Visco-Elastic and Thermal Properties of Microbiologically Synthesized Polyhydroxyalkanoate Plasticized with Triethyl Citrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plasticized Systems
2.3. Characterization Methods
2.3.1. Molecular Weight (Mw)
2.3.2. Fourier Transform Infrared Spectroscopy (FT−IR)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Oscillatory Shear Rheology
2.3.6. Tensile Properties
2.3.7. Dynamic Mechanical Thermal Analysis (DMTA)
3. Results
3.1. Molecular Weight
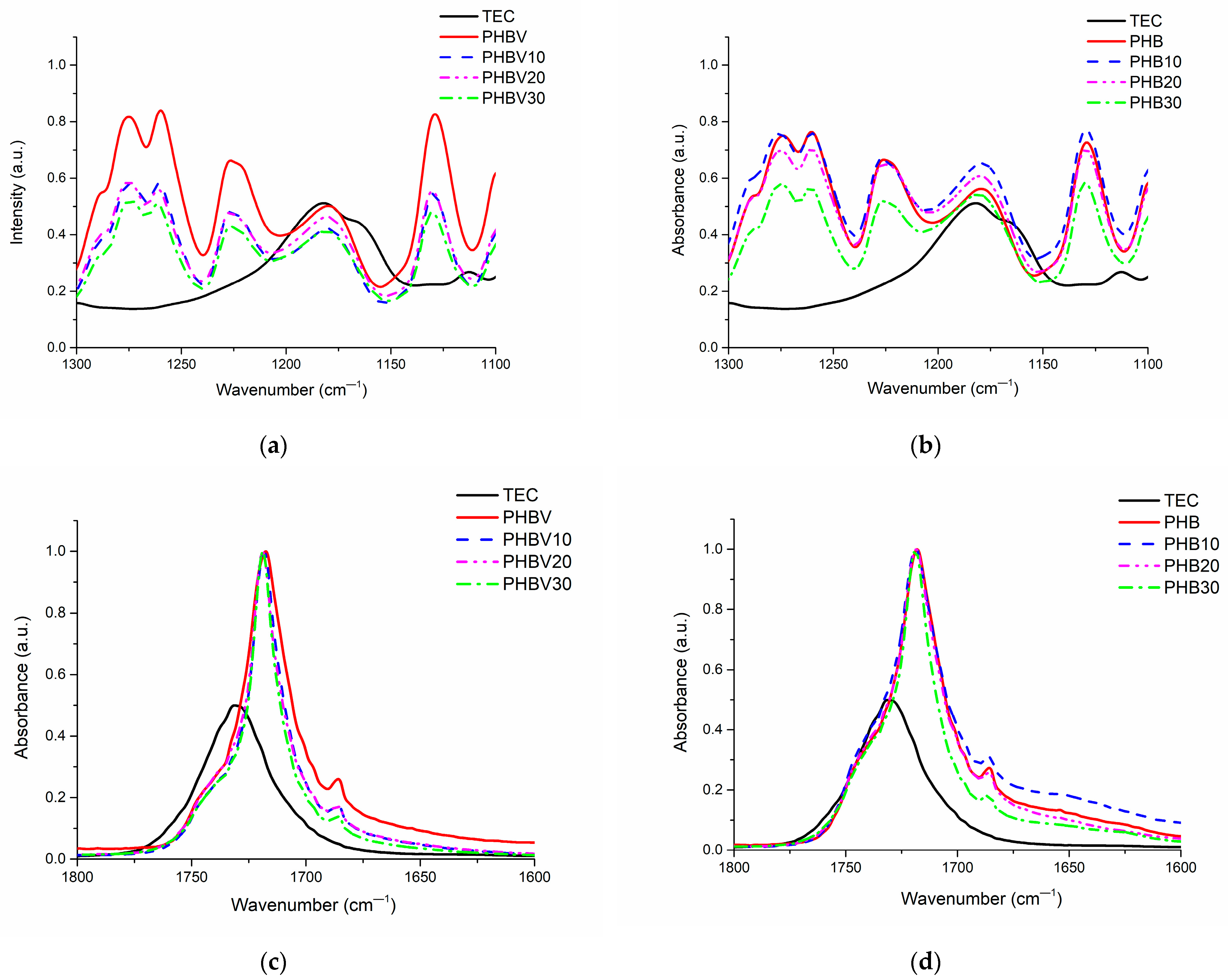
3.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.3. Thermal Gravimetric Analysis (TGA)
3.4. Differential Scanning Calorimetry (DSC)
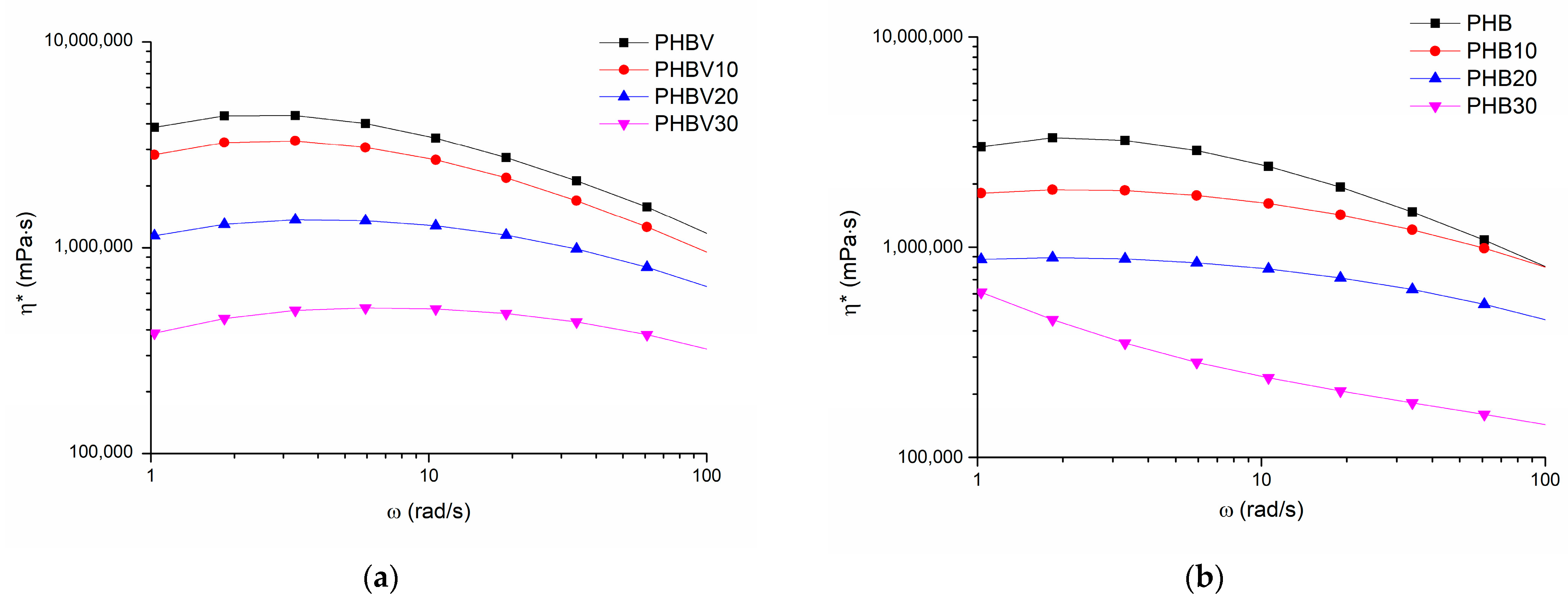
3.5. Oscillatory Shear Rheology
3.6. Tensile Properties
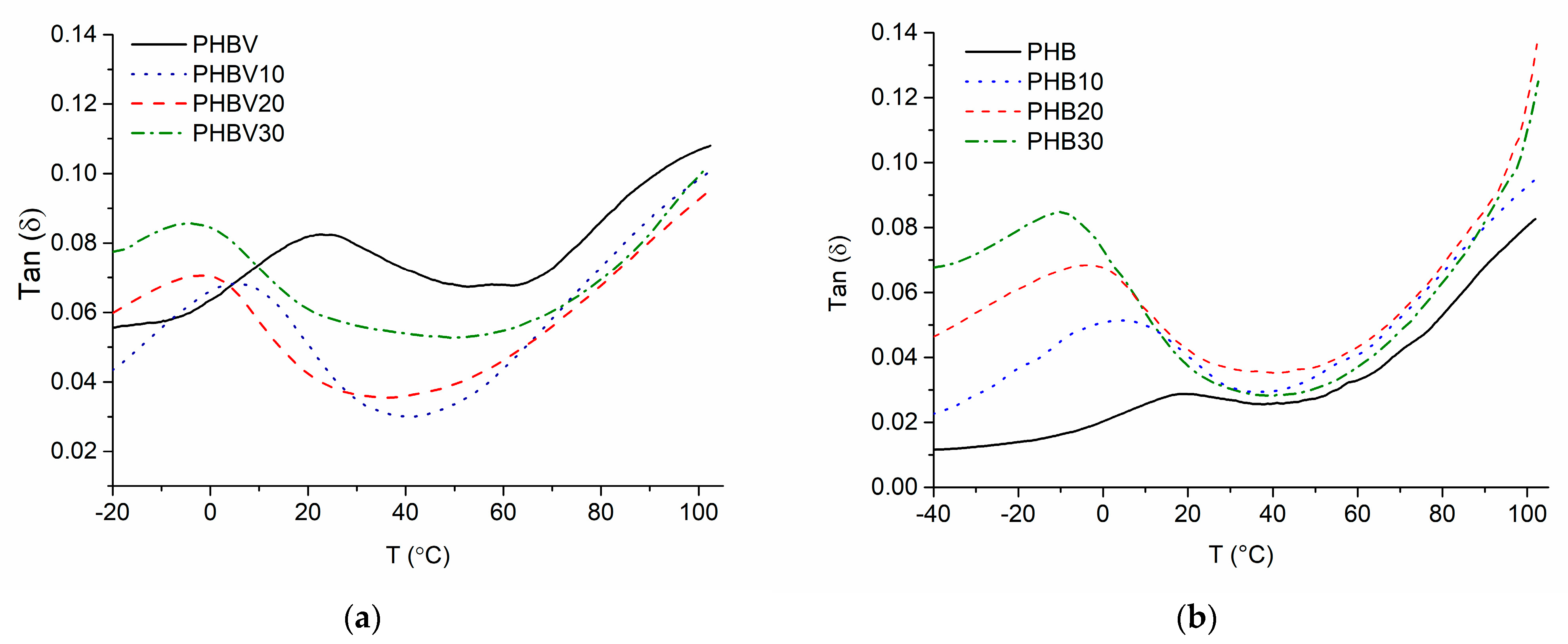
3.7. Dynamic Mechanical Analysis (DMA)
4. Conclusions
- (1)
- Considerable thermooxidative degradation in the air of the investigated plasticized systems does not occur until 240–260 °C, while the minimum onset thermal degradation temperature is 264 °C;
- (2)
- The rate of thermooxidative degradation of the plasticized systems is decreased to a certain extent due to the contribution of TEC in the building of the gas-impermeable char layer;
- (3)
- Increased shear forces cause decrement of melt viscosity as well as storage and loss modules of both PHB and especially PHB- based systems due to lower activation energy of the latter and weakened interaction between the polymer chains because of plasticization;
- (4)
- The melting range of the plasticized systems is considerably decreased (by ca 10 °C at the maximum peak value), thus relieving the processability of the investigated systems;
- (5)
- Ultimate elongation εB values of the investigated plasticized systems increase on average 2.5 times by increasing TEC content, reaching values as high as 9% (for PHBV-based systems);
- (6)
- Modulus of elasticity E as well as tensile strength σB values experience certain decrements, especially for PHBV-based systems above glass transition temperature Tg.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eurostat. EU Recycled 41% of Plastic Packaging Waste in 2019. 2021. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20211027-2 (accessed on 15 May 2023).
- van der Harst, E.; Potting, J. A critical comparison of ten disposable cup LCAs. Environ. Impact Assess. Rev. 2013, 43, 86–96. [Google Scholar] [CrossRef]
- Utriainen, M.; Application, F.; Data, P.; Oy, E. (12) Patent Application Publication (10) Pub. No.: US 2009/0312954 A1. D Pat. Appl. Publ. 2009, 1, 1–6. Available online: https://patentimages.storage.googleapis.com/9d/30/f7/7b725f3f41be3c/US20090082491A1.pdf (accessed on 28 March 2023).
- Aramvash, A.; Moazzeni Zavareh, F.; Gholami Banadkuki, N. Comparison of different solvents for extraction of polyhydroxybutyrate from Cupriavidus necator. Eng. Life Sci. 2018, 18, 20–28. [Google Scholar] [CrossRef]
- Myshkina, V.L.; Nikolaeva, D.A.; Makhina, T.K.; Bonartsev, A.P.; Bonartseva, G.A. Effect of growth conditions on the molecular weight of poly-3- hydroxybutyrate produced by Azotobacter chroococcum 7B. Appl. Biochem. Microbiol. 2008, 44, 482–486. [Google Scholar] [CrossRef]
- McAdam, B.; Fournet, B.M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Prakash Pandey, J.; Kumar Gaur, V.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef]
- Aragosa, A.; Specchia, V.; Frigione, M. Isolation of two bacterial species from argan soil in morocco associated with polyhydroxybutyrate (Phb) accumulation: Current potential and future prospects for the bio-based polymer production. Polymers 2021, 13, 1870. [Google Scholar] [CrossRef]
- Nova and Institute. Global Production Capacities. 2021. Available online: https://primebiopol.com/aumenta-produccion-bioplasticos/?lang=en (accessed on 16 May 2023).
- Nova-Institute. For the First Time: Growth Rate for Bio-based Polymers with 8% CAGR Far above Overall Polymer Market Growth. 2021. Available online: https://renewable-carbon.eu/news/for-the-first-time-growth-rate-for-bio-based-polymers-with-8-cagr-far-above-overall-polymer-market-growth/ (accessed on 16 May 2023).
- Requena, R.; Jiménez, A.; Vargas, M.; Chiralt, A. Effect of plasticizers on thermal and physical properties of compression-moulded poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] films. Polym. Test. 2016, 56, 45–53. [Google Scholar] [CrossRef]
- Stanley, A.; Murthy, P.S.K.; Vijayendra, S.V.N. Characterization of Polyhydroxyalkanoate Produced by Halomonas venusta KT832796. J. Polym. Environ. 2020, 28, 973–983. [Google Scholar] [CrossRef]
- Quagliano, J.C.; Amarilla, F.; Fernandes, E.G.; Mata, D.; Miyazaki, S.S. “Effect of simple and complex carbon sources, low temperature culture and complex carbon feeding policies on poly-3-hydroxybutyric acid (PHB) content and molecular weight (Mw) from Azotobacter chroococcum 6B. World J. Microbiol. Biotechnol. 2001, 17, 9–14. [Google Scholar] [CrossRef]
- Umemura, R.T.; Felisberti, M.I. Plasticization of poly(3-hydroxybutyrate) with triethyl citrate: Thermal and mechanical properties, morphology, and kinetics of crystallization. J. Appl. Polym. Sci. 2021, 138, 49990. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W.; Wang, X.; Chen, X.; Chen, G.-Q. Processability Modifications of Poly(3-hydroxybutyrate) by Plasticizing, Blending, and Stabilizing. J. Appl. Polym. Sci. 2008, 107, 166–173. [Google Scholar] [CrossRef]
- Nosal, H.; Moser, K.; Warzała, M.; Holzer, A.; Stańczyk, D.; Sabura, E. Selected Fatty Acids Esters as Potential PHB-V Bioplasticizers: Effect on Mechanical Properties of the Polymer. J. Polym. Environ. 2020, 29, 38–53. [Google Scholar] [CrossRef]
- Mangeon, C.; Michely, L.; Rios De Anda, A.; Thevenieau, F.; Renard, E.; Langlois, V. Natural Terpenes Used as Plasticizers for Poly(3-hydroxybutyrate). ACS Sustain. Chem. Eng. 2018, 6, 16160–16168. [Google Scholar] [CrossRef]
- Scalioni, L.V.; Gutiérrez, M.C.; Felisberti, M.I. Green composites of poly(3-hydroxybutyrate) and curaua fibers: Morphology and physical, thermal, and mechanical properties. J. Appl. Polym. Sci. 2017, 134, 1–13. [Google Scholar] [CrossRef]
- Râpă, M.; Darie-Nita, R.; Grosu, E.; Popa, E.; Trifoi, A.; Pap, T.; Vasile, C. Effect of plasticizers on melt processability and properties of PHB. J. Optoelectron. Adv. Mater. 2015, 17, 1778–1784. [Google Scholar]
- Garcia-Garcia, D.; Ferri, J.M.; Montanes, N.; Lopez-Martinez, J.; Balart, R. Plasticization effects of epoxidized vegetable oils on mechanical properties of poly(3-hydroxybutyrate). Polym. Int. 2016, 65, 1157–1164. Available online: http://hdl.handle.net/10251/82871 (accessed on 17 May 2023). [CrossRef]
- Barbosa, J.L.; Perin, G.B.; Felisberti, M.I. Plasticization of Poly(3-hydroxybutyrate- co-3-hydroxyvalerate) with an Oligomeric Polyester: Miscibility and Effect of the Microstructure and Plasticizer Distribution on Thermal and Mechanical Properties. ACS Omega 2021, 6, 3278–3290. [Google Scholar] [CrossRef]
- Haas, R.; Jin, B.; Zepf, F.T. Production of Poly(3-hydroxybutyrate) from Waste Potato Starch. Biosci. Biotechnol. Biochem. 2008, 72, 253–256. [Google Scholar] [CrossRef]
- Yang, Y.H.; Brigham, C.; Willis, L.; Rha, C.K.; Sinskey, A. Improved detergent-based recovery of polyhydroxyalkanoates (PHAs). Biotechnol. Lett. 2011, 33, 937–942. [Google Scholar] [CrossRef]
- Vandi, L.J.; Chan, C.M.; Werker, A.; Richardson, D.; Laycock, B.; Pratt, S. Extrusion of wood fibre reinforced poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV) biocomposites: Statistical analysis of the effect of processing conditions on mechanical performance. Polym. Degrad. Stab. 2018, 159, 1–14. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In vivo and Post-synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Sato, S.; Ushimaru, K.; Saika, A.; Fukuoka, T.; Ohshiman, K.; Morita, T. Mechanical properties of cold-drawn films of ultrahigh-molecular-weight poly(3-hydroxybutyrate-co-3-hydroxyvalerate) produced by Haloferax mediterranei. Polym. J. 2020, 52, 1299–1306. [Google Scholar] [CrossRef]
- Chen, Y.; Chou, I.N.; Tsai, Y.H.; Wu, H.S. Thermal degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate- co-3-hydroxyvalerate) in drying treatment. J. Appl. Polym. Sci. 2013, 130, 3659–3667. [Google Scholar] [CrossRef]
- Abbasi, M.; Pokhrel, D.; Coats, E.R.; Guho, N.M.; McDonald, A.G. Effect of 3-Hydroxyvalerate Content on Thermal, Mechanical, and Rheological Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Biopolymers Produced from Fermented Dairy Manure. Polymers 2022, 14, 4140. [Google Scholar] [CrossRef]
- Ramezani, M.; Amoozegar, M.A.; Ventosa, A. Screening and comparative assay of poly-hydroxyalkanoates produced by bacteria isolated from the Gavkhooni Wetland in Iran and evaluation of poly-β-hydroxybutyrate production by halotolerant bacterium Oceanimonas sp. GK1. Ann. Microbiol. 2015, 65, 517–526. [Google Scholar] [CrossRef]
- Chotchindakun, K.; Pathom-Aree, W.; Dumri, K.; Ruangsuriya, J.; Pumas, C.; Pekkoh, J. Low Crystallinity of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Bioproduction by Hot Spring Cyanobacterium Cyanosarcina sp. AARL T020. Plants 2021, 10, 503. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; de Soares, F.F.N. Glycerol and triethyl citrate plasticizer effects on molecular, thermal, mechanical, and barrier properties of cellulose acetate films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Sánchez, K.D.T.; Allen, N.S.; Liauw, C.M.; Johnson, B. Effects of type of polymerization catalyst system on the degradation of polyethylenes in the melt state. Part 1: Unstabilized polyethylenes (including metallocene types). J. Vinyl Addit. Technol. 2011, 17, 8–39. [Google Scholar] [CrossRef]
- Xiang, H.; Wen, X.; Miu, X.; Li, Y.; Zhou, Z.; Zhu, M. Thermal depolymerization mechanisms of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Prog. Nat. Sci. Mater. Int. 2016, 26, 58–64. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Sajkiewicz, P.; La Pietra, P.; Gradys, A. Irregularly shaped DSC exotherms in the analysis of polymer crystallization. Polym. Bull. 2006, 57, 713–721. [Google Scholar] [CrossRef]
- Briassoulis, D.; Tserotas, P.; Athanasoulia, I.G. Alternative optimization routes for improving the performance of poly(3-hydroxybutyrate) (PHB) based plastics. J. Clean. Prod. 2021, 318, 128555. [Google Scholar] [CrossRef]
- Hsiao, C.S.B.S.; Zuo, F.; Mao, Y. Handbook of Polymer Crystallization; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 15. [Google Scholar]
- Sauer, B.B.; Kampert, W.G.; Blanchard, N.E.; Threefoot, S.A.; Hsiao, B.S. Temperature modulated DSC studies of melting and recrystallization in polymers exhibiting multiple endotherms. Polymer 2000, 41, 1099–1108. [Google Scholar] [CrossRef]
- Jost, V.; Langowski, H.C. Effect of different plasticisers on the mechanical and barrier properties of extruded cast PHBV films. Eur. Polym. J. 2015, 68, 302–312. [Google Scholar] [CrossRef]
- Scandola, M.; Ceccorulli, G.; Doi, Y. Viscoelastic relaxations and thermal properties of bacterial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Int. J. Biol. Macromol. 1990, 12, 112–117. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Mansour, A.A.; Abdou, N.Y. Molecular dynamics of amorphous/crystalline polymer blends studied by broadband dielectric spectroscopy. Eur. Polym. J. 2007, 43, 1892–1904. [Google Scholar] [CrossRef]








| PHAs | TEC(wt. Parts) | Plasticizer | Tm (°C) | X (%) | εB (%) | Reference |
|---|---|---|---|---|---|---|
| PHB | 0 | Epoxidized linseed oil (ELO) | 175 | 52 | 9.7 | [20] |
| 0.05 | 173 | 47 | 12.7 | |||
| 0.1 | 171 | 46 | 13.6 | |||
| 0.05 | Epoxidized soybean oil (ESBO) | 173 | 47 | 9.2 | ||
| 0.1 | 172 | 48 | 8.9 | |||
| PHB | 0 | Triethyl citrate (TEC) | 180 | 81 | 5.8 ± 0.6 | [14] |
| 0.1 | 173 | 71 | 5.6 ± 0.4 | |||
| 0.2 | 171 | 62 | 7.4 ± 0.9 | |||
| 0.3 | 162 | 53 | 6.9 ± 1.6 | |||
| PHB | 0 | Dioctyl (o-)phthalate (DOS) | 169 | 56 * | 2.5 ± 0.5 | [15] |
| 0.25 | 164 | 54 * | 3.9 ± 0.3 | |||
| 0.3 | 163 | 57 * | 4.3 ± 0.6 | |||
| 0.35 | 165 | 50 * | 5.4 ± 0.9 | |||
| 0.4 | 165 | 57 * | 5.2 ± 0.6 | |||
| 0.5 | 165 | 51 * | - | |||
| 0 | Acetyl tributyl citrate (ATBC) | 169 | 56 * | 2.5 ± 0.5 | ||
| 0.1 | 163 | 61 * | 6.1 ± 0.8 | |||
| 0.2 | 160 | 58 * | 8.5 ± 0.9 | |||
| 0.25 | 158 | 60 * | 8.8 ± 0.9 | |||
| 0.3 | 157 | 59 * | 9.7 ± 0.7 | |||
| PHBV | 0 | Biodegradable oligomeric polyester based on lactic acid, adipic acid, and 1,2-propanediol at a molar ratio of 20:40:40 (PLAP) | 174 | 53 | 8 ± 0.4 | [21] |
| 0.1 | 173 | 55 | 8.2 ± 0.4 | |||
| 0.2 | 170 | 56 | 8.1 ± 0.4 | |||
| 0.3 | 174 | 61 | 6.6 ± 0.5 |
| Code | PHBV (wt.%) | PHB (wt.%) | TEC (wt.%) |
|---|---|---|---|
| PHBV | 100 | — | 0 |
| PHBV10 | 90 | — | 10 |
| PHBV20 | 80 | — | 20 |
| PHBV30 | 70 | — | 30 |
| PHB | — | 100 | 0 |
| PHB10 | — | 90 | 10 |
| PHB20 | — | 80 | 20 |
| PHB30 | — | 70 | 30 |
| Sample Code | Mw (kDa) | η (Pa·s) |
|---|---|---|
| PHBV | 540 | 0.69 |
| PHB | 66 | 3.86 |
| Mode of Molecular Vibration | PHB | PHBV | TEC | |||
|---|---|---|---|---|---|---|
| Current Research | Reference | Current Research | Reference | Current Research | Reference | |
| C–C backbone stretching | 978 | — | 977 | 977 [28] | — | — |
| O−C−C stretching | 1043/1054 | — | 1044/1054 | 1054 [28] | 1023 | — |
| O–C–C asymmetric stretching | 1100 | 1000–1300 [29] | 1099 | 1099 [28] | 1096 1113 | 1097 [31] 1114 [31] |
| C–O–C symmetric stretching | 1130 | 1129 | 1129 [28] | — | 1050-1300 [31] | |
| C–O–C asymmetric stretching | 1180 | 1181 | 1179 [28] | 1182 | ||
| C–O symmetric stretching | 1226 | 1226 | 1226 [28] | — | ||
| C–O symmetric stretching of aliphatic esters | 1260 | 1261 | 1261 [28] | — | ||
| C–O symmetric stretching | 1274 | 1274 | 1275 [28] | — | ||
| C–H symmetric bending of methyl (-CH3) groups | 1379 | 1377 [29] | 1379 | 1379 [28] | 1370 | 1373 [31] |
| C–H asymmetric stretching and bending vibrations of methyl (-CH3) and methylene (-CH2-) groups | 1453 | 1452 [29] | 1452 | 1452 [28] | — | — |
| C=O stretching of ester groups | 1718 | 1727 [29] | 1718 | 1720 [28] 1722 [30] | 1730 | 1735 [31] |
| —CH3 symmetric stretching | 2851/2873 | — | 2851/2873 | 2881 [30] | — | — |
| —CH2 symmetric stretching | 2390 | — | 2932 | 2933 [28] 2925/2945 [30] | — | — |
| C−H asymmetric vibration of methyl (-CH3) groups | 2975 | 2927/2969 [29] | 2976 | 2975 [28] | 2982 | 2983 [31] |
| Terminal –OH group | 3434 | 3434 [29] | 3435 | 3434 [28] | 3484 | 3502 [31] |
| Sample Code | Residual Mass at Fixed Temperature, wt.% | Ton, °C | Percent Mass Loss Temperatures, °C | ||||
|---|---|---|---|---|---|---|---|
| 180 °C | 190 °C | 200 °C | T1% | T5% | Tdeg | ||
| PHB | 100 | 100 | 100 | 288 | 227 | 279 | 298 |
| PHB10 | 100 | 99 | 99 | 280 | 202 | 246 | 258 |
| PHB20 | 100 | 99 | 98 | 264 | 185 | 241 | 274 |
| PHB30 | 100 | 99 | 98 | 275 | 181 | 229 | 286 |
| PHBV | 100 | 100 | 100 | 283 | 259 | 276 | 295 |
| PHBV10 | 99 | 99 | 99 | 285 | 208 | 257 | 299 |
| PHBV20 | 99 | 99 | 98 | 279 | 184 | 234 | 283 |
| PHBV30 | 99 | 99 | 98 | 275 | 187 | 228 | 287 |
| TEC | 96 | 95 | 94 | 276 | 120 | 190 | 276 |
| Sample Code | wt. % | 1st Heating Run | ||||||
|---|---|---|---|---|---|---|---|---|
| χ | ΔHm | Tm1 | Tm2 | Tonset | Toffset | ΔT | ||
| (%) | (J/g) | (°C) | (°C) | (°C) | (°C) | (°C) | ||
| PHB | 0 | 53 | 77 | - | 174 | 136 | 183 | 47 |
| PHB10TEC | 10 | 58 | 76 | 165 * | 172 | 133 | 181 | 48 |
| PHB20TEC | 20 | 59 | 69 | 160 * | 169 | 127 | 177 | 50 |
| PHB30TEC | 30 | 60 | 61 | 157 * | 166 | 122 | 173 | 51 |
| PHBV | 0 | 64 | 94 | - | 175/185 * | 135 | 192 | 57 |
| PHBV10%TEC | 10 | 59 | 78 | - | 168 | 118 | 177 | 59 |
| PHBV20%TEC | 20 | 57 | 66 | - | 163 | 114 | 174 | 60 |
| PHBV30%TEC | 30 | 59 | 60 | 151* | 161 | 106 | 168 | 62 |
| Sample Code | wt.% | Cooling Run | |||||
|---|---|---|---|---|---|---|---|
| χ (%) | ΔHm (J/g) | Tm1 (°C) | Tonset (°C) | Toffset (°C) | ΔT (°C) | ||
| PHB | 0 | 45 | 66 | 91 | 67 | 112 | 45 |
| PHB10TEC | 10 | 44 | 58 | 71 | 51 | 100 | 49 |
| PHB20TEC | 20 | 47 | 55 | 75 | 49 | 100 | 51 |
| PHB30TEC | 30 | 45 | 46 | 69 | 43 | 96 | 53 |
| PHBV | 0 | 46 | 68 | 83 | 55 | 108 | 53 |
| PHBV10%TEC | 10 | 47 | 61 | 81 | 52 | 106 | 54 |
| PHBV20%TEC | 20 | 44 | 51 | 75 | 44 | 99 | 55 |
| PHBV30%TEC | 30 | 45 | 46 | 71 | 31 | 98 | 67 |
| Sample Code | wt.% | 2nd Heating Run | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| χcc (%) | ΔHcc (J/g) | Tcc (°C) | χ * (%) | ΔHm (J/g) | Tm1 (°C) | Tm2 (°C) | Tonset (°C) | Toffset (°C) | ΔT (°C) | ||
| PHB | 0 | 3 | 4 | 99 | 54 (51) | 78 | 169 | 173 | 139 | 183 | 44 |
| PHB10TEC | 10 | 2 | 2 | 89 | 59 (57) | 77 | 156 | 167 | 131 | 176 | 45 |
| PHB20TEC | 20 | 3 | 4 | 89 | 59 (56) | 69 | 154 | 166 | 129 | 175 | 46 |
| PHB30TEC | 30 | 4 | 4 | 83 | 62 (58) | 63 | 157 | 165 | 127 | 174 | 47 |
| PHBV | 0 | 5 | 8 | 95 | 61 (56) | 90 | 167 | 172 | 128 | 185 | 57 |
| PHBV10%TEC | 10 | 3 | 4 | 94 | 62 (58) | 81 | 162 | 170 | 118 | 176 | 58 |
| PHBV20%TEC | 20 | 5 | 6 | 91 | 63 (58) | 74 | 154 | 165 | 117 | 175 | 58 |
| PHBV30%TEC | 30 | 4 | 5 | 90 | 62 (58) | 64 | 148 | 162 | 112 | 172 | 60 |
| Sample | Tg, °C | Sample | Tg, °C |
|---|---|---|---|
| PHBV | 22 | PHB | 19 |
| PHBV10 | 5 | PHB10 | 12 |
| PHBV20 | −1 | PHB20 | −5 |
| PHBV30 | −4 | PHB30 | −7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žiganova, M.; Merijs-Meri, R.; Zicāns, J.; Bochkov, I.; Ivanova, T.; Vīgants, A.; Ence, E.; Štrausa, E. Visco-Elastic and Thermal Properties of Microbiologically Synthesized Polyhydroxyalkanoate Plasticized with Triethyl Citrate. Polymers 2023, 15, 2896. https://doi.org/10.3390/polym15132896
Žiganova M, Merijs-Meri R, Zicāns J, Bochkov I, Ivanova T, Vīgants A, Ence E, Štrausa E. Visco-Elastic and Thermal Properties of Microbiologically Synthesized Polyhydroxyalkanoate Plasticized with Triethyl Citrate. Polymers. 2023; 15(13):2896. https://doi.org/10.3390/polym15132896
Chicago/Turabian StyleŽiganova, Madara, Remo Merijs-Meri, Jānis Zicāns, Ivan Bochkov, Tatjana Ivanova, Armands Vīgants, Enno Ence, and Evita Štrausa. 2023. "Visco-Elastic and Thermal Properties of Microbiologically Synthesized Polyhydroxyalkanoate Plasticized with Triethyl Citrate" Polymers 15, no. 13: 2896. https://doi.org/10.3390/polym15132896
APA StyleŽiganova, M., Merijs-Meri, R., Zicāns, J., Bochkov, I., Ivanova, T., Vīgants, A., Ence, E., & Štrausa, E. (2023). Visco-Elastic and Thermal Properties of Microbiologically Synthesized Polyhydroxyalkanoate Plasticized with Triethyl Citrate. Polymers, 15(13), 2896. https://doi.org/10.3390/polym15132896








