Space Charge Characteristics and Breakdown Properties of Nanostructured SiO2/PP Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. FTIR Characterization
2.3. SEM Characterization
2.4. Space Charge Test
2.5. Volume Resistivity Test
2.6. Breakdown Strength Test
3. Results
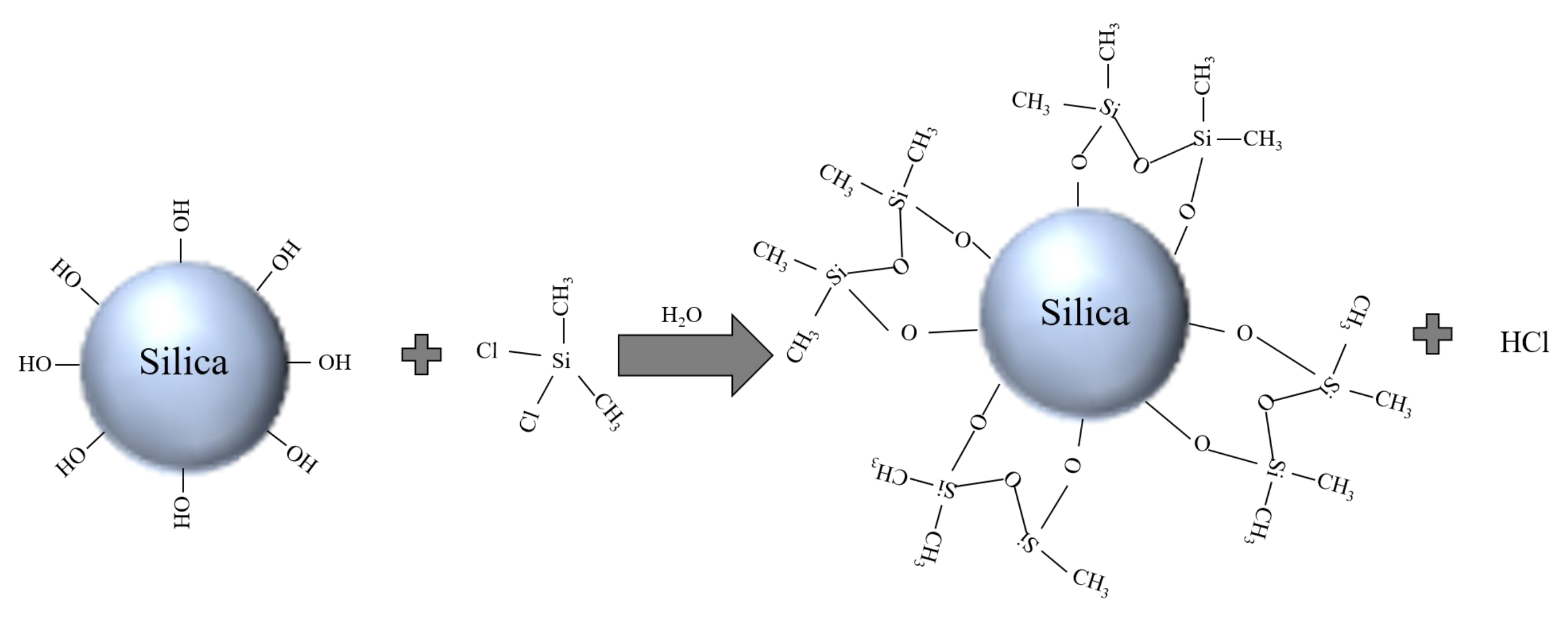
3.1. Structural Characterization of Functional Groups
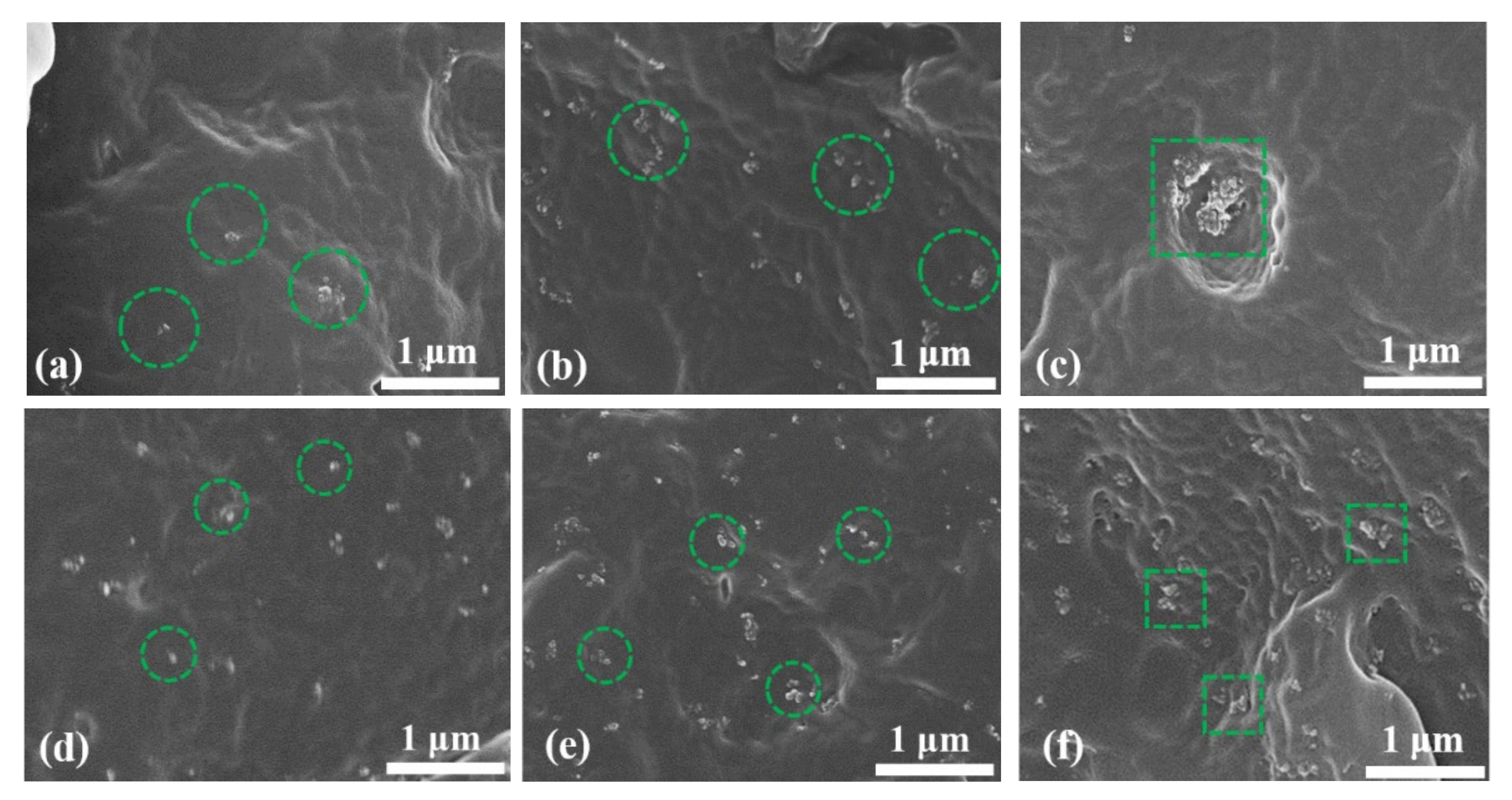
3.2. SEM Morphology
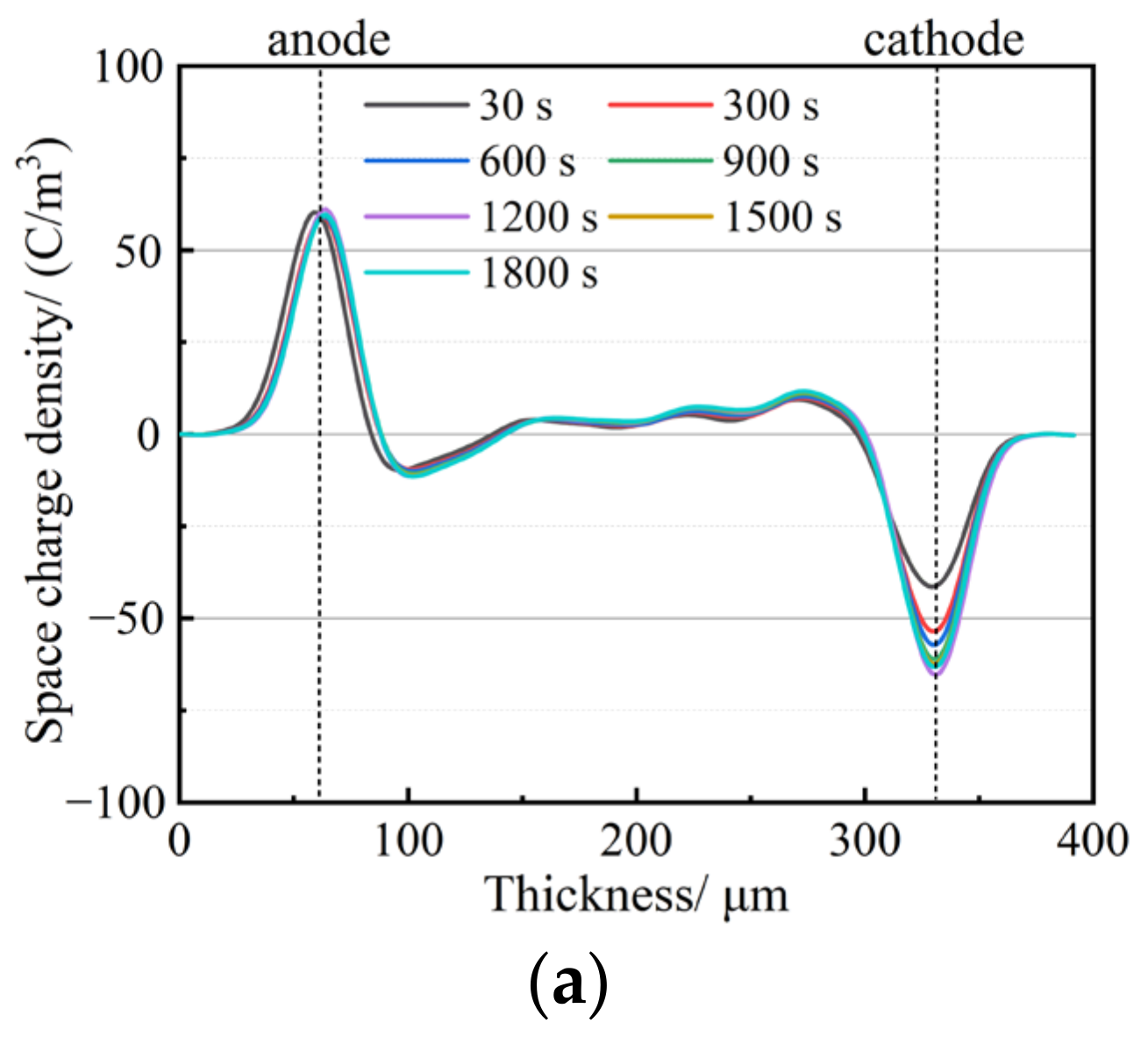
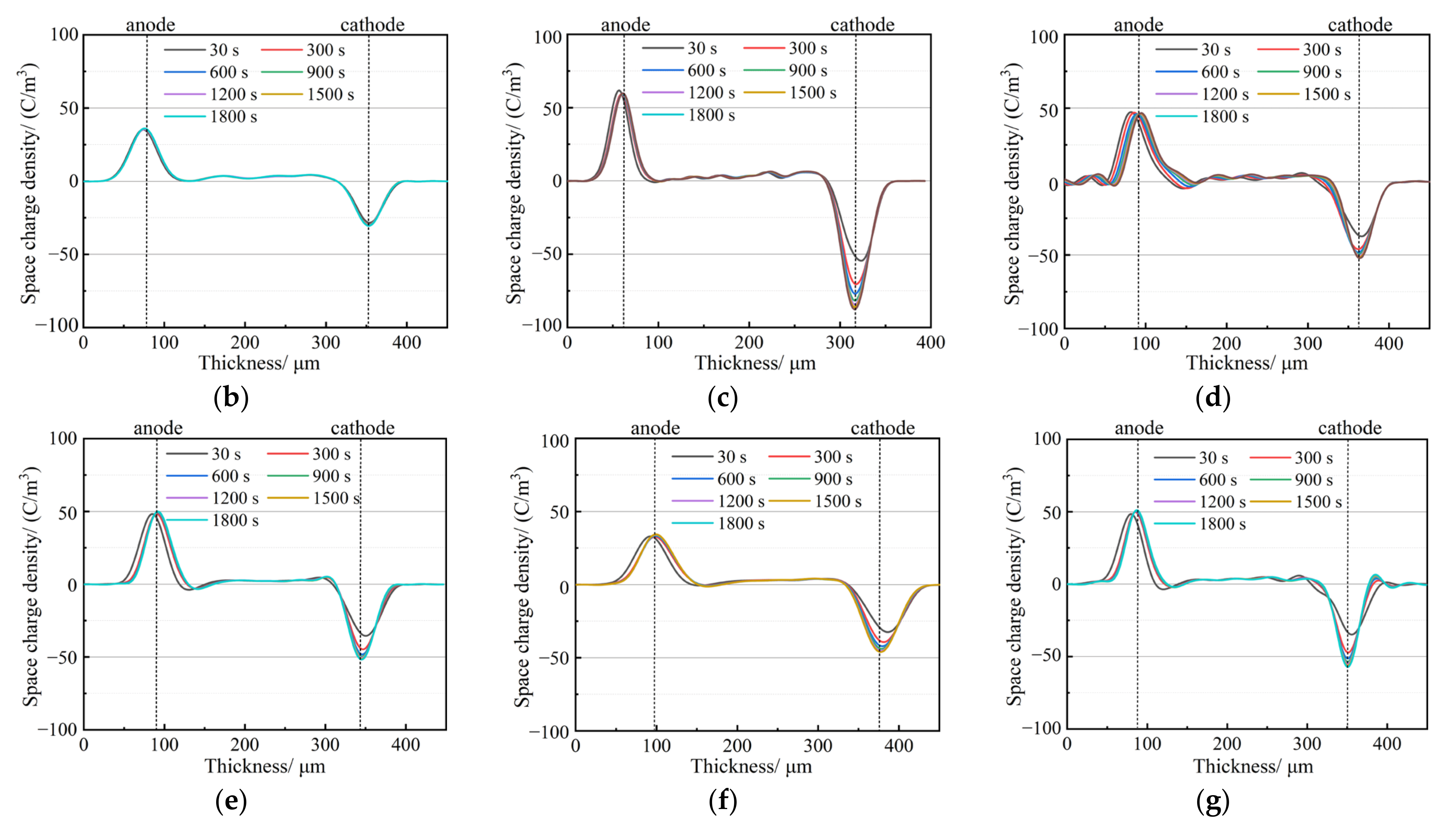
3.3. Space Charge Characteristics
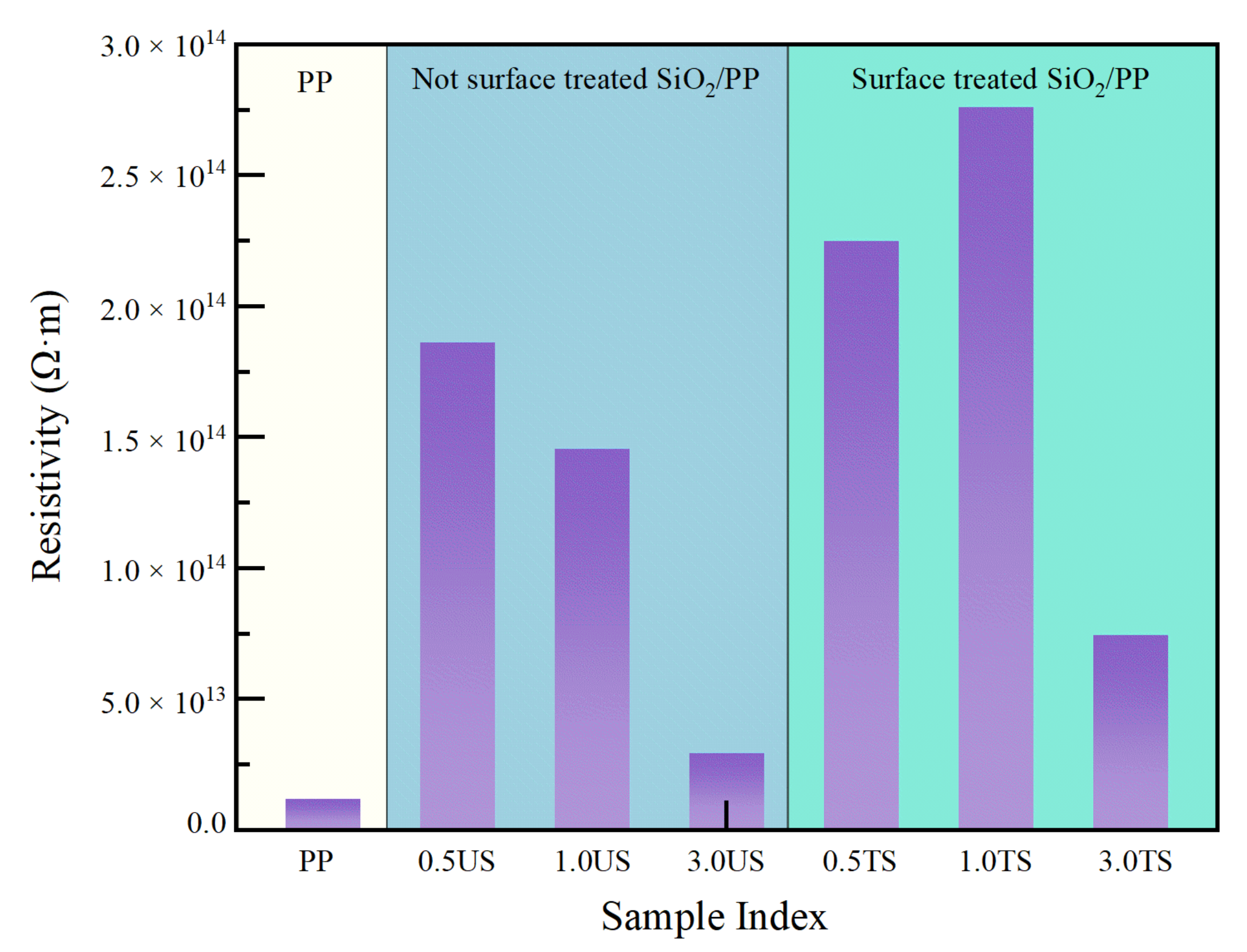
3.4. Volume Resistivity
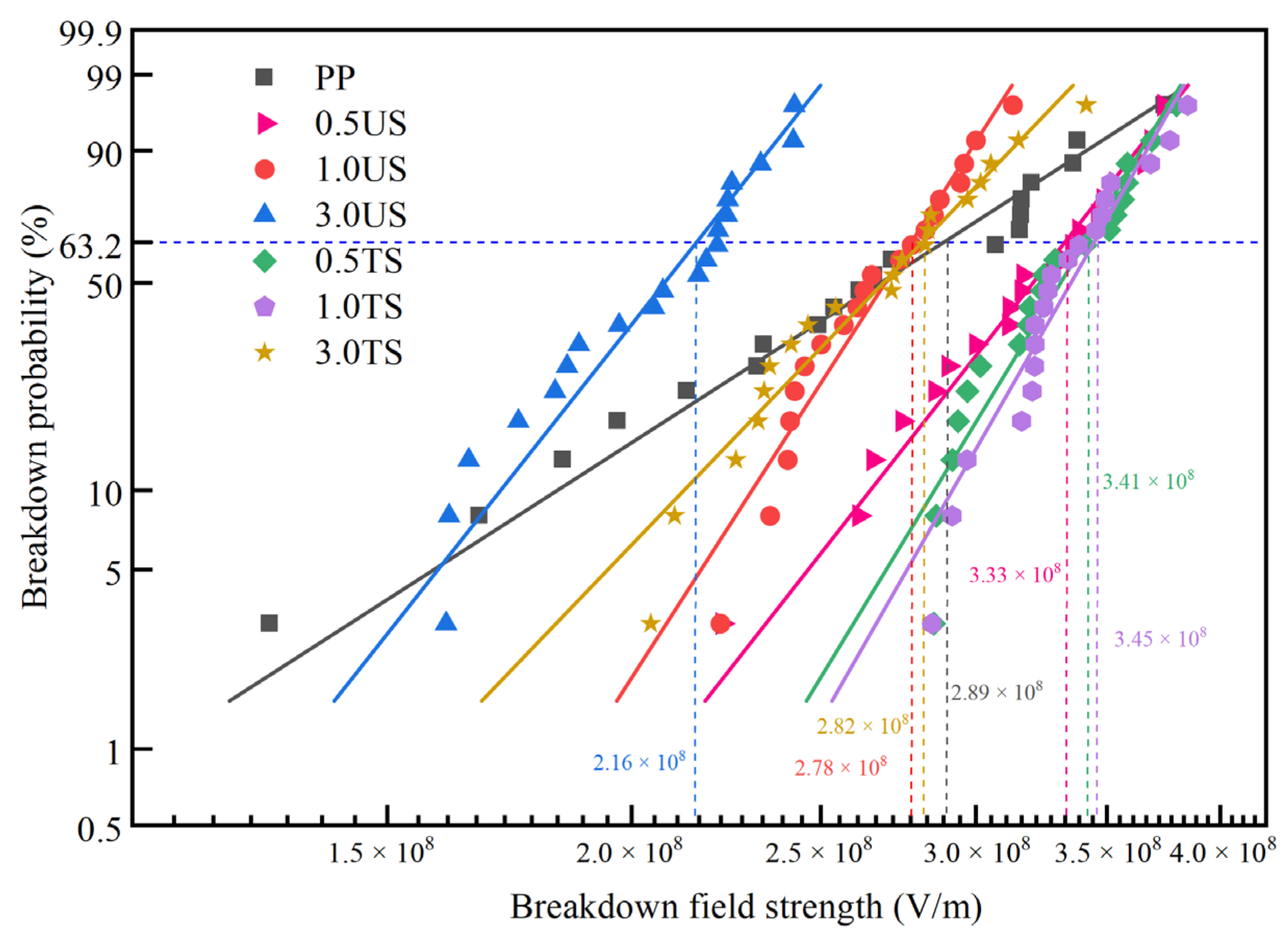
3.5. DC Breakdown Strength
4. Discussion
5. Conclusions
- SEM images reveal that when the nanoparticle content reaches 3.0 wt%, agglomeration occurs in the matrix, which introduces impurities and reduces the breakdown field strength. However, with the addition of SiO2 R974, the surface hydrophobic treatment greatly reduced the hydroxyl content and improved the agglomeration phenomenon, resulting in a 30.58% increase in breakdown field strength and an enhanced space charge suppression compared to SiO2 200 at the same ratio.
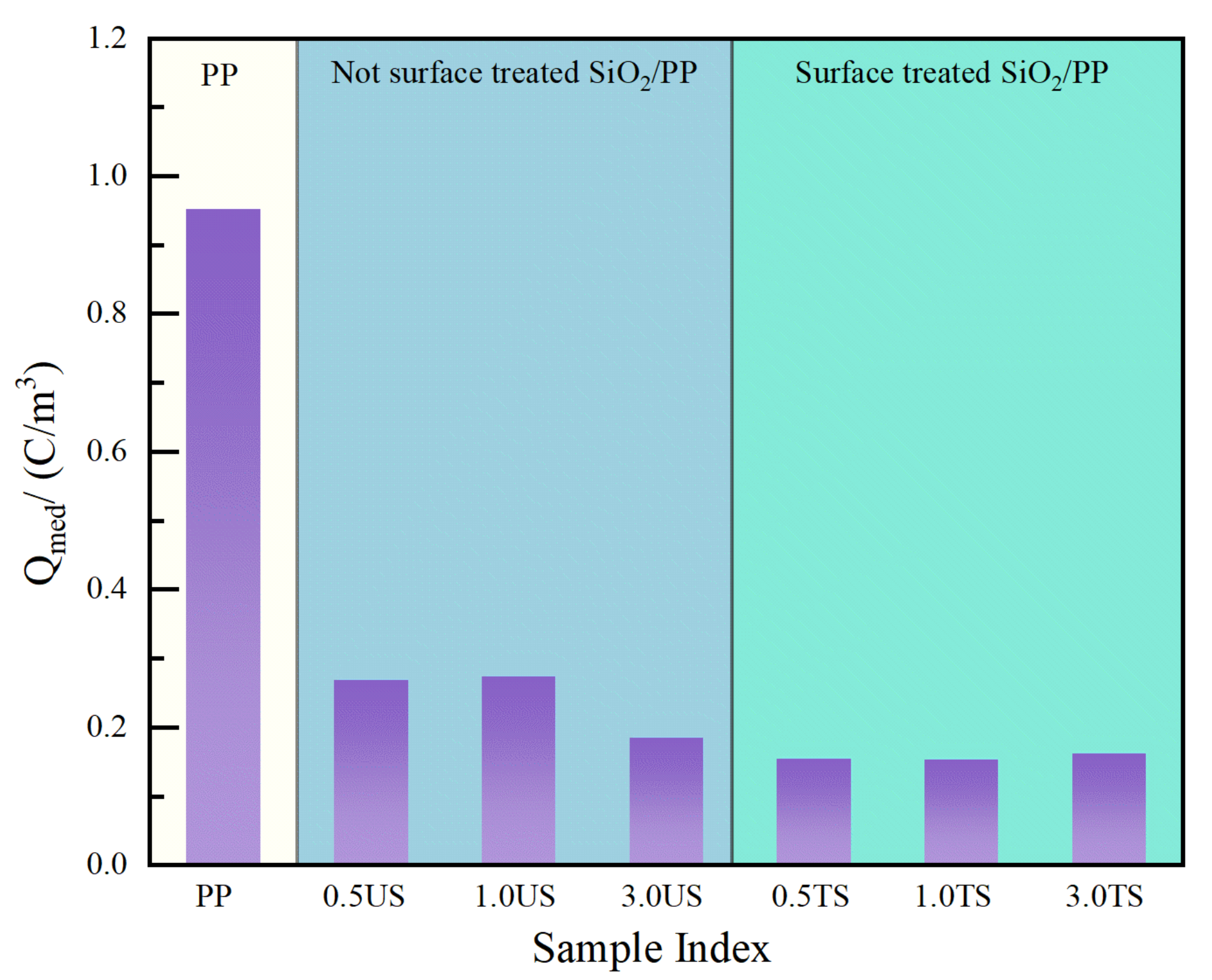
- The addition of a small amount of nanostructured SiO2 effectively inhibits space charge accumulation, weakens electrode charge injection, significantly reduces residual charge after short-circuiting discharge, and enhances charge capture capability. The hydrophobic siloxane group formed after surface treatment improves the compatibility between SiO2 and non-polar PP and reduces structural defects in the amorphous zone. Consequently, SiO2 R974 exhibits lower charge accumulation and residual charge after discharge compared to SiO2 200. The composite with 1.0 wt% SiO2 R974 shows the least space charge accumulation and residual charge after discharge, with an average charge density of only 0.153 C·m−3 after 1800 s of a short-circuit discharge, which is 83.9% lower than that of PP.
- The addition of a small amount of nanostructured SiO2 into the PP matrix increases the DC breakdown field strength. When 0.5 wt% SiO2 200 is added, the breakdown field strength increases by 15.3% compared to pure PP. Similarly, the addition of SiO2 R974 at 0.5 wt% and 1.0 wt% leads to an increase of 18.2% and 19.4% in the breakdown field strength, respectively, compared to PP. The surface treatment reduces the hydrophilicity of nanomaterials, improves their bonding with the polymer matrix, forms a higher polarity interface, and enhances dispersion. Therefore, SiO2 R974 exhibits a more significant effect in improving the breakdown field strength of PP.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, J.G.; Liu, L.W.; Sun, W.F. Dielectric Characteristics of Crosslinked Polyethylene Modified by Grafting Polar-Group Molecules. Polymers 2023, 15, 231. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.W.; Wang, Y.; Li, W.K. Electrical properties of polypropylene/styrene-ethylene-butylene-styrene block copolymer/MgO nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1457–1464. [Google Scholar] [CrossRef]
- Wang, X.; Hao, J.Q.; Xiong, J.Z. Comparison and analysis of three pulse injection methods in the pulsed electroacoustic technique used for long cables. IEEE Electr. Insul. Mag. 2018, 34, 17–31. [Google Scholar] [CrossRef]
- Rehman, B.; Rehman, A.; Khan, W.A. Operation and Challenges of Multi-Infeed LCC–HVDC System: Commutation Failure, AC/DC Power Flow, and Voltage Stability. Appl. Sci. 2021, 11, 8637. [Google Scholar] [CrossRef]
- He, J.; Chen, G. Insulation materials for HVDC polymeric cables. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1307. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Jiang, P. Material progress toward recyclable insulation of power cables part 2: Polypropylene-based thermoplastic materials. IEEE Electr. Insul. Mag. 2019, 36, 8–18. [Google Scholar] [CrossRef]
- Adeniran, A.A.; Shakantu, W. The health and environmental impact of plastic waste disposal in South African Townships: A review. Int. J. Environ. Res. Public Health 2022, 19, 779. [Google Scholar] [CrossRef]
- Du, B.X.; Hou, Z.H.; Li, Z.L. Temperature dependent space charge and breakdown strength of PP/ULDPE/graphene nanocomposites for HVDC extruded cable insulation. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 876–884. [Google Scholar] [CrossRef]
- Zhou, Y.; Dang, B.; Wang, H. Polypropylene-based ternary nanocomposites for recyclable high-voltage direct-current cable insulation. Compos. Sci. Technol. 2018, 165, 168–174. [Google Scholar] [CrossRef]
- Yan, H.D.; Zhang, C.; Li, W.K. Effect of trap level density on breakdown strength and space charge distribution of polypropylene/low-density polyethylene composites. Polym. Compos. 2020, 41, 780–787. [Google Scholar] [CrossRef]
- Liang, Y.; Weng, L.; Zhang, W. Preparation and electrical properties of 4-allyloxy-2-hydroxybenzophenone grafted polypropylene for HVDC cables. J. Electron. Mater. 2021, 50, 6228–6236. [Google Scholar] [CrossRef]
- Wu, J.; Dang, B.; Hu, J. Comparison of Effects of Ethylene-Based and Propylene-Based Copolymer on Tailoring the Properties of Polypropylene. IEEE Access 2020, 8, 123507–123513. [Google Scholar] [CrossRef]
- Aumnate, C.; Rudolph, N.; Sarmadi, M. Recycling of polypropylene/polyethylene blends: Effect of chain structure on the crystallization behaviors. Polymers 2019, 11, 1456. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.H.; Du, B.X.; Li, Z.L. Effects of radical scavenger on space charge accumulation of PP/ULDPE composites for HVDC cable insulation. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 989–997. [Google Scholar] [CrossRef]
- Jiang, X.; Sima, W.; Peng, Q. Effect of thermal ageing on space charge characteristics in double-layered polyester film. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3156–3164. [Google Scholar] [CrossRef]
- Tan, D.Q. The search for enhanced dielectric strength of polymer-based dielectrics: A focused review on polymer nanocomposites. J. Appl. Polym. Sci. 2020, 137, 49379. [Google Scholar] [CrossRef]
- Zadehnazari, A. Metal oxide/polymer nanocomposites: A review on recent advances in fabrication and applications. Polym.-Plast. Technol. Mater. 2023, 62, 655–700. [Google Scholar] [CrossRef]
- Zhang, L.; Khani, M.M.; Krentz, T.M. Suppression of space charge in crosslinked polyethylene filled with poly (stearyl methacrylate)-grafted SiO2 nanoparticles. Appl. Phys. Lett. 2017, 110, 132903. [Google Scholar] [CrossRef]
- Adhikari, C. Polymer nanoparticles-preparations, applications, and future insights: A concise review. Polym.-Plast. Technol. Mater. 2021, 60, 1996–2024. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Xu, N. Dielectric properties of P (VDF-TrFE-CTFE) composites filled with surface-coated TiO2 nanowires by SnO2 nanoparticles. Polymers 2020, 12, 85. [Google Scholar] [CrossRef]
- Zeng, J.; Yan, J.; Li, B.W. Improved breakdown strength and energy storage performances of PEI-based nanocomposite with core-shell structured PI@ BaTiO3 nanofillers. Ceram. Int. 2022, 48, 20526–20533. [Google Scholar] [CrossRef]
- Jain, S.; Goossens, H.; van Duin, M. Effect of in situ prepared silica nano-particles on non-isothermal crystallization of polypropylene. Polymer 2005, 46, 8805–8818. [Google Scholar] [CrossRef]
- Fuse, N.; Sato, H.; Ohki, Y. Effects of nanofiller loading on the molecular motion and carrier transport in polyamide. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 524–530. [Google Scholar] [CrossRef]
- Chi, X.H.; Cheng, L.; Liu, W.F. Dynamic mechanism of breakdown in polypropylene-based nano-dielectric. AIP Adv. 2019, 9, 015135. [Google Scholar] [CrossRef]
- Kon, H.; Suzuoki, Y.; Mizutani, T. Packet-like space charges and conduction current in polyethylene cable insulation. IEEE Trans. Dielectr. Electr. Insul. 1996, 3, 380–385. [Google Scholar] [CrossRef]
- Li, G.; Gu, Z.; Xing, Z. Space Charge and Trap Distributions and Charge Dynamic Migration Characteristics in Polypropylene under Strong Electric Field. ECS J. Solid State Sci. Technol. 2022, 11, 083003. [Google Scholar] [CrossRef]
- Gao, J.G.; Liu, H.S.; Lee, T.T. Effect of Hydrophilic/Hydrophobic Nanostructured TiO2 on Space Charge and Breakdown Properties of Polypropylene. Polymers 2022, 14, 2762. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.W.; Wu, Y.H.; Wang, S.J. Improvement of space charge suppression of polypropylene for potential application in HVDC cables. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2337–2343. [Google Scholar] [CrossRef]
- Andersen, A.; Dennison, J.R. Mixed Weibull distribution model of DC dielectric breakdowns with dual defect modes. In Proceedings of the 2015 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Ann Arbor, MI, USA, 18–21 October 2015; pp. 570–573. [Google Scholar]
- Zhang, X.; Wang, M.; Gao, J. Crystallization morphology and space charge property of silica/low density polyethylene composites. AIP Adv. 2020, 10, 015043. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Dang, B. Titanium oxide nanoparticle increases shallow traps to suppress space charge accumulation in polypropylene dielectrics. RSC Adv. 2016, 6, 48720–48727. [Google Scholar] [CrossRef]
- Li, Z.; Cao, W.; Sheng, G. Experimental study on space charge and electrical strength of MgO nano-particles/polypropylene composite. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1812–1819. [Google Scholar] [CrossRef]
- Krentz, T.; Khani, M.M.; Bell, M. Morphologically dependent alternating-current and direct-current breakdown strength in silica–polypropylene nanocomposites. J. Appl. Polym. Sci. 2017, 134, 44347. [Google Scholar] [CrossRef]
- Lau, K.Y.; Vaughan, A.S.; Chen, G. On the space charge and DC breakdown behavior of polyethylene/silica nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 340–351. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, J.; Zhang, X. Composite micro-nanoarchitectonics of MMT-SiO2: Space charge characteristics under tensile state. Polymers 2021, 13, 4354. [Google Scholar] [CrossRef]
- Seiler, J.; Kindersberger, J. Insight into the interphase in polymer nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 537–547. [Google Scholar] [CrossRef]
- Gong, S.; Chen, Q.; Moll, J.F. Segmental dynamics of polymer melts with spherical nanoparticles. ACS Macro Lett. 2014, 3, 773–777. [Google Scholar] [CrossRef]
- Li, S.; Yin, G.; Chen, G. Short-term breakdown and long-term failure in nanodielectrics: A review. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1523–1535. [Google Scholar] [CrossRef]




| Specimen | PP/wt% | SiO2 200/wt% | SiO2 R974/wt% |
|---|---|---|---|
| PP | 100.0 | 0.0 | 0.0 |
| 0.5 US | 99.5 | 0.5 | 0.0 |
| 1.0 US | 99.0 | 1.0 | 0.0 |
| 3.0 US | 97.0 | 3.0 | 0.0 |
| 0.5 TS | 99.5 | 0.0 | 0.5 |
| 1.0 TS | 99.0 | 0.0 | 1.0 |
| 3.0 TS | 97.0 | 0.0 | 3.0 |
| Specimen | DC Breakdown Field Strength Eb (V/m) | Shape Parameter β |
|---|---|---|
| PP | 2.89 × 108 | 4.95 |
| 0.5 US | 3.33 × 108 | 9.83 |
| 1.0 US | 2.78 × 108 | 12.01 |
| 3.0 US | 2.16 × 108 | 9.76 |
| 0.5 TS | 3.41 × 108 | 12.69 |
| 1.0 TS | 3.45 × 108 | 13.50 |
| 3.0 TS | 2.82 × 108 | 8.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.-W.; Gao, J.-G.; Wang, R.; Lee, T.-t.; Schachtely, U.; Kobayashi, H.; Wang, W.-W. Space Charge Characteristics and Breakdown Properties of Nanostructured SiO2/PP Composites. Polymers 2023, 15, 2826. https://doi.org/10.3390/polym15132826
Zhang G-W, Gao J-G, Wang R, Lee T-t, Schachtely U, Kobayashi H, Wang W-W. Space Charge Characteristics and Breakdown Properties of Nanostructured SiO2/PP Composites. Polymers. 2023; 15(13):2826. https://doi.org/10.3390/polym15132826
Chicago/Turabian StyleZhang, Guang-Wei, Jun-Guo Gao, Ran Wang, Ting-tai Lee, Uwe Schachtely, Hitoshi Kobayashi, and Wei-Wang Wang. 2023. "Space Charge Characteristics and Breakdown Properties of Nanostructured SiO2/PP Composites" Polymers 15, no. 13: 2826. https://doi.org/10.3390/polym15132826
APA StyleZhang, G.-W., Gao, J.-G., Wang, R., Lee, T.-t., Schachtely, U., Kobayashi, H., & Wang, W.-W. (2023). Space Charge Characteristics and Breakdown Properties of Nanostructured SiO2/PP Composites. Polymers, 15(13), 2826. https://doi.org/10.3390/polym15132826







