Hydrogenation of High-Density Polyethylene during Decompression of Pressurized Hydrogen at 90 MPa: A Molecular Perspective
Abstract
:1. Introduction
2. Materials and Methods
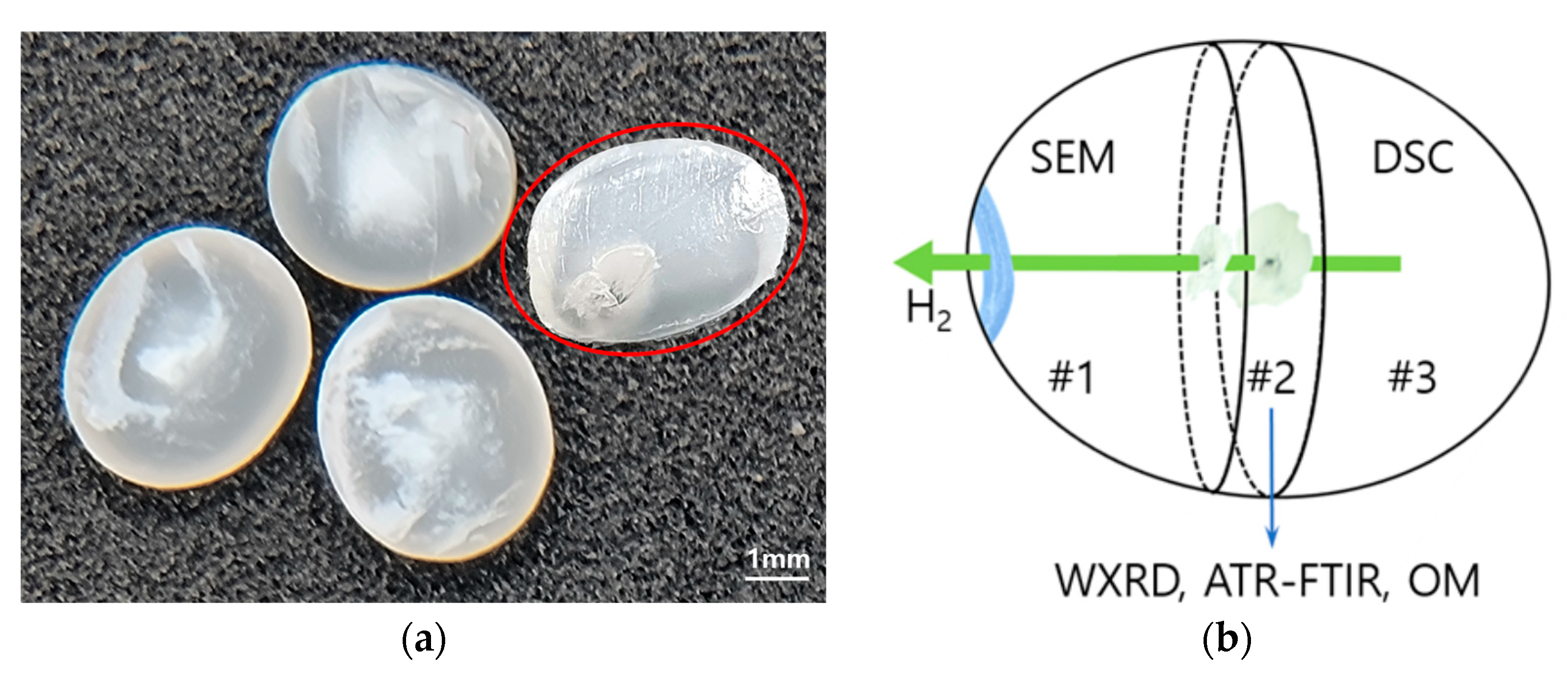
2.1. HDPE Preparation
2.2. Hydrogen Pressure Treatment
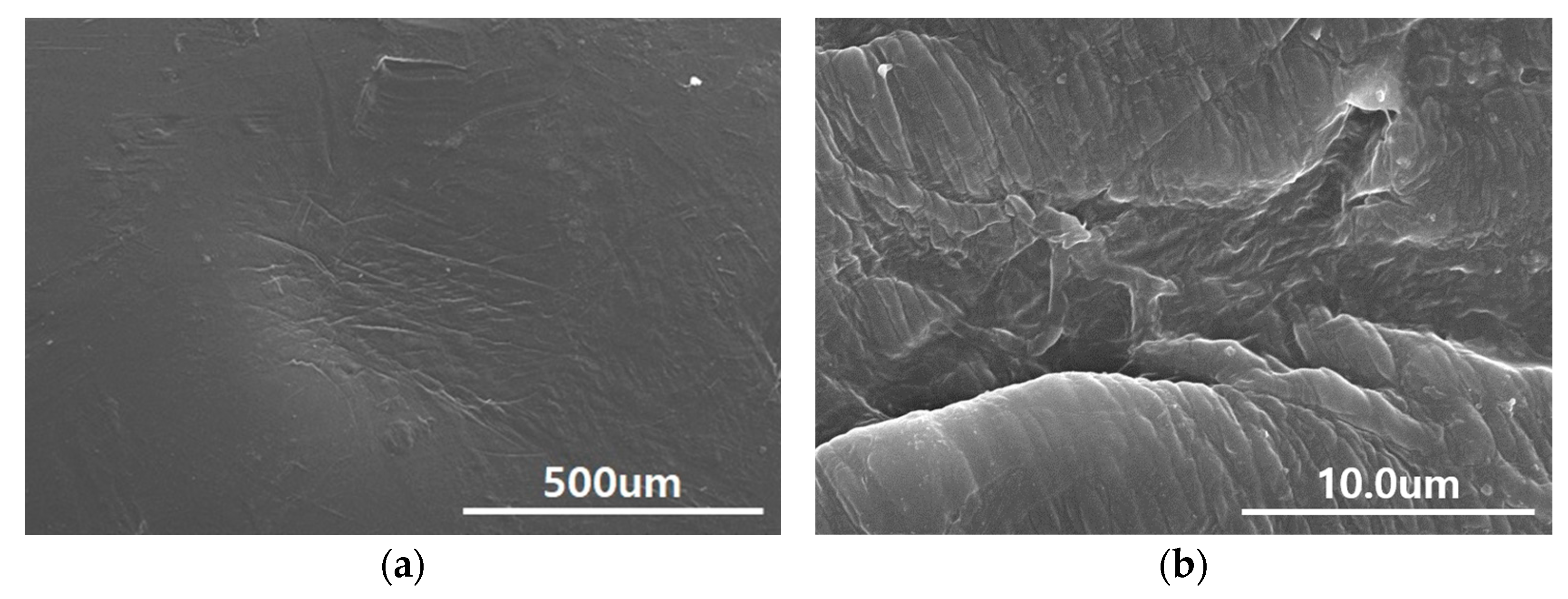
2.3. Microscopy Analysis on Surface Morphology
2.4. X-ray Diffraction (XRD)
2.5. Differential Scanning Calorimetry (DSC)
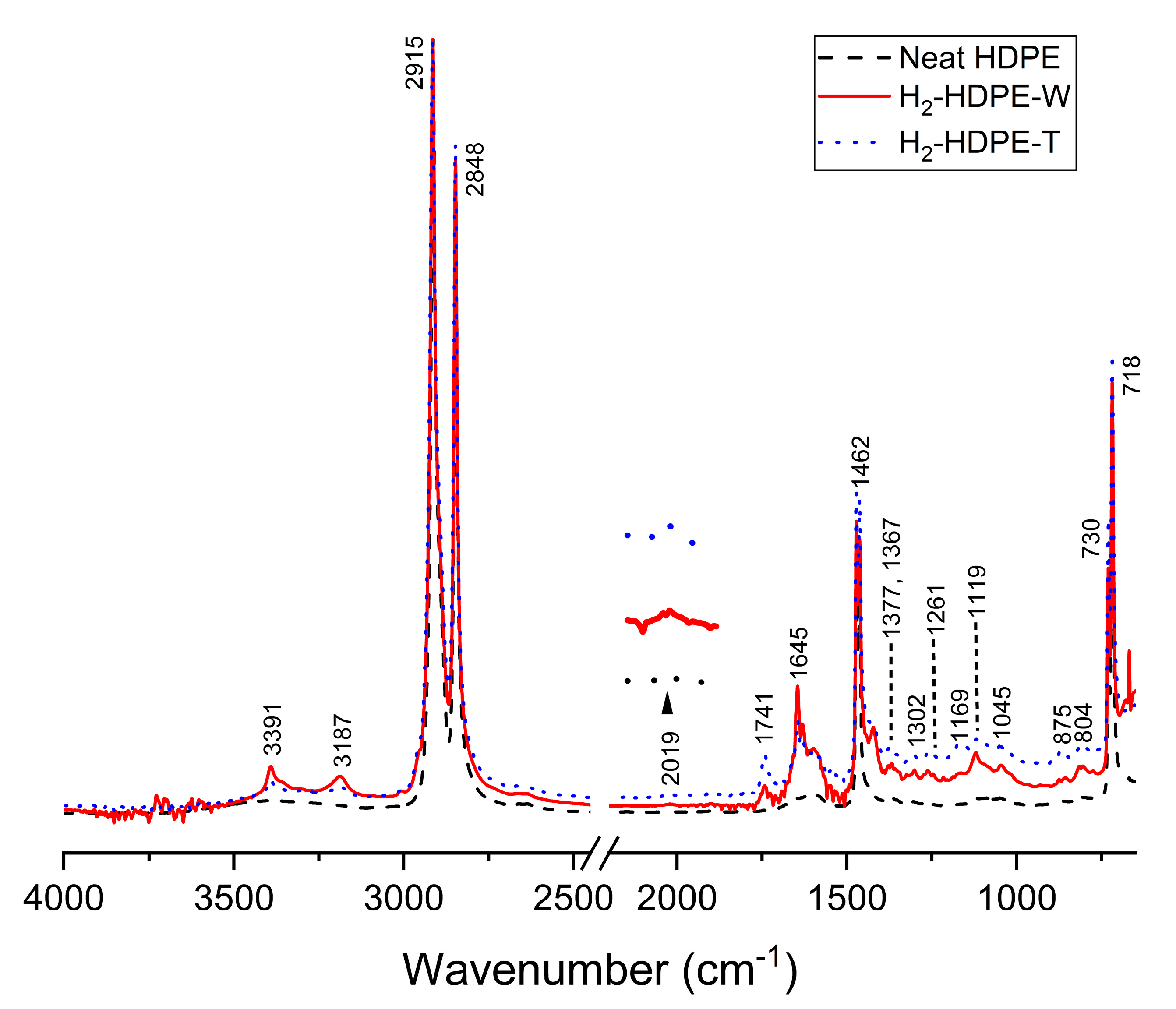
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Microscopy of Surface Morphology
3.2. XRD
3.3. DSC
3.4. ATR-FTIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrog. Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrog. Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Berro Ramirez, J.P.; Halm, D.; Grandidier, J.-C.; Villalonga, S.; Nony, F. 700 bar type iv high pressure hydrogen storage vessel burst—Simulation and experimental validation. Int. J. Hydrog. Energy 2015, 40, 13183–13192. [Google Scholar] [CrossRef]
- Melnichuk, M.; Gardavaud, Q.; Thiébaud, F.; Perreux, D. Temperature effect in cavitation risk assessments of polymers for hydrogen systems. Int. J. Hydrog. Energy 2020, 45, 23020–23026. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, M.; Pan, B. Simulation and burst validation of 70 mpa type iv hydrogen storage vessel with dome reinforcement. Int. J. Hydrog. Energy 2021, 46, 23779–23794. [Google Scholar] [CrossRef]
- Su, Y.; Lv, H.; Zhou, W.; Zhang, C. Review of the hydrogen permeability of the liner material of type iv on-board hydrogen storage tank. World Electr. Veh. J. 2021, 12, 130. [Google Scholar] [CrossRef]
- Yersak, T.A.; Baker, D.R.; Yanagisawa, Y.; Slavik, S.; Immel, R.; Mack-Gardner, A.; Herrmann, M.; Cai, M. Predictive model for depressurization-induced blistering of type iv tank liners for hydrogen storage. Int. J. Hydrog. Energy 2017, 42, 28910–28917. [Google Scholar] [CrossRef]
- Ono, H.; Fujiwara, H.; Onoue, K.; Nishimura, S. Influence of repetitions of the high-pressure hydrogen gas exposure on the internal damage quantity of high-density polyethylene evaluated by transmitted light digital image. Int. J. Hydrog. Energy 2019, 44, 23303–23319. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Choi, K.S.; Kuang, W.; Menon, N.; Mills, B.; Soulami, A.; Simmons, K. Damage evolution in polymer due to exposure to high-pressure hydrogen gas. Int. J. Hydrog. Energy 2021, 46, 19001–19022. [Google Scholar] [CrossRef]
- Zheng, Y.; Tan, Y.; Zhou, C.; Chen, G.; Li, J.; Liu, Y.; Liao, B.; Zhang, G. A review on effect of hydrogen on rubber seals used in the high-pressure hydrogen infrastructure. Int. J. Hydrog. Energy 2020, 45, 23721–23738. [Google Scholar] [CrossRef]
- Yuan, K.; Luo, X. A density functional theory study of the structural and electronic properties of pure and h2 doped polyethylene under high pressure and high temperature conditions of earth layers. AIP Adv. 2020, 10, 065326. [Google Scholar] [CrossRef]
- Voyiatzis, E.; Stroeks, A. Atomistic modeling of hydrogen and oxygen solubility in semicrystalline pa-6 and hdpe materials. J. Phys. Chem. B 2022, 126, 6102–6111. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A. Cavitation during tensile deformation of high-density polyethylene. Polymer 2007, 48, 1397–1409. [Google Scholar] [CrossRef]
- Rastogi, S.; Odell, J.A. Stress stabilization of the orthorhombic and hexagonal phases of uhm pe gel-spun fibres. Polymer 1993, 34, 1523–1527. [Google Scholar] [CrossRef]
- Pennings, A.J.; Zwijnenburg, A. Longitudinal growth of polymer crystals from flowing solutions. Vi. Melting behavior of continuous fibrillar polyethylene crystals. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 1011–1032. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sajwan, M.; Singh, R. Crystallinity of hdpe pipes by dsc, xrd and ftir spectroscopy—A forensic comparison. Indian J. Criminol. Crim. 2008, 29, 141–148. [Google Scholar]
- Fontana, L.; Vinh, D.Q.; Santoro, M.; Scandolo, S.; Gorelli, F.A.; Bini, R.; Hanfland, M. High-pressure crystalline polyethylene studied by x-ray diffraction and ab initio simulations. Phys. Rev. B 2007, 75, 174112. [Google Scholar] [CrossRef]
- Yamamoto, T.; Miyaji, H.; Asai, K. Structure and properties of high pressure phase of polyethylene. Jpn. J. Appl. Phys. 1977, 16, 1891. [Google Scholar] [CrossRef]
- Tsubakihara, S.; Nakamura, A.; Yasuniwa, M. Hexagonal phase of polyethylene fibers under high pressure. Polym. J. 1991, 23, 1317–1324. [Google Scholar] [CrossRef]
- Uehara, H.; Kanamoto, T.; Kawaguchi, A.; Murakami, S. Real-time X-ray diffraction study on two-stage drawing of ultra-high molecular weight polyethylene reactor powder above the static melting temperature. Macromolecules 1996, 29, 1540–1547. [Google Scholar] [CrossRef]
- Pae, K.D.; Newman, B.A.; Sham, T.P. High pressure x-ray studies of polymers. J. Mater. Sci. 1977, 12, 1793–1797. [Google Scholar] [CrossRef]
- Bassett, D.C.; Block, S.; Piermarini, G.J. A high-pressure phase of polyethylene and chain-extended growth. J. Appl. Phys. 2003, 45, 4146–4150. [Google Scholar] [CrossRef]
- Tsubakihara, S.; Nakamura, A.; Yasuniwa, M. Melting and crystallization of ultra-high molecular weight polyethylene with appearance of hexagonal phase i. Melting processes of fibers under constrained state. Polym. J. 1996, 28, 489–495. [Google Scholar] [CrossRef]
- Hindeleh, A.M.; Johnson, D.J. The resolution of multipeak data in fibre science. J. Phys. D Appl. Phys. 1971, 4, 259. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified scherrer equation to estimate more accurately nano-crystallite size using xrd. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Loomis, J.; Ghasemi, H.; Huang, X.; Thoppey, N.; Wang, J.; Tong, J.K.; Xu, Y.; Li, X.; Lin, C.-T.; Chen, G. Continuous fabrication platform for highly aligned polymer films. Technology 2014, 2, 189–199. [Google Scholar] [CrossRef]
- Wunderlich, B.; Czornyj, G. A study of equilibrium melting of polyethylene. Macromolecules 1977, 10, 906–913. [Google Scholar] [CrossRef]
- Weeks, J.J. Melting temperature and change of lamellar thickness with time for bulk polyethylene. J. Res. Natl. Bur. Stand. A Phys. Chem. 1963, 67, 441–451. [Google Scholar] [CrossRef]
- Kuwabara, K.; Horii, F. Solid-state 13c nmr analyses of the orthorhombic-to-hexagonal phase transition for constrained ultradrawn polyethylene fibers. Macromolecules 1999, 32, 5600–5605. [Google Scholar] [CrossRef]
- Roiron, C.; Lainé, E.; Grandidier, J.-C.; Garois, N.; Vix-Guterl, C. A review of the mechanical and physical properties of polyethylene fibers. Textiles 2021, 1, 86–151. [Google Scholar] [CrossRef]
- Rein, D.M.; Khalfin, R.L.; Cohen, Y. Analysis of melting transitions in extended-chain polymer crystals. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 2238–2244. [Google Scholar] [CrossRef]
- Hikosaka, M.; Tsukijima, K.; Rastogi, S.; Keller, A. Equilibrium triple point pressure and pressure-temperature phase diagram of polyethylene. Polymer 1992, 33, 2502–2507. [Google Scholar] [CrossRef]
- Fontana, L.; Santoro, M.; Bini, R.; Vinh, D.Q.; Scandolo, S. High-pressure vibrational properties of polyethylene. J. Chem. Phys. 2010, 133, 204502. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Dias, M.L.; Mano, E.B. Orthorhombic-hexagonal phase transition in high density polyethylene crystals. Int. J. Polym. Mater. 2000, 45, 69–78. [Google Scholar] [CrossRef]
- Bassett, D.C.; Khalifa, B.A.; Turner, B. Chain-extended crystallization of polyethylene. Nat. Phys. Sci. 1972, 239, 106–108. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Liu, C.; Yang, J.; Li, L. Understanding structure-mechanics relationship of high density polyethylene based on stress induced lattice distortion. Polymer 2019, 160, 170–180. [Google Scholar] [CrossRef]
- Seto, T.; Hara, T.; Tanaka, K. Phase transformation and deformation processes in oriented polyethylene. Jpn. J. Appl. Phys. 1968, 7, 31. [Google Scholar] [CrossRef]
- Russell, K.E.; Hunter, B.K.; Heyding, R.D. Monoclinic polyethylene revisited. Polymer 1997, 38, 1409–1414. [Google Scholar] [CrossRef]
- Keller, A.; Ungar, G. Radiation effects and crystallinity in polyethylene. Radiat. Phys. Chem. (1977) 1983, 22, 155–181. [Google Scholar] [CrossRef]
- Da Silva, D.J.; Wiebeck, H. Atr-ftir spectroscopy combined with chemometric methods for the classification of polyethylene residues containing different contaminants. J. Polym. Environ. 2022, 30, 3031–3044. [Google Scholar] [CrossRef]
- Motawie, M.; Hanafi, S.A.; Elmelawy, M.S.; Ahmed, S.M.; Mansour, N.A.; Darwish, M.S.A.; Abulyazied, D.E. Wax co-cracking synergism of high density polyethylene to alternative fuels. Egypt. J. Pet. 2015, 24, 353–361. [Google Scholar] [CrossRef]
- Pagés, P. Characterization of Polymer Materials Using Ft-Ir and Dsc Techniques. Universiadae da Coruna, Presented at 01 01 2005. Available online: https://core.ac.uk/works/34077741 (accessed on 25 June 2023).
- Charles, J.; Ramkumaar, G.R. Qualitative analysis of high density polyethylene using ftir spectroscopy. Asian J. Chem. 2009, 21, 4477–4484. [Google Scholar]
- Alsabbagh, A.; Saleem, R.A.; Almasri, R.; Aljarrah, S.; Awad, S. Effects of gamma irradiation on 3d-printed polylactic acid (pla) and high-density polyethylene (hdpe). Polym. Bull. 2020, 78, 4931–4945. [Google Scholar] [CrossRef]
- Vasylieva, A.; Doroshenko, I.; Vaskivskyi, Y.; Chernolevska, Y.; Pogorelov, V. Ftir study of condensed water structure. J. Mol. Struct. 2018, 1167, 232–238. [Google Scholar] [CrossRef]
- Smith, B. The infrared spectroscopy of alkenes. Spectroscopy 2016, 31, 28–34. [Google Scholar]
- Yang, R.; Liu, Y.; Yu, J.; Wang, K. Thermal oxidation products and kinetics of polyethylene composites. Polym. Degrad. Stab. 2006, 91, 1651–1657. [Google Scholar] [CrossRef]
- Winter, A. How to Identify Carbonyls, Alkenes, Alkynes, and Aromatics in the ir Spectrum. Dummies. 2020. Available online: https://www.dummies.com/education/science/chemistry/how-to-identify-carbonyls-alkenes-alkynes-and-aromatics-inthe-ir-spectrum/ (accessed on 22 March 2021).
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by ftir. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Yang, R.; Li, Y.; Yu, J. Photo-stabilization of linear low density polyethylene by inorganic nano-particles. Polym. Degrad. Stab. 2005, 88, 168–174. [Google Scholar] [CrossRef]
- Sarker, M.; Rashid, M.M.; Molla, M. Abundant high-density polyethylene (hdpe-2) turns into fuel by using of hzsm-5 catalyst. J. Fundam. Renew. Energy Appl. 2011, 1, 1–12. [Google Scholar] [CrossRef]
- Pischedda, V.; Dawe, A.M.; Lowther, J.E.; Hearne, G.R. Study of the high pt phase diagram of crystalline polyethylene. Found. Crystallogr. 2005, 61, C461–C462. [Google Scholar] [CrossRef]
- Mallapragada, S.K.; Narasimhan, B. Infrared spectroscopy in analysis of polymer crystallinity. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Zerbi, G.; Gallino, G.; Del Fanti, N.; Baini, L. Structural depth profiling in polyethylene films by multiple internal reflection infra-red spectroscopy. Polymer 1989, 30, 2324–2327. [Google Scholar] [CrossRef]



| XRD | %Xc | Lamella Thickness (Å) | d-Spacing (Å) | I(200)/I(110) | ||
|---|---|---|---|---|---|---|
| L110 | L200 | (110) | (200) | |||
| Neat HDPE | 65 | 187 | 167 | 4.15 | 3.74 | 0.31 |
| H2-HDPE-W | 57 | 248 | 208 | 4.13 | 3.72 | 0.53 |
| H2-HDPE-T | 47 | 263 | 221 | 4.15 | 3.73 | 0.37 |
| DSC | Hm (J/g) | %Xc | Melting Temperature (°C) | Thickness of Lamellae (Å) |
|---|---|---|---|---|
| Neat HDPE | 226.49 | 77 | 132 | 311 |
| H2-HDPE | 217.04 | 74 | 131 | 311 |
| Assigned Wavenumber (cm−1) | Functional Group and Vibration Mode | New/Existing | Reference |
|---|---|---|---|
| 718, 730 | Split -CH2- (rocking) | Existing | [41] |
| 804 | -CH3 (bending) | New | [41] |
| 875 | -CH=CH2 (C-H bending) | New | [42] |
| 1045 | -CHOH- (C-O stretching) | Existing | [43] |
| 1119 | -COH(CH3)- (C-O stretching) | Existing | [43] |
| 1169 | Symmetric CCH bending | Existing | [44] |
| 1261 | Asymmetric CCH bending | Existing | [44] |
| 1302 | -CH3 (twisting and wagging) | New | [41] |
| 1367 | -CH3 (wagging) | Existing | [41] |
| 1377 | -CH3 (Symmetric C-H bending) | New | [42] |
| 1462, 1472 | -CH2- (bending) | Existing | [45] |
| 1605 | O-H (bending) | Existing | [46] |
| 1645 | C=C (stretching) | New | [47] |
| 1741 | C=O (ester) | New | [48] |
| 2019 | C≡C (stretching) | New | [49] |
| 2848 | -CH2- (Symmetric C-H stretching) | Existing | [41] |
| 2915 | -CH2- (Asymmetric C-H stretching) | Existing | [41] |
| 3187 | C=C-H (C-H stretching) | New | [47] |
| 3351 | O-H (stretching) | Existing | [46] |
| 3391 | C≡C-H (C-H stretching) | New | [49] |
| Chemical change | Molecular | Wavenumber (cm−1) |
|---|---|---|
| Hydrogenation |  | 875, 1645, 3187 |
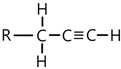 | 2019, 3391 | |
 | 804, 1302, 1367, 1377 | |
| Crosslinking |  | 1169, 1261 |
| Oxidation |  | 1741 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Lee, C.H. Hydrogenation of High-Density Polyethylene during Decompression of Pressurized Hydrogen at 90 MPa: A Molecular Perspective. Polymers 2023, 15, 2880. https://doi.org/10.3390/polym15132880
Kim M, Lee CH. Hydrogenation of High-Density Polyethylene during Decompression of Pressurized Hydrogen at 90 MPa: A Molecular Perspective. Polymers. 2023; 15(13):2880. https://doi.org/10.3390/polym15132880
Chicago/Turabian StyleKim, Mina, and Chang Hoon Lee. 2023. "Hydrogenation of High-Density Polyethylene during Decompression of Pressurized Hydrogen at 90 MPa: A Molecular Perspective" Polymers 15, no. 13: 2880. https://doi.org/10.3390/polym15132880
APA StyleKim, M., & Lee, C. H. (2023). Hydrogenation of High-Density Polyethylene during Decompression of Pressurized Hydrogen at 90 MPa: A Molecular Perspective. Polymers, 15(13), 2880. https://doi.org/10.3390/polym15132880





