Environmental Impacts Assessment in Suspension PVC Production Process Using Computer-Aided Process Engineering
Abstract
1. Introduction
2. Materials and Methods
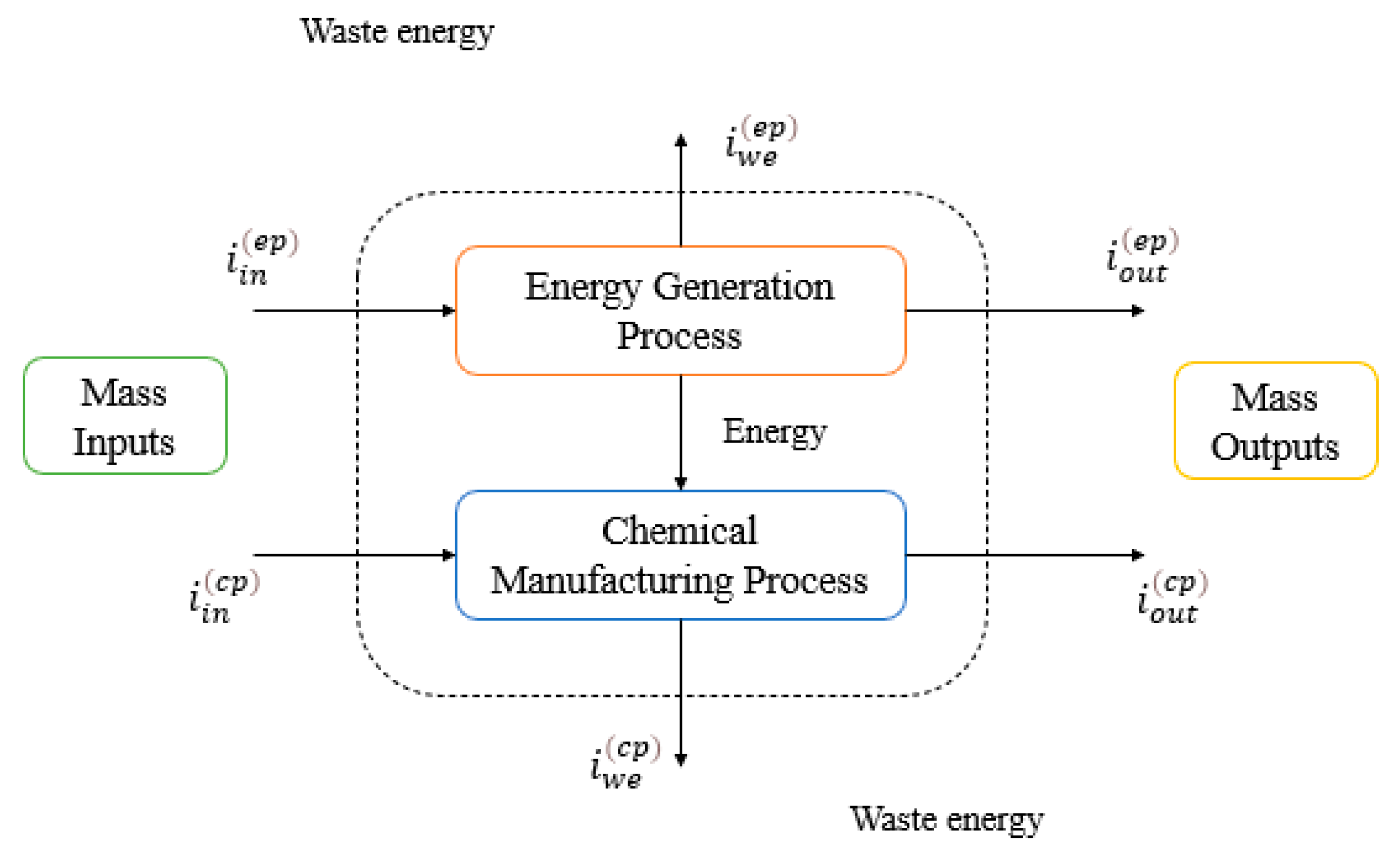
2.1. Environmental Assessment Using WAR Algorithm
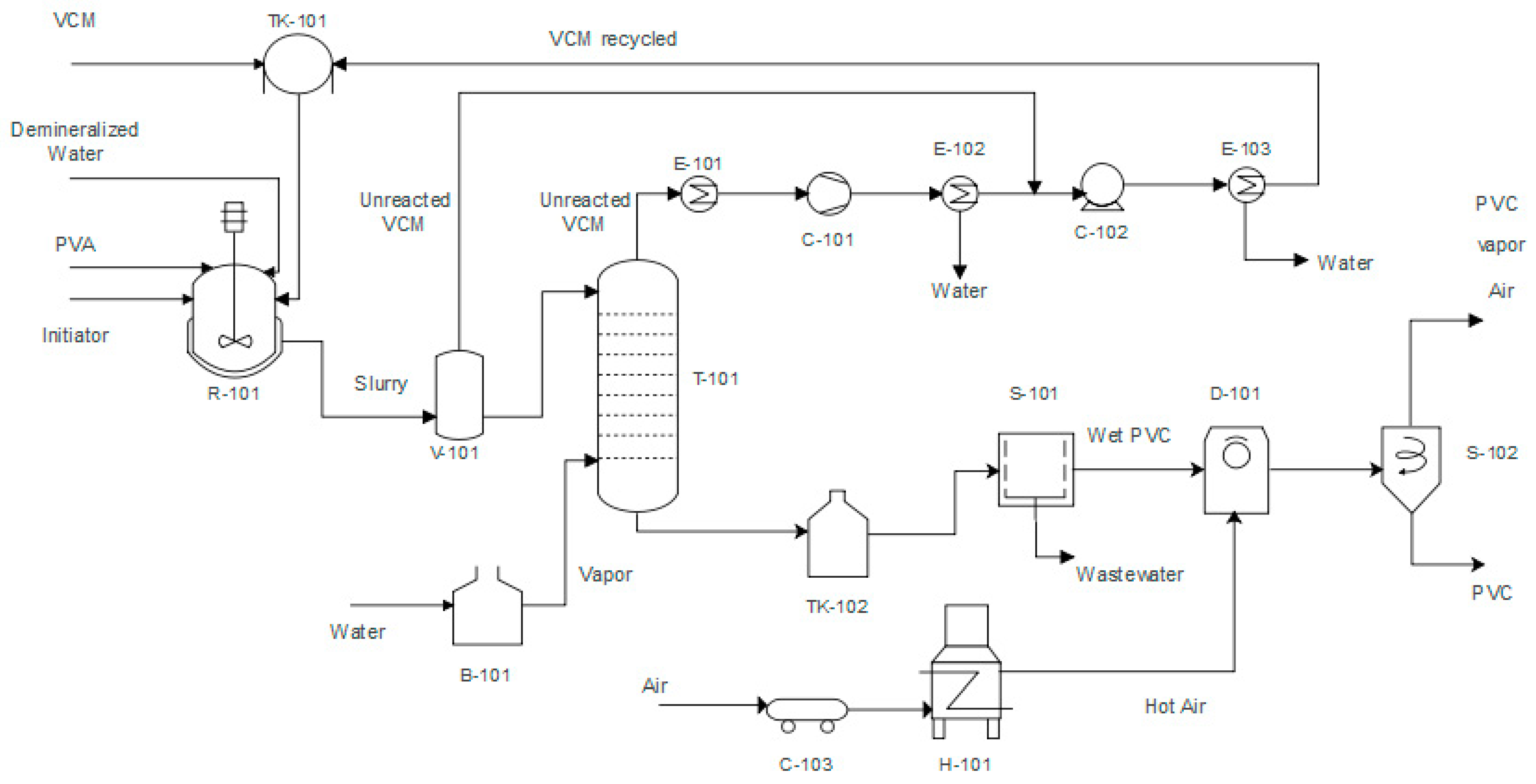
2.2. Process Description
2.3. Environmental Assessment Using Computer-Aided Process Engineering
3. Results and Discussion
3.1. Toxicological Impacts of PVC Suspension Production Process
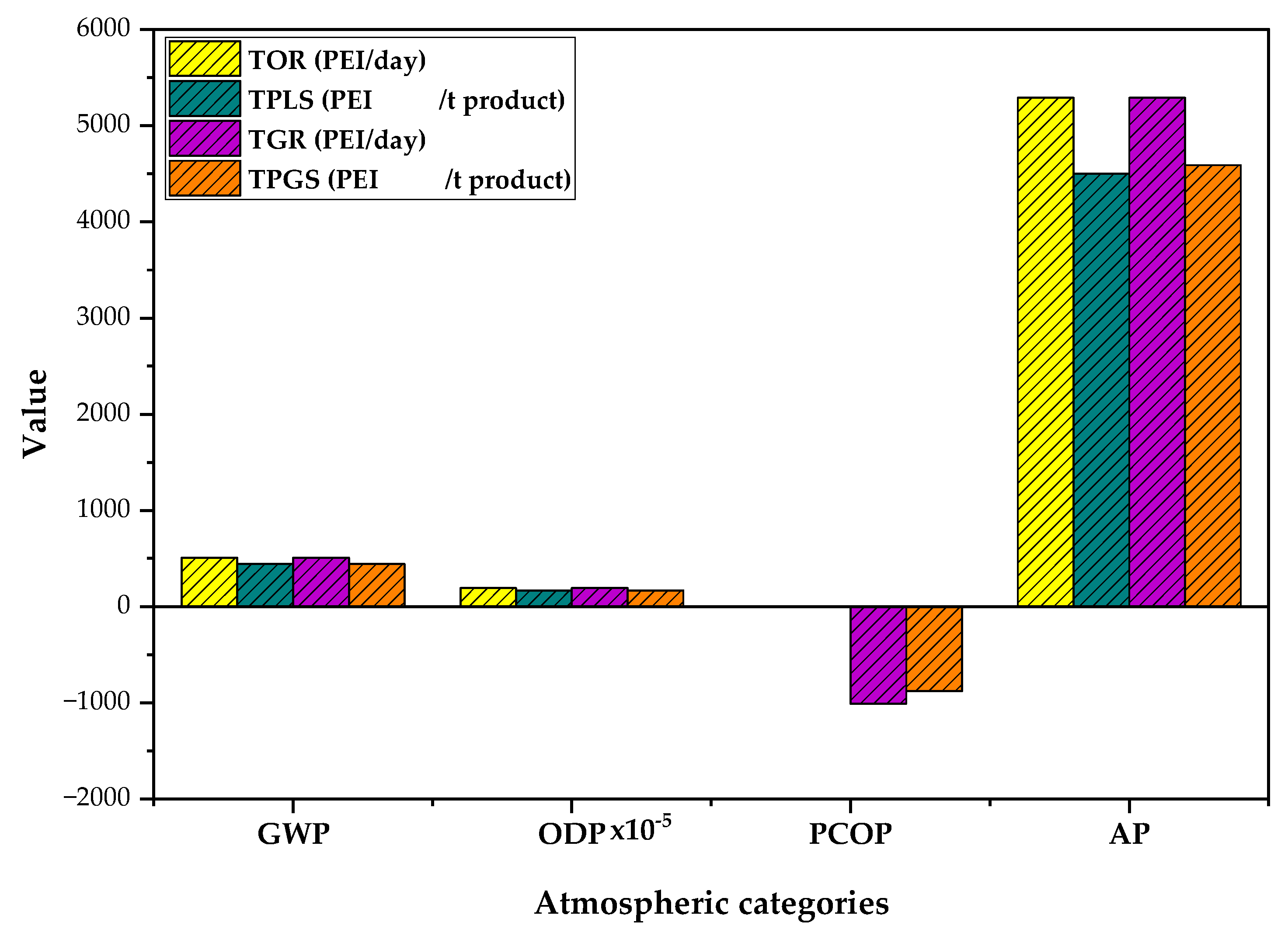
3.2. Atmospheric Impacts of PVC Suspension Production Process
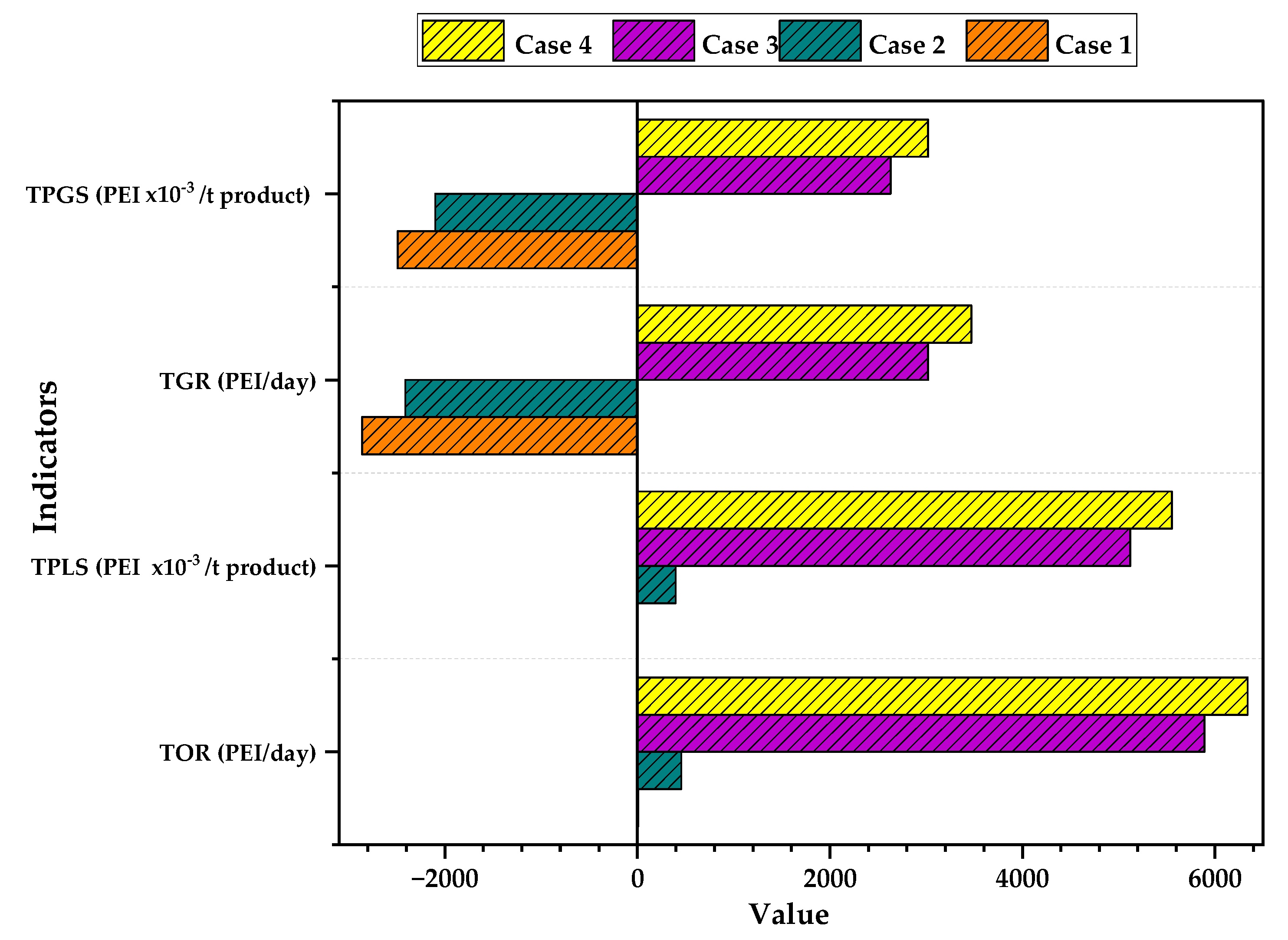
3.3. Impacts According to the Energy Source Used by the Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| PEI | Potential environmental impact |
| PVC | Poly(vinyl chloride) |
| PE | Polyethylene |
| PP | Polypropylene |
| WAR | Waste reduction algorithm |
| AP | Acidification potential |
| GWP | Global warming potential |
| PCOP | Photochemical oxidation potential |
| ODP | Ozone depletion potential |
| HTPI | Human toxicity potential by ingestion |
| HTPE | Human toxicity potential by exposition |
| TTP | Terrestrial toxicity potential |
| ATP | Aquatic toxicity potential |
| ORE | Output rate of PEI from energy usage (PEI/h) |
| PAH | Polycyclic aromatic hydrocarbon |
| TGR | Total generation rate of PEI (PEI/day) |
| TOR | Total output rate of PEI (PEI/day) |
| TPGS | Total PEI generated within a system per mass of products (PEI/t product) |
| TPLS | Total PEI leaving the system per mass of products (PEI/t product) |
| VOC | Volatile organic compounds |
| VCM | Vinyl chloride monomer |
| OSHA | Occupational Safety and Health Administration |
| ACGIH | American Conference of Governmental Industrial Hygienists |
| NIOSH | National Institute for Occupational Safety and Health |
References
- European Commission. Strategy for Plastics in a Circular Economy. Seikei-Kakou 2018, 30, 577–580. [Google Scholar] [CrossRef]
- Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Degradation of Polyvinyl Chloride (PVC) Waste with Supercritical Water. Processes 2022, 10, 1940. [Google Scholar] [CrossRef]
- Carroll, W.F.; Johnson, R.W.; Moore, S.S.; Paradis, R.A. Poly(Vinyl Chloride). In Applied Plastics Engineering Handbook: Processing, Materials, and Applications, 2nd ed.; William Andrew: Waltham, MA, USA, 2017; pp. 73–89. [Google Scholar]
- Mijangos, C.; Calafel, I.; Santamaría, A. Poly(vinyl chloride), a historical polymer still evolving. Polymer 2023, 266, 125610. [Google Scholar] [CrossRef]
- Brooks, B. Suspension Polymerization Processes. Chem. Eng. Technol. 2010, 33, 1737–1744. [Google Scholar] [CrossRef]
- Saeki, Y.; Emura, T. Technical progresses for PVC production. Prog. Polym. Sci. 2002, 27, 2055–2131. [Google Scholar] [CrossRef]
- Leadbitter, J. PVC and sustainability. Prog. Polym. Sci. 2002, 27, 2197–2226. [Google Scholar] [CrossRef]
- Carvalho, A.; Mimoso, A.F.; Mendes, A.N.; Matos, H.A. From a literature review to a framework for environmental process impact assessment index. J. Clean. Prod. 2014, 64, 36–62. [Google Scholar] [CrossRef]
- Mahmud, R.; Moni, S.M.; High, K.; Carbajales-Dale, M. Integration of techno-economic analysis and life cycle assessment for sustainable process design—A review. J. Clean. Prod. 2021, 317, 128247. [Google Scholar] [CrossRef]
- González-Delgado, Á.D.; Moreno-Sader, K.A.; Martínez-Consuegra, J.D. Sustainable Biorefining of Shrimp: Developments from Computer Aided Process Engineering; Corporación Universitaria Minuto de Dios—UNIMINUTO: Bogotá, Colombia, 2022. (In Spanish) [Google Scholar] [CrossRef]
- Sammons, N.; Yuan, W.; Bommareddy, S.; Eden, M.R.; Aksoy, B.; Cullinan, H. A Systematic Framework to Calculate Economic Value and Environmental Impact of Biorefining Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 27. [Google Scholar] [CrossRef]
- Moreno-Sader, K.; Alarcón-Suesca, C.; González-Delgado, A. Application of environmental and hazard assessment methodologies towards the sustainable production of crude palm oil in North-Colombia. Sustain. Chem. Pharm. 2020, 15, 100221. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, A.D.; Cuenca, M.; Martinez, E.; Rincón, B. Evaluación ambiental asistida por computador del proceso de producción de hidromiel a escala piloto en el Departamento de Boyacá y Bolívar (Colombia). Ing. Compet. 2021, 24, e22111112. [Google Scholar] [CrossRef]
- Cardona, C.; Marulanda, V.; Young, D. Analysis of the environmental impact of butylacetate process through the WAR algorithm. Chem. Eng. Sci. 2004, 59, 5839–5845. [Google Scholar] [CrossRef]
- Pájaro, M.A.C.; Ramos, K.J.; González-Delgado, Á.D. Computer aided environmental evaluation of a refinery unit for sulfide absorption and mercaptans oxidation. Chem. Eng. Trans. 2018, 70, 1999–2004. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Alarcón-Suesca, C.; González-Delgado, Á.D. Exergetic sensibility analysis and environmental evaluation of chitosan production from shrimp exoskeleton in Colombia. J. Clean. Prod. 2020, 248, 119285. [Google Scholar] [CrossRef]
- Duarte, A.; Uribe, J.C.; Sarache, W.; Calderón, A. Economic, environmental, and social assessment of bioethanol production using multiple coffee crop residues. Energy 2021, 216, 119170. [Google Scholar] [CrossRef]
- Comanita, E.-D.; Ghinea, C.; Rosca, M.; Simion, I.M.; Petraru, M.; Gavrilescu, M. Environmental impacts of polyvinyl chloride (PVC) production process. In Proceedings of the 2015 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 19–21 November 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Franklin Associates. Cradle-to-Gate Life Cycle Analysis of Polyvinyl Chloride (PVC) Resin; Franklin Associates: Baton Rouge, LA, USA, 2020. [Google Scholar]
- Baitz, M.; Kreissig, J.; Makishi, C. Life cycle assessment of PVC in product optimisation and green procurement—Fact-based decisions towards sustainable solutions. Plast. Rubber Compos. 2005, 34, 95–98. [Google Scholar] [CrossRef]
- Shadiya, O.O.; Satish, V.; High, K.A. Process enhancement through waste minimization and multiobjective optimization. J. Clean. Prod. 2012, 31, 137–149. [Google Scholar] [CrossRef]
- Herrera-Aristizábal, R.; Salgado-Dueñas, J.; Peralta-Ruiz, Y.; González-Delgado, Á. Environmental evaluation of a palm-based biorefinery under north-colombian conditions. Chem. Eng. Trans. 2017, 57, 193–198. [Google Scholar] [CrossRef]
- Young, D.M.; Cabezas, H. Designing sustainable processes with simulation: The waste reduction (WAR) algorithm. Comput. Chem. Eng. 1999, 23, 1477–1491. [Google Scholar] [CrossRef]
- Cabezas, H.; Bare, J.C.; Mallick, S.K. Pollution prevention with chemical process simulators: The generalized waste reduction (WAR) algorithm—Full version. Comput. Chem. Eng. 1999, 23, 623–634. [Google Scholar] [CrossRef]
- Moreno-Sader, K.; Meramo-Hurtado, S.; González-Delgado, A. Computer-aided environmental and exergy analysis as decision-making tools for selecting bio-oil feedstocks. Renew. Sustain. Energy Rev. 2019, 112, 42–57. [Google Scholar] [CrossRef]
- Bracho Carmona, M.L.; Van Der Biest Rodriguez, F.R. Requerimientos Maximos de Agua de Enfriamiento en el Area de Polimerizacion de la Planta PVC II, el Tablazo; Universidad Rafael Urdaneta: Maracaibo, Zulia, Venezuela, 2007. [Google Scholar]
- Wieme, J.; De Roo, T.; Marin, G.B.; Heynderickx, G.J. Simulation of Pilot- and Industrial-Scale Vinyl Chloride Batch Suspension Polymerization Reactors. Ind. Eng. Chem. Res. 2007, 46, 1179–1196. [Google Scholar] [CrossRef]
- Hoa, C.J.; Sivakumar, D.C.Y.F.; Adffin, M.; Iassan, A.; Azizr, A. Process Modelling of A PVC Production Plant. In Proceedings of the International Conference Bioprocess & Engineering, Kota Kinabalu, Malaysia, 9–12 December 2015; pp. 881–886. [Google Scholar]
- Agaciak, P.; Yahiaoui, S.; Djabourov, M.; Lasuye, T. Dehydration and drying poly(vinyl)chloride (PVC) porous grains: 1. Centrifugation and drying in controlled humid atmospheres. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 132–140. [Google Scholar] [CrossRef]
- Bottausci, S.; Ungureanu-Comanita, E.-D.; Gavrilescu, M.; Bonoli, A. Environmental impacts quantification of PVC production. Environ. Eng. Manag. J. 2021, 20, 1693–1702. [Google Scholar] [CrossRef]
- Jimenez-Varon, C.F.; Soto, R.T. Design of a production plant of LDPE in Colombia. Vector 2017, 10, 59–64. [Google Scholar]
- Velásquez-Barrios, A.; Rueda-Duran, C.; Marín-Valencia, P.; Mogollón-Rincón, E.; Alvarez-Giraldo, J.; Cardona-Cabarcas, R.; Giraldo-Osorio, Ó.H.; Cardona-Alzate, C.A. Analysis of the environmental impact using the waste reduction algorithm in polypropylene production by applying grade transitions strategies in Colombia. Environ. Sci. Pollut. Res. 2019, 26, 35533–35542. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, R.B.; Wolfe, A.; Hendryx, M.S. The future environmental and health impacts of coal. Energy Geosci. 2021, 2, 99–112. [Google Scholar] [CrossRef]
- Agrawal, K.K.; Jain, S.; Jain, A.K.; Dahiya, S. Assessment of greenhouse gas emissions from coal and natural gas thermal power plants using life cycle approach. Int. J. Environ. Sci. Technol. 2014, 11, 1157–1164. [Google Scholar] [CrossRef]
- Lattanzio, R.K. An overview of air quality issues in natural gas systems. In The Natural Gas Sector: Life-Cycle Greenhouse Gas and Air Quality Issues; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 39–65. [Google Scholar]
- EPA Nitrogen Oxides (NOx), Why and How They Are Controlled. Epa-456/F-99-006R 1999, November, 48. Available online: http://www.epa.gov/ttncatc1/dir1/fnoxdoc.pdf (accessed on 15 April 2023).
- Munawer, M.E. Human health and environmental impacts of coal combustion and post-combustion wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
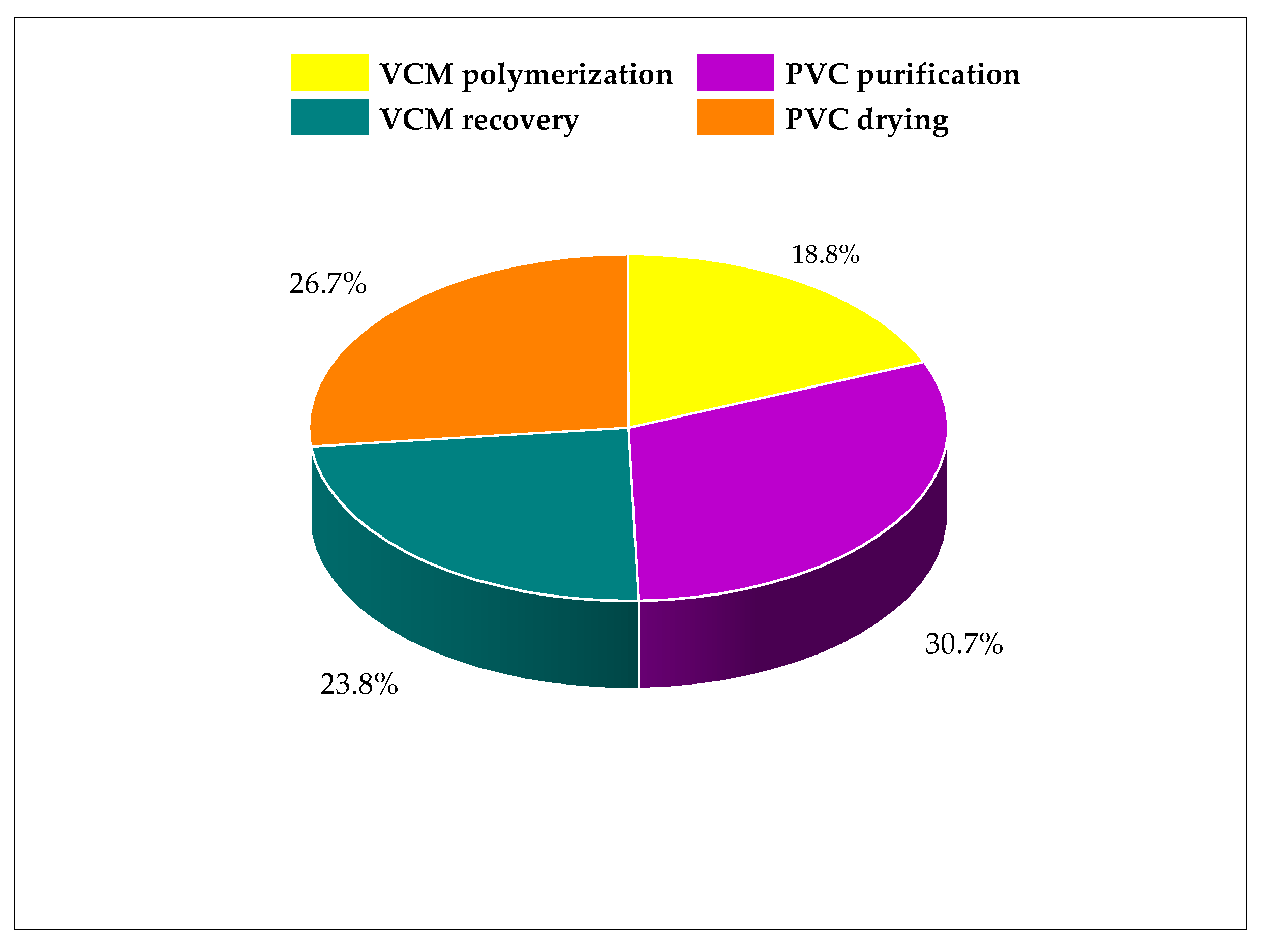


| Stage | Output Rate of PEI Per Day | Contribution (%) |
|---|---|---|
| VCM polymerization | 0 | 0% |
| PVC purification | 0 | 0% |
| VCM recovery | 0 | 0% |
| PVC drying | 6.5 | 100% |
| total | 6.5 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Delgado, Á.D.; Ramos-Olmos, M.; Aguilar-Vásquez, E. Environmental Impacts Assessment in Suspension PVC Production Process Using Computer-Aided Process Engineering. Polymers 2023, 15, 2902. https://doi.org/10.3390/polym15132902
González-Delgado ÁD, Ramos-Olmos M, Aguilar-Vásquez E. Environmental Impacts Assessment in Suspension PVC Production Process Using Computer-Aided Process Engineering. Polymers. 2023; 15(13):2902. https://doi.org/10.3390/polym15132902
Chicago/Turabian StyleGonzález-Delgado, Ángel Darío, Miguel Ramos-Olmos, and Eduardo Aguilar-Vásquez. 2023. "Environmental Impacts Assessment in Suspension PVC Production Process Using Computer-Aided Process Engineering" Polymers 15, no. 13: 2902. https://doi.org/10.3390/polym15132902
APA StyleGonzález-Delgado, Á. D., Ramos-Olmos, M., & Aguilar-Vásquez, E. (2023). Environmental Impacts Assessment in Suspension PVC Production Process Using Computer-Aided Process Engineering. Polymers, 15(13), 2902. https://doi.org/10.3390/polym15132902







