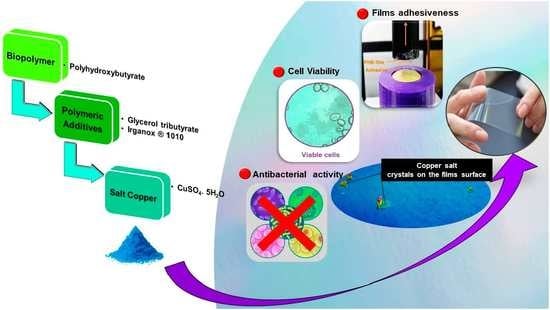
Films of Poly(Hydroxybutyrate) (PHB) and Copper with Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Films Obtaining
2.2. Films’ Microstructure
2.3. Films’ Tensile Properties
2.4. Films’ Antimicrobial Capacity
2.5. Films’ Cytotoxicity
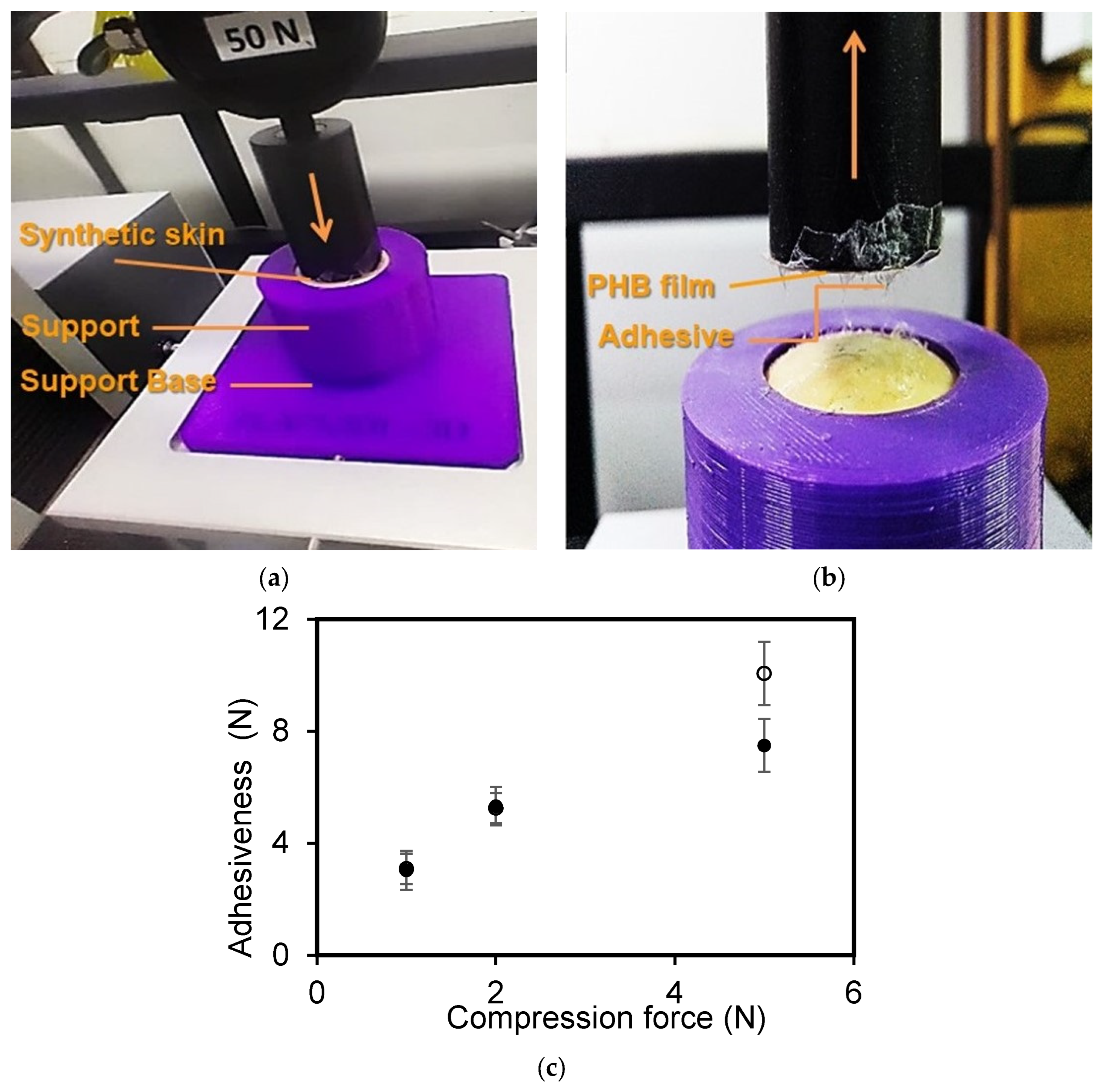
2.6. Films’ Adhesive Strength to Human Synthetic Skin
2.7. Statistical Analysis
3. Results
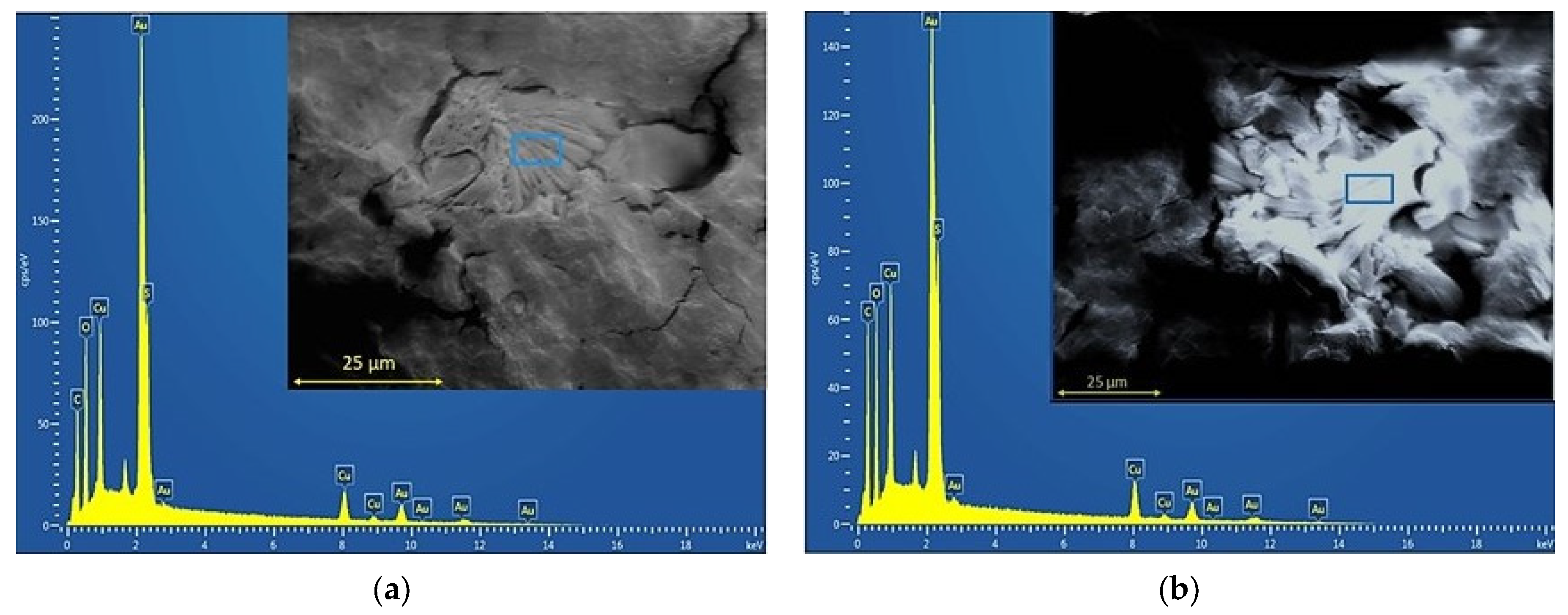
3.1. Films’ Microstructure
3.2. Films’ Tensile Properties
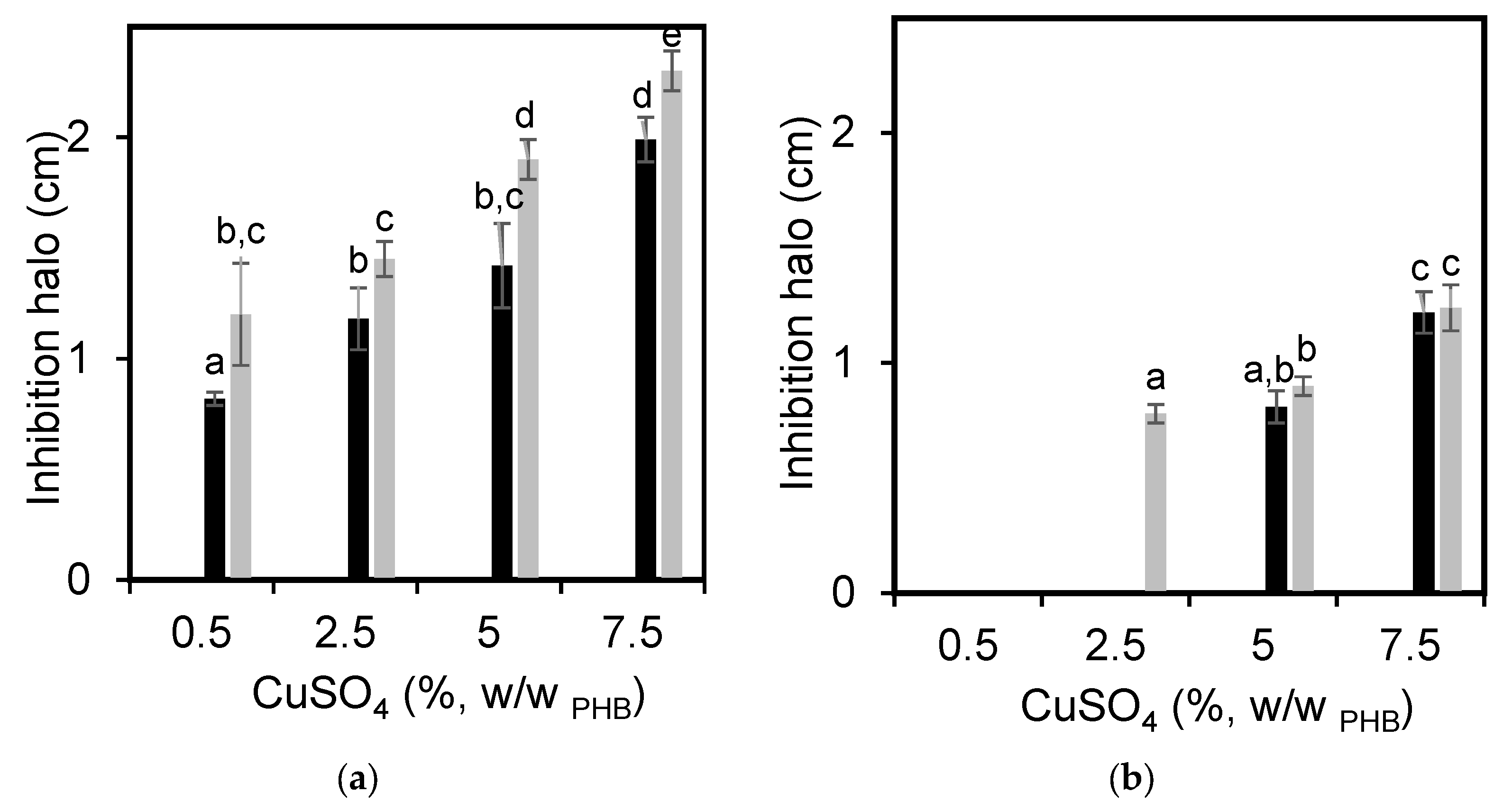
3.3. Films’ Antimicrobial Capacity
3.4. Films’ Adhesiveness
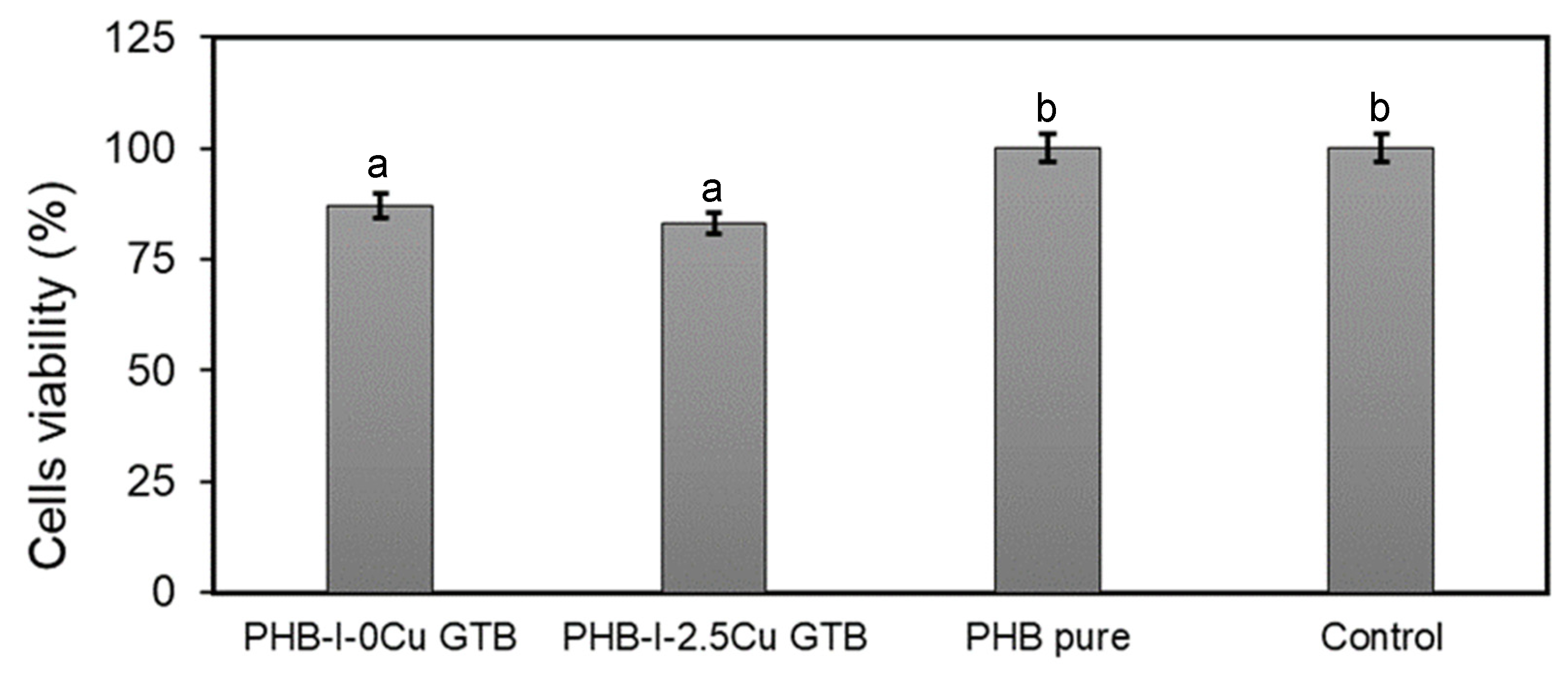
3.5. Films’ Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varghese, S.A.; Siengchin, S.; Parameswaranpillai, J. Essential oils as antimicrobial agents in biopolymer-based food packaging, A comprehensive review. Food Biosci. 2020, 38, 100785. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Costa, I.F.M.; Jorge, P.; Sousa, A.C.; André, V.; Cerca, N.; Kirillov, A.M. Silver(I) Coordination Polymers Immobilized into Biopolymer Films for Antimicrobial Applications. ACS Appl. Mater. Interfaces 2021, 13, 12836–12844. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Montero, M.P. Enhancement of oral bioavailability of natural compounds and probiotics by mucoadhesive tailored biopolymer-based nanoparticles: A review. Food Hydrocoll. 2021, 118, 106772. [Google Scholar] [CrossRef]
- Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymer 2021, 13, 1962. [Google Scholar] [CrossRef]
- Morgan, N.B. Medical shape memory alloy applications, the market and its products. Mater. Sci. Eng. A 2004, 378, 16–23. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; De la Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Can, A.S.; Erdal, M.S.; Güngör, S.; Özsoy, Y. Optimization, and characterization of chitosan films for transdermal delivery of ondansetron. Molecules 2013, 18, 5455–5471. [Google Scholar] [CrossRef]
- Rojas Cortés, M.G.; Vallejo Díaz, B.M.; Perilla, J.E. Los biopolímeros como materiales para el desarrollo de productos en aplicaciones farmacéuticas y de uso biomédico. Ing. Investig. 2008, 28, 57–71. [Google Scholar] [CrossRef]
- Olivas-Armendariz, I.; Valencia-Gómez, L.E.; Martel-Estrada, S.A.; Vargas-Requena, C.L.; Rodriguez-González, C.A. Natural polymers aposites for skin regeneration. Mex. J. Biomed. Eng. 2016, 37, 235–249. [Google Scholar] [CrossRef]
- Leaper, D.J. Silver dressings: Their role in wound management. Int. Wound J. 2006, 3, 282–294. [Google Scholar] [CrossRef]
- Woo, K.Y.; Heil, J. A prospective evaluation of methylene blue and gentian violet dressing for management of chronic wounds with local infection. Int. Wound J. 2017, 14, 1029–1035. [Google Scholar] [CrossRef]
- Aluigi, A.; Sotgiu, G.; Torreggiani, A.; Guerrini, A.; Orlandi, V.T.; Corticelli, F.; Varchi, G. Methylene blue doped films of wool keratin with antimicrobial photodynamic activity. ACS Appl. Mater. Interfaces 2015, 7, 17416–17424. [Google Scholar] [CrossRef]
- Lundin, J.G.; McGann, C.L.; Weise, N.K.; Estrella, L.A.; Balow, R.B.; Streifel, B.C.; Wynne, J.H. Iodine binding and release from antimicrobial hemostatic polymer foams. React. Funct. Polym. 2019, 135, 44–51. [Google Scholar] [CrossRef]
- Woo, K.Y.; Alam, T.; Marin, J. Topical antimicrobial toolkit for wound infection. Surg. Technol. Int. 2014, 25, 45–52. [Google Scholar]
- Holbrook, R.D.; Rykaczewski, K.; Staymates, M.E. Dynamics of silver nanoparticle release from wound dressings revealed via in situ nanoscale imaging. J. Mater. Sci. Mater. Med. 2014, 25, 2481–2489. [Google Scholar] [CrossRef]
- Yücedag, F.; Atalay-Oral, C.; Erkal, S.; Sirkecioglu, A.; Karasartova, D.; Sahin, F.; Tantekin-Ersolmaz, S.B.; Güner, F.S. Antibacterial oil-based polyurethane films for wound dressing applications. J. Appl. Polym. Sci. 2010, 115, 1347–1357. [Google Scholar] [CrossRef]
- Febré, N.; Silva, V.; Báez, A.; Palza, H.; Delgado, K.; Aburto, I.; Silva, V. Comportamiento antibacteriano de partículas de cobre frente a microorganismos obtenidos de úlceras crónicas infectadas y su relación con la resistencia a antimicrobianos de uso común. Rev. Med. Chil. 2016, 144, 1523–1530. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Wehrschuetz-Sigl, E.; Hasmann, A.; Guebitz, G.; Gedanken, A. CuO–cotton nanocomposite: Formation, morphology, and antibacterial activity. Surf. Coat. Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8, S4–S9. [Google Scholar] [CrossRef]
- Ozkaynak, M.U.; Atalay-Oral, C.; Tantekin-Ersolmaz, S.B.; Güner, F.S. Polyurethane Films for Wound Dressing Applications. Macromol. Symp. 2005, 228, 177–184. [Google Scholar] [CrossRef]
- Israni, N.; Shivakumar, S. Chapter 13—Polyhydroxybutyrate: Development and Applications as a Biodegradable biotextile. In Materials for Biomedical Engineering; Grumezescu, B.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 405–444. [Google Scholar] [CrossRef]
- Constantinides, C.; Basnett, P.; Lukasiewicz, B.; Carnicer, R.; Swider, E.; Majid, Q.A.; Srinivas, M.; Carr, C.A.; Roy, I. In Vivo Tracking and 1H/19F Magnetic Resonance Imaging of Biodegradable Polyhydroxyalkanoate/Polycaprolactone Blend Scaffolds Seeded with Labeled Cardiac Stem Cells. ACS Appl. Mater. Interfaces. 2018, 10, 25056–25068. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Quispe, M.M.; López, O.V.; Villar, M.A. Antioxidant Efficiency of Irganox® 1010 on Processing and Properties of Plasticized Poly(3-Hydroxybutyrate) Films. J. Compos. Biodegrad. Polym. 2021, 9, 7–16. [Google Scholar] [CrossRef]
- Ishizawa, C.; Nakamatsu, J. Matrices Poliméricas para Liberación Controlada de Sustancias Activas. Rev. Química 2002, 16, 13–23. [Google Scholar]
- Escobar, L.M.; Rivera, A.; Aristizábal, F.A. Estudio comparativo de los métodos de resazurina y MTT en estudios de citotoxicidad en líneas celulares tumorales humanas. Vitae 2010, 17, 67–74. [Google Scholar]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S.; McCoy, K.D.; Wang, R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica 1996, 4, 14–19. [Google Scholar]
- Wong, C.F.; Yuen, K.H.; Peh, K.K. Formulation, and evaluation of controlled release Eudragit buccal patches. Int. J. Pharm. 1999, 178, 11–22. [Google Scholar] [CrossRef]
- Bhushan, B.; Tang, W. Surface, tribological, and mechanical characterization of synthetic skins for tribological applications in cosmetic science. J. Appl. Polym. Sci. 2011, 120, 2881–2890. [Google Scholar] [CrossRef]
- Chan, R.; Garvey, C.; Marcal, H.; Russell, R.; Holden, P.; Foster, L. Manipulation of Polyhydroxybutyrate Properties through Blending with Ethyl-Cellulose for a Composite Biomaterial. Int. J. Polym. Sci. 2011, 2011, 651549. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate-polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Quispe, M.M.; Lopez, O.V.; Boina, D.A.; Stumbé, J.F.; Villar, M.A. Glycerol-based additives of poly(3-hydroxybutyrate) films. Polym. Test. 2021, 93, 107005. [Google Scholar] [CrossRef]
- Mittal, V. Polymer Layered Silicate Nanocomposites: A Review. Materials 2009, 2, 992–1057. [Google Scholar] [CrossRef]
- Pukánszky, B. Interfaces, and interphases in multicomponent materials: Past, present, future. Eur. Polym. J. 2005, 41, 645–662. [Google Scholar] [CrossRef]
- Spanoudakis, J.; Young, R.J. Crack propagation in a glass particle-filled epoxy resin. J. Mater. Sci. 1984, 19, 473–486. [Google Scholar] [CrossRef]
- Tafur, J.D.; Torres, J.A.; Villegas, M.V. Mecanismos de resistencia a los antibióticos en bacterias Gram Negativas. Infectio 2008, 12, 227–232. [Google Scholar]
- España-Sánchez, B.L.; Rodríguez-González, J.A.; González-Morones, P.; Neira-Velázquez, M.G.; Franco-Bárcenas, B.; Anaya-Velázquez, F.; Mendoza-Macía, C.L.; Ávila-Orta, C.A.; Padilla-Vaca, F. Nanocomposites based on Polypropylene and Copper Nanoparticles: Preparation, Surface Activation by Plasma and Antibacterial Activity. Acta Univ. 2014, 24, 13–24. [Google Scholar] [CrossRef]
- Toloue, E.B.; Karbasi, S.; Salehi, H.; Rafienia, M. Potential of an electrospun composite scaffold of poly (3-hydroxybutyrate)-chitosan/alumina nanowires in bone tissue engineering applications. Mater. Sci. Eng. C 2019, 99, 1075–1091. [Google Scholar] [CrossRef]
- Rius Tarruella, J.; López Bertrán, R. Evaluación in vitro de las propiedades de seis apósitos para la cura en ambiente húmedo de heridas exudativas. Gerokomos 2008, 19, 30–40. [Google Scholar] [CrossRef]
- Tavakoli, J. Physico-mechanical, morphological, and biomedical properties of a novel natural wound dressing material. J. Mech. Behav. Biomed. Mater. 2017, 65, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Girdhar, M.; Kumar, R.; Chaturvedi, H.S.; Vadhel, A.; Solanki, P.R.; Mamidi, N. Polyhydroxybutyrate-based nanocomposites for bone tissue engineering. Pharmaceuticals 2021, 14, 1163. [Google Scholar] [CrossRef] [PubMed]


| Sample | Irganox 1010 (I) (%, w/wPHB) | Glycerol Tributyrate (GTB) (%, w/wPHB) | CuSO4·5H2O (Cu) (%, w/wPHB) |
|---|---|---|---|
| PHB-I-0Cu | 0.3 | - | - |
| PHB-I-0.5Cu | 0.3 | - | 0.5 |
| PHB-I-2.5Cu | 0.3 | - | 2.5 |
| PHB-I-5.0Cu | 0.3 | - | 5.0 |
| PHB-I-7.5Cu | 0.3 | - | 7.5 |
| PHB-I-0CuGTB | 0.3 | 20 | - |
| PHB-I-0.5CuGTB | 0.3 | 20 | 0.5 |
| PHB-I-2.5CuGTB | 0.3 | 20 | 2.5 |
| PHB-I-5.0CuGTB | 0.3 | 20 | 5.0 |
| PHB-I-7.5CuGTB | 0.3 | 20 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe, M.M.; Villanueva, M.E.; Copello, G.J.; López, O.V.; Villar, M.A. Films of Poly(Hydroxybutyrate) (PHB) and Copper with Antibacterial Activity. Polymers 2023, 15, 2907. https://doi.org/10.3390/polym15132907
Quispe MM, Villanueva ME, Copello GJ, López OV, Villar MA. Films of Poly(Hydroxybutyrate) (PHB) and Copper with Antibacterial Activity. Polymers. 2023; 15(13):2907. https://doi.org/10.3390/polym15132907
Chicago/Turabian StyleQuispe, Mayte M., María E. Villanueva, Guillermo J. Copello, Olivia V. López, and Marcelo A. Villar. 2023. "Films of Poly(Hydroxybutyrate) (PHB) and Copper with Antibacterial Activity" Polymers 15, no. 13: 2907. https://doi.org/10.3390/polym15132907
APA StyleQuispe, M. M., Villanueva, M. E., Copello, G. J., López, O. V., & Villar, M. A. (2023). Films of Poly(Hydroxybutyrate) (PHB) and Copper with Antibacterial Activity. Polymers, 15(13), 2907. https://doi.org/10.3390/polym15132907