. Polym. Sci. 1994, 52, 1137–1151. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamaguchi, M.; Iko, K.; Okubo, M.; Matsumoto, T. Effect of Particle Size on Mechanical Properties of Epoxy Resin Filled with Angular-Shaped Silica. J. Mater. Sci. 1990, 52, 2711. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Partridge, L.K. Effects of morphology in the epoxy/PES matrix on the fatigue behaviour of uniaxial CFRP. Composites 1984, 15, 129–133. [Google Scholar] [CrossRef]
- Zhuo, H.; He, Z.; Liu, J.; Ma, G.; Ren, Z.; Zeng, Y.; Chen, S. Bulk Polymerization of Thermoplastic Shape Memory Epoxy Polymer for Recycling Applications. Polymers 2023, 15, 809. [Google Scholar] [CrossRef]
- Cara, F.; Dumon, M.; Pascault, J.P. Porous Epoxy Thermosets Obtained by a Polymerization-Induced Phase Separation Process of a Degradable Thermoplastic Polymer. Macromolecules 2002, 35, 6291–6297. [Google Scholar]
- Liu, X.; Jiang, Y.; Tang, Z.; Zhang, C.; Zhu, J. Bio-based epoxy resin from itaconic acid and its thermosets cured with anhydride and comonomers. Green Chem. 2013, 15, 245–254. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2015, 37, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.G.; Chow, W.S. Biobased Epoxidized Vegetable Oils and Its Greener Epoxy Blends: A Review Biobased Epoxidized Vegetable Oils and Its Greener Epoxy Blends: A Review. Polym.-Plast. Technol. Eng. 2010, 49, 1581–1590. [Google Scholar] [CrossRef]
- Kalita, D.J.; Tarnavchyk, I.; Chisholm, B.J.; Webster, D.C. Novel Bio-Based Epoxy Resins from Eugenol as an Alternative to BPA Epoxy and High Throughput Screening of the Cured Coatings. Polymer 2021, 233, 124191. [Google Scholar] [CrossRef]
- Kalita, D.J.; Tarnavchyk, I.; Kalita, H.; Chisholm, B.J.; Webster, D.C. Novel Bio-Based Epoxy Resins from Eugenol Derived Copolymers as an Alternative to DGEBA Resin. Prog. Org. Coat. 2023, 178, 107471. [Google Scholar] [CrossRef]
- Saitta, L.; Prasad, V.; Tosto, C.; Murphy, N.; Ivankovic, A.; Cicala, G.; Scarselli, G. Characterization of biobased epoxy resins to manufacture eco-composites showing recycling properties. Polym. Compos. 2022, 43, 9179–9192. [Google Scholar] [CrossRef]
- Cicala, G.; Pergolizzi, E.; Piscopo, F.; Carbone, D.; Recca, G. Hybrid composites manufactured by resin infusion with a fully recyclable bioepoxy resin. Compos. Part B Eng. 2018, 132, 69–76. [Google Scholar] [CrossRef]
- Cicala, G.; Mannino, S.; La Rosa, A.D.; Banatao, D.R.; Pastine, S.J.; Kosinski, S.T.; Scarpa, F. Hybrid Biobased Recyclable Epoxy Composites for Mass Production. Polym. Compos. 2017, 39, E2217–E22252017. [Google Scholar] [CrossRef]
- Daniela, A.; Rosa, L.; Greco, S.; Tosto, C.; Cicala, G. LCA and LCC of a chemical recycling process of waste CF-thermoset composites for the production of novel CF-thermoplastic composites. Open loop and closed loop scenarios. J. Clean. Prod. 2021, 304, 127158. [Google Scholar] [CrossRef]
- Qin, J.; Woloctt, M.; Zhang, J. Use of Polycarboxylic Acid Derived from Partially Depolymerized Lignin As a Curing Agent for Epoxy Application. ACS Sustain. Chem. Eng. 2014, 2, 188–193. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Fox, B. Thermal and Mechanical Properties of a Dendritic Hydroxyl-Functional Hyperbranched Polymer and Tetrafunctional Epoxy Resin Blends. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 417–424. [Google Scholar] [CrossRef]
- Yang, Y.; Xian, G.; Li, H.; Sui, L. Thermal aging of an anhydride-cured epoxy resin. Polym. Degrad. Stab. 2015, 118, 111–119. [Google Scholar] [CrossRef]
- Samper, M.D.; Fombuena, V.; Boronat, T.; García-Sanoguera, D.; Balart, R. Thermal and Mechanical Characterization of Epoxy Resins (ELO and ESO) Cured with Anhydrides. J. Am. Oil Chem. Soc. 2012, 89, 1521–1528. [Google Scholar] [CrossRef]
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Jin, F.; Li, X.; Park, S. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Cicala, G.; La Spina, R.; Recca, A.; Sturiale, S. Influence of Copolymer’s End Groups and Molecular Weights on the Rheological and Thermomechanical Properties of Blends of Novel Thermoplastic Copolymers and Epoxy Resins. J. Appl. Polym. Sci. 2006, 101, 250–257. [Google Scholar] [CrossRef]
- Shechter, L.; Wynstra, J. Glycidyl Ether Reactions with Alcohols, Phenols, Carboxylic Acids. Ind. Eng. Chem. 1956, 48, 86–93. [Google Scholar] [CrossRef]
- Bowen, D.O.; Whiteside, R.C. Effect of Hydroxyl Compounds on Reactivity of Epoxy Resins; ACS Publications: Washington, DC, USA, 1970; pp. 48–59. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Gabbot, P. Principles and Applications of Thermal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Paul, D.R. Control of Phase Structure in Polymer Blends. In Functional Polymers; Bergbreiter, D.E., Martin, C.R., Eds.; Springer US: Boston, MA, USA, 1989; pp. 1–18. [Google Scholar] [CrossRef]
- Grohens, Y.; George, B.; Touyeras, F.; Vebrel, J.; Laude, B. Experimental Design as a Route for Improving the Performances of Formulated Epoxy Adhesives. Polym. Test. 1997, 16, 417–427. [Google Scholar] [CrossRef]
- Zucchi, I.A.; Galante, M.J.; Williams, R.J.J. Comparison of morphologies and mechanical properties of crosslinked epoxies modified by polystyrene and poly(methyl methacrylate)) or by the corresponding block copolymer polystyrene-b-poly(methyl methacrylate). Polymer 2005, 46, 2603–2609. [Google Scholar] [CrossRef]
- Poncet, S.; Boiteux, G.; Pascault, J.P.; Sautereau, H.; Seytre, G.; Rogozinski, J.; Kranbuehl, D. Monitoring phase separation and reaction advancement in situ in thermoplastic/epoxy blends. Polymer 1999, 40, 6811–6820. [Google Scholar] [CrossRef]
- Rodríguez, M.T.; García, S.J.; Cabello, R.; Naber, J.J.G. Effect of Plasticizer on the Thermal, Mechanical, and Anticorrosion Properties Of an Epoxy Primer. JCT Res. 2005, 2, 557–564. [Google Scholar] [CrossRef]
- Blanco, I.; Cicala, G.; Motta, O.; Recca, A. Influence of a Selected Hardener on the Phase Separation in Epoxy/Thermoplastic Polymer Blends. J. Appl. Polym. Sci. 2004, 94, 361–371. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Hailatihan, B.; Mi, L.; Baheti, Y.; Yan, Y. Effect of the Addition of Thermoplastic Resin and Composite on Mechanical and Thermal Properties of Epoxy Resin. Polymers 2022, 14, 1087. [Google Scholar] [CrossRef]
- Giannotti, M.I.; Galante, M.J.; Oyanguren, P.A.; Vallo, C.I. Role of intrinsic flaws upon flexural behaviour of a thermoplastic modified epoxy resin. Polym. Test. 2003, 22, 429–437. [Google Scholar] [CrossRef]
- Essmeister, J.; Taublaender, M.J.; Thomas Koch Cerròn-Infantes, D.A.; Unterlass, M.M.; Konegger, T. High modulus polyimide particle-reinforcement of epoxy composites. Mater. Adv. 2021, 2, 2278–2288. [Google Scholar] [CrossRef]
- Carolina, S.; Carolina, S. Raman and Near-Infrared Studies of an Epoxy Resin. Appl. Spectrosc. 1993, 47, 1631–1635. [Google Scholar]
- Hay, M.B.; Myneni, S.C.B. Structural environments of carboxyl groups in natural organic molecules from terrestrial systems. Part 1: Infrared spectroscopy. Geochim. Cosmochim. Acta 2007, 71, 3518–3532. [Google Scholar] [CrossRef]
- Stevens, G.C. Cure kinetics of a low epoxide/hydroxyl group-ratio bisphenol a epoxy resin–anhydride system by infrared absorption spectroscopy. J. Appl. Polym. Sci. 1981, 26, 4259–4278. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Jian, X. Introduction to Epoxy/Thermoplastic Blends. In Handbook of Epoxy Blends; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Yu, Y.F.; Cui, J.; Chen, W.J.; Li, S.J. Pure and Applied Chemistry Studies on the Phase Separation of Polyetherimide Modified Tetrafunctional Epoxy Resin. II. Effects of the Molecular Weight. J. Macromol. Sci. Part A 1998, 35, 121–135. [Google Scholar] [CrossRef]


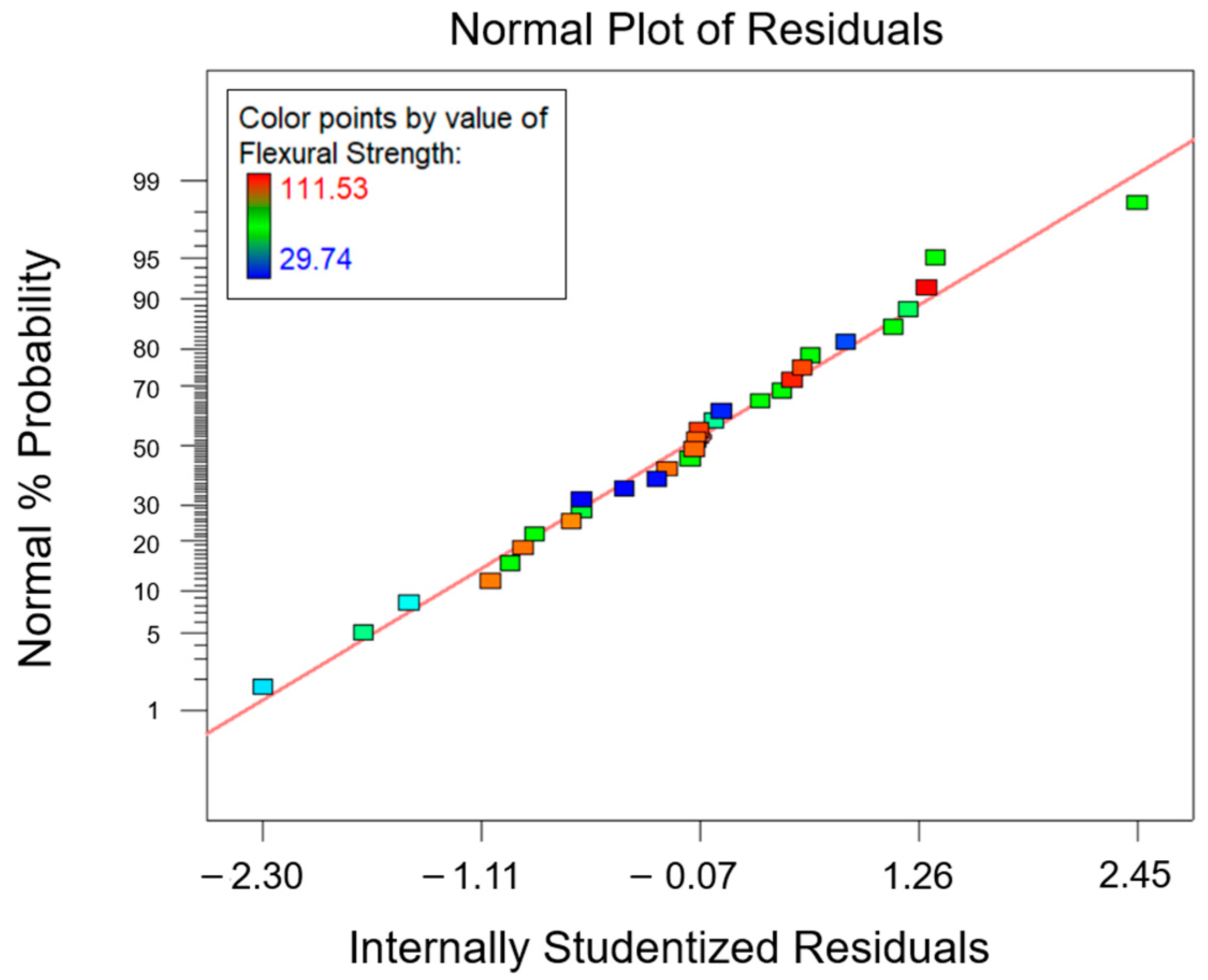

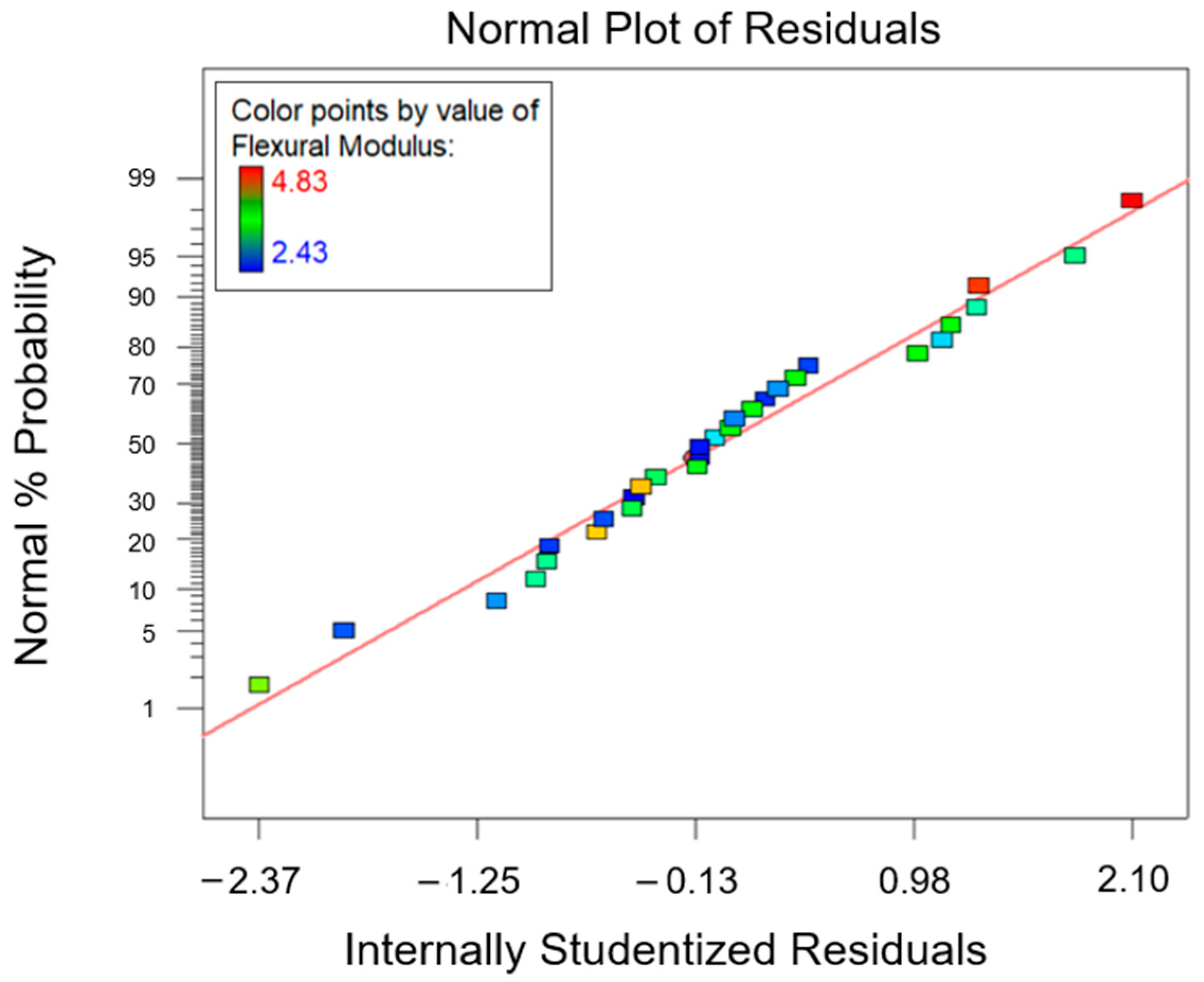




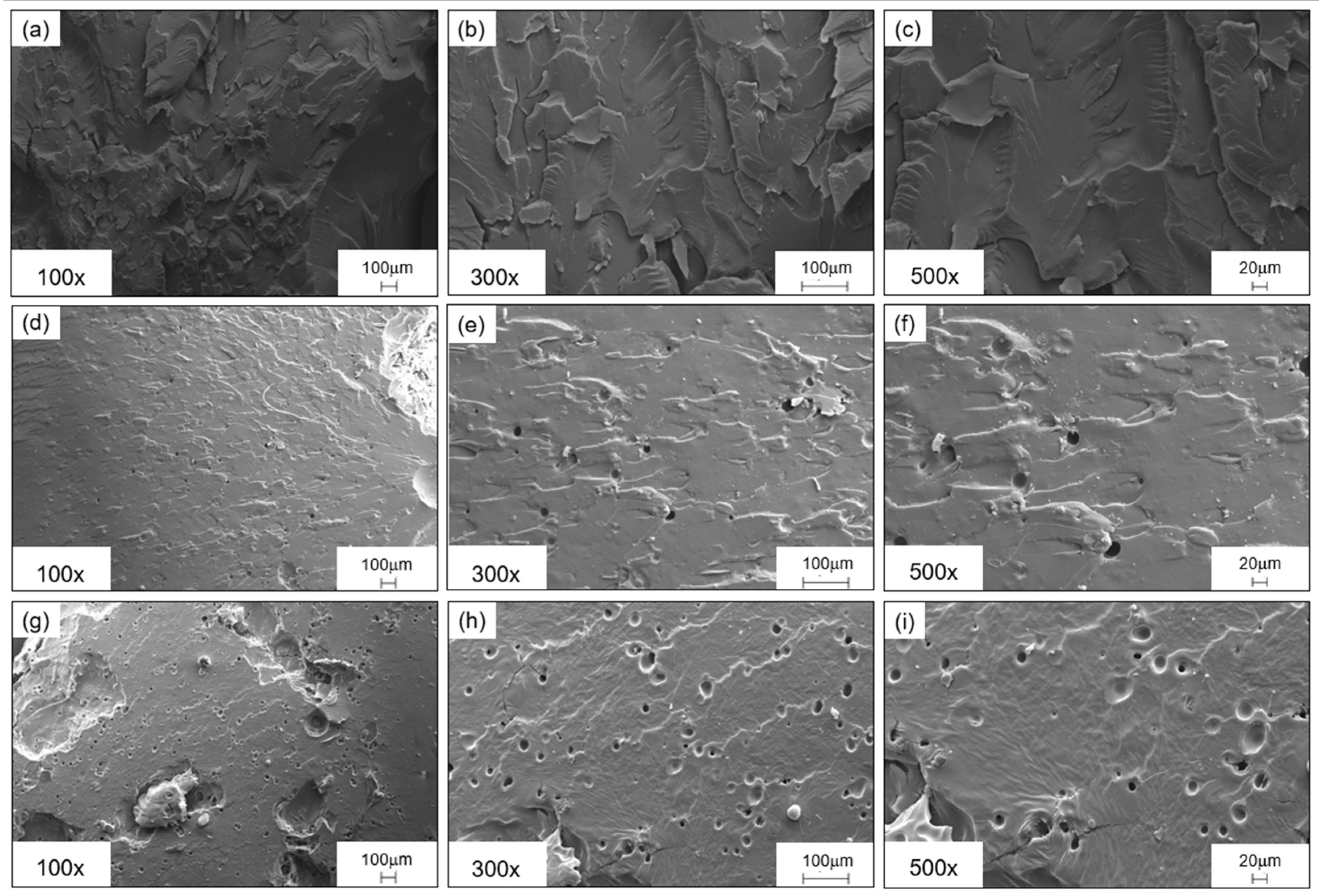

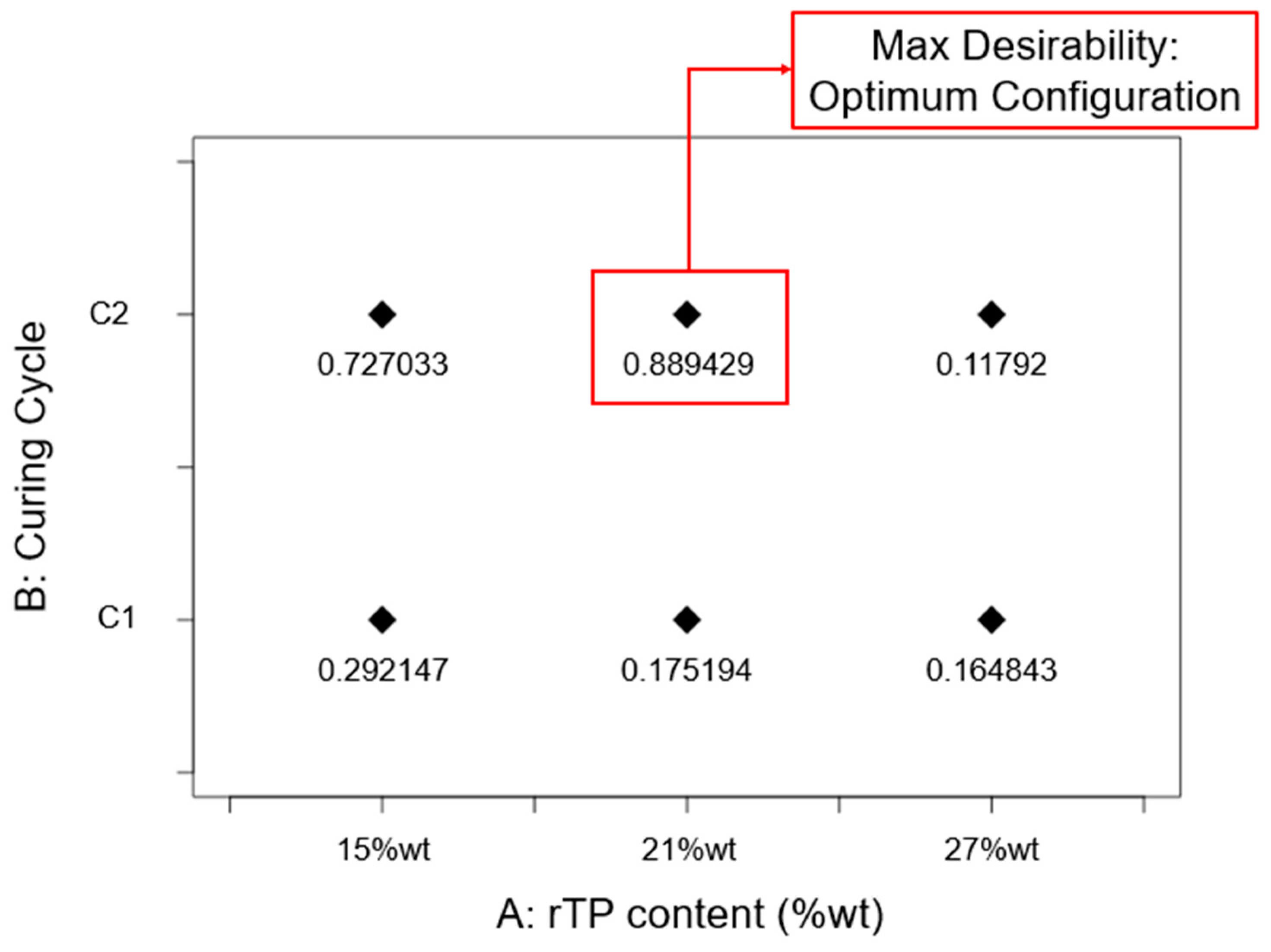
| Response | Factor | Symbol | Type | Unit | Level (−1) | Level (0) | Level (+1) |
|---|---|---|---|---|---|---|---|
| Tg | rTP content | A | Categorical | (%wt) | 15 | 21 | 27 |
| Curing cycle | B | Categorical | (-) | C1 | - | C2 | |
| Flexural strength | rTP content | A | Categorical | (%wt) | 15 | 21 | 27 |
| Curing cycle | B | Categorical | (-) | C1 | - | C2 | |
| Flexural modulus | rTP content | A | Categorical | (%wt) | 15 | 21 | 27 |
| Curing cycle | B | Categorical | (-) | C1 | - | C2 | |
| Deformation at break | rTP content | A | Categorical | (%wt) | 15 | 21 | 27 |
| Curing cycle | B | Categorical | (-) | C1 | - | C2 |
| Parameter | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
|---|---|---|---|---|---|---|
| rTP content | In range | 15%wt | 27%wt | 1 | 1 | 3 |
| Curing cycle | In range | C1 | C2 | 1 | 1 | 3 |
| Tg | Maximize | Lowest value collected | Highest value collected | 1 | 1 | 3 |
| Flexural strength | Maximize | Lowest value collected | Highest value collected | 1 | 1 | 3 |
| Flexural modulus | Maximize | Lowest value collected | Highest value collected | 1 | 1 | 3 |
| Deformation at break | Maximize | Lowest value collected | Highest value collected | 1 | 1 | 3 |
| Sample ID | Thermoset System | Curing Cycle | Tg (°C) |
|---|---|---|---|
| P_A_rTP15_C | Polar + R*101 + 15%wt rTP | C1 | 60.80 ± 0.56 |
| P_A_rTP15_PC | Polar + R*101 + 15%wt rTP | C2 | 85.10 ± 0.42 |
| P_A_rTP21_C | Polar + R*101 + 21%wt rTP | C1 | 59.42 ± 0.98 |
| P_A_rTP21_PC | Polar + R*101 + 21%wt rTP | C2 | 82.54 ± 0.21 |
| P_A_rTP27_C | Polar + R*101 + 27%wt rTP | C1 | 59.76 ± 0.57 |
| P_A_rTP27_PC | Polar + R*101 + 27%wt rTP | C2 | 81.34 ± 0.44 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 4007.42 | 5 | 801.48 | 2431.19 | <0.0001 | significant |
| A–rTP content | 33.05 | 2 | 16.52 | 50.12 | <0.0001 | |
| B–Curing Cycle | 3965.20 | 1 | 3965.20 | 12,027.91 | <0.0001 | |
| AB | 9.17 | 2 | 4.59 | 13.91 | <0.0001 | |
| Pure Error | 7.91 | 24 | 0.33 | |||
| Cor Total | 4015.33 | 29 | ||||
| Std. Dev. | 0.57 | R-Squared | 0.9980 | |||
| Mean | 71.50 | Adj R-Squared | 0.9976 |
| Sample ID | Thermoset System | Curing State | Onset (°C) | Endset (°C) | Peak (°C) | Heat (J/g) |
|---|---|---|---|---|---|---|
| P_A_rTP15 | Polar + R*101 + 15%wt rTP | Uncured | 82.26 | 157.84 | 120.19 | 226.83 |
| P_A_rTP21 | Polar + R*101 + 21%wt rTP | Uncured | 79.38 | 156.19 | 117.78 | 245.21 |
| P_A_rTP27 | Polar + R*101 + 27%wt rTP | Uncured | 77.94 | 153.17 | 116.91 | 179.38 |
| ID | Thermoset System | Flexural Strength (MPa) | Flexural Modulus (GPa) | Deformation at Break (%) |
|---|---|---|---|---|
| P_A_rTP15_C | Polar + R*101 + 15%wt rTP | 73.44 ± 3.93 | 3.44 ± 0.18 | 2.22 ± 0.11 |
| P_A_rTP15_PC | Polar + R*101 + 15%wt rTP | 106.11 ± 4.13 | 3.50 ± 0.06 | 3.41 ± 0.41 |
| P_A_rTP21_C | Polar + R*101 + 21%wt rTP | 57.44 ± 8.04 | 2.99 ± 0.28 | 1.95 ± 0.16 |
| P_A_rTP21_PC | Polar + R*101 + 21%wt rTP | 103.16 ± 1.86 | 4.45 ± 0.31 | 4.21 ± 0.25 |
| P_A_rTP27_C | Polar + R*101 + 27%wt rTP | 66.68 ± 4.82 | 2.51 ± 0.06 | 3.69 ± 0.24 |
| P_A_rTP27_PC | Polar + R*101 + 27%wt rTP | 31.09 ± 2.32 | 2.73 ± 0.14 | 1.40 ± 0.16 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 19,808.09 | 5 | 3961.62 | 184.02 | <0.0001 | significant |
| A–rTP content | 8921.93 | 2 | 4460.97 | 207.22 | <0.0001 | |
| B–Curing Cycle | 1598.85 | 1 | 1598.85 | 74.27 | <0.0001 | |
| AB | 9287.31 | 2 | 4643.66 | 215.70 | <0.0001 | |
| Pure Error | 516.67 | 24 | 21.52 | |||
| Cor Total | 20,324.76 | 29 | ||||
| Std. Dev. | 4.64 | R-Squared | 0.9746 | |||
| Mean | 73.16 | Adj R-Squared | 0.9693 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 12.18 | 5 | 2.44 | 60.92 | <0.0001 | significant |
| A—rTP content | 6.67 | 2 | 3.33 | 83.34 | <0.0001 | |
| B—Curing Cycle | 2.55 | 1 | 2.55 | 63.66 | <0.0001 | |
| AB | 2.97 | 2 | 1.48 | 37.12 | <0.0001 | |
| Pure Error | 0.96 | 24 | 0.04 | |||
| Cor Total | 13.14 | 29 | ||||
| Std. Dev. | 0.20 | R-Squared | 0.9270 | |||
| Mean | 3.27 | Adj R-Squared | 0.9117 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 30.86 | 5 | 6.17 | 105.67 | <0.0001 | significant |
| A—rTP content | 1.4 | 2 | 0.72 | 12.30 | 0.0002 | |
| B—Curing Cycle | 1.12 | 1 | 1.12 | 19.14 | 0.0002 | |
| AB | 28.30 | 2 | 14.15 | 242.31 | <0.0001 | |
| Pure Error | 1.40 | 24 | 0.06 | |||
| Cor Total | 32.26 | 29 | ||||
| Std. Dev. | 0.24 | R-Squared | 0.9566 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitta, L.; Rizzo, G.; Tosto, C.; Cicala, G.; Blanco, I.; Pergolizzi, E.; Ciobanu, R.; Recca, G. Chemical Recycling of Fully Recyclable Bio-Epoxy Matrices and Reuse Strategies: A Cradle-to-Cradle Approach. Polymers 2023, 15, 2809. https://doi.org/10.3390/polym15132809
Saitta L, Rizzo G, Tosto C, Cicala G, Blanco I, Pergolizzi E, Ciobanu R, Recca G. Chemical Recycling of Fully Recyclable Bio-Epoxy Matrices and Reuse Strategies: A Cradle-to-Cradle Approach. Polymers. 2023; 15(13):2809. https://doi.org/10.3390/polym15132809
Chicago/Turabian StyleSaitta, Lorena, Giuliana Rizzo, Claudio Tosto, Gianluca Cicala, Ignazio Blanco, Eugenio Pergolizzi, Romeo Ciobanu, and Giuseppe Recca. 2023. "Chemical Recycling of Fully Recyclable Bio-Epoxy Matrices and Reuse Strategies: A Cradle-to-Cradle Approach" Polymers 15, no. 13: 2809. https://doi.org/10.3390/polym15132809
APA StyleSaitta, L., Rizzo, G., Tosto, C., Cicala, G., Blanco, I., Pergolizzi, E., Ciobanu, R., & Recca, G. (2023). Chemical Recycling of Fully Recyclable Bio-Epoxy Matrices and Reuse Strategies: A Cradle-to-Cradle Approach. Polymers, 15(13), 2809. https://doi.org/10.3390/polym15132809










