Phosphonium-Based Polyelectrolytes: Preparation, Properties, and Usage in Lithium-Ion Batteries
Abstract
1. Introduction
- (i)
- Improve the ILs’ thermo-mechanical strength, which would allow for thinner membranes.
- (ii)
- Improve the PILs’ ion conductivities.
2. Phosphorus
3. Phosphonium Ionic Liquid Polymer Electrolytes in Lithium-Ion Batteries
3.1. Polymer Electrolytes
3.2. IL-Based Polymer Electrolyte
3.2.1. Ionic Liquid (ILs)/Polymer Doped (Blending Technique)
3.2.2. Polymeric Ionic Liquids (PILs)
4. Phosphonium-Based Polymeric Ionic Liquid Electrolyte
4.1. Synthesis Route and Conductivities
4.2. Analytical Tools
4.3. Comparison between Phosphonium-Based PILs and Nitrogen-Based PILs
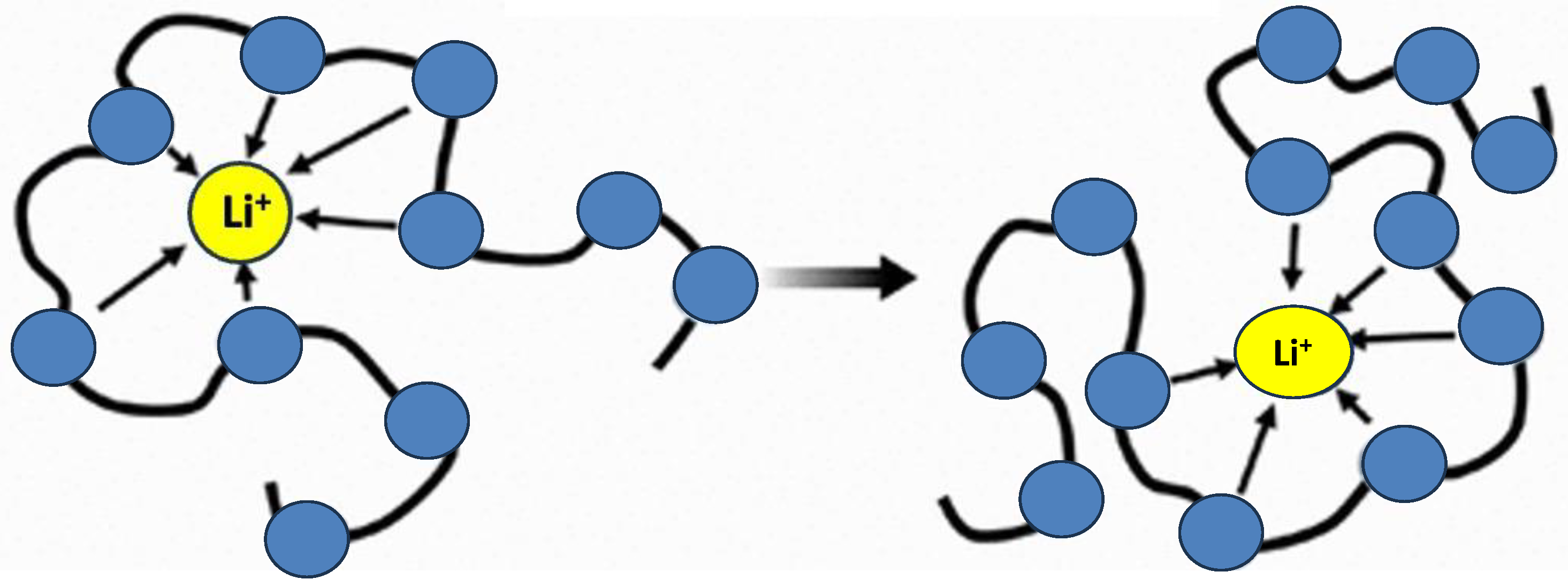
4.4. Counter-Ion Effect in Conductivity
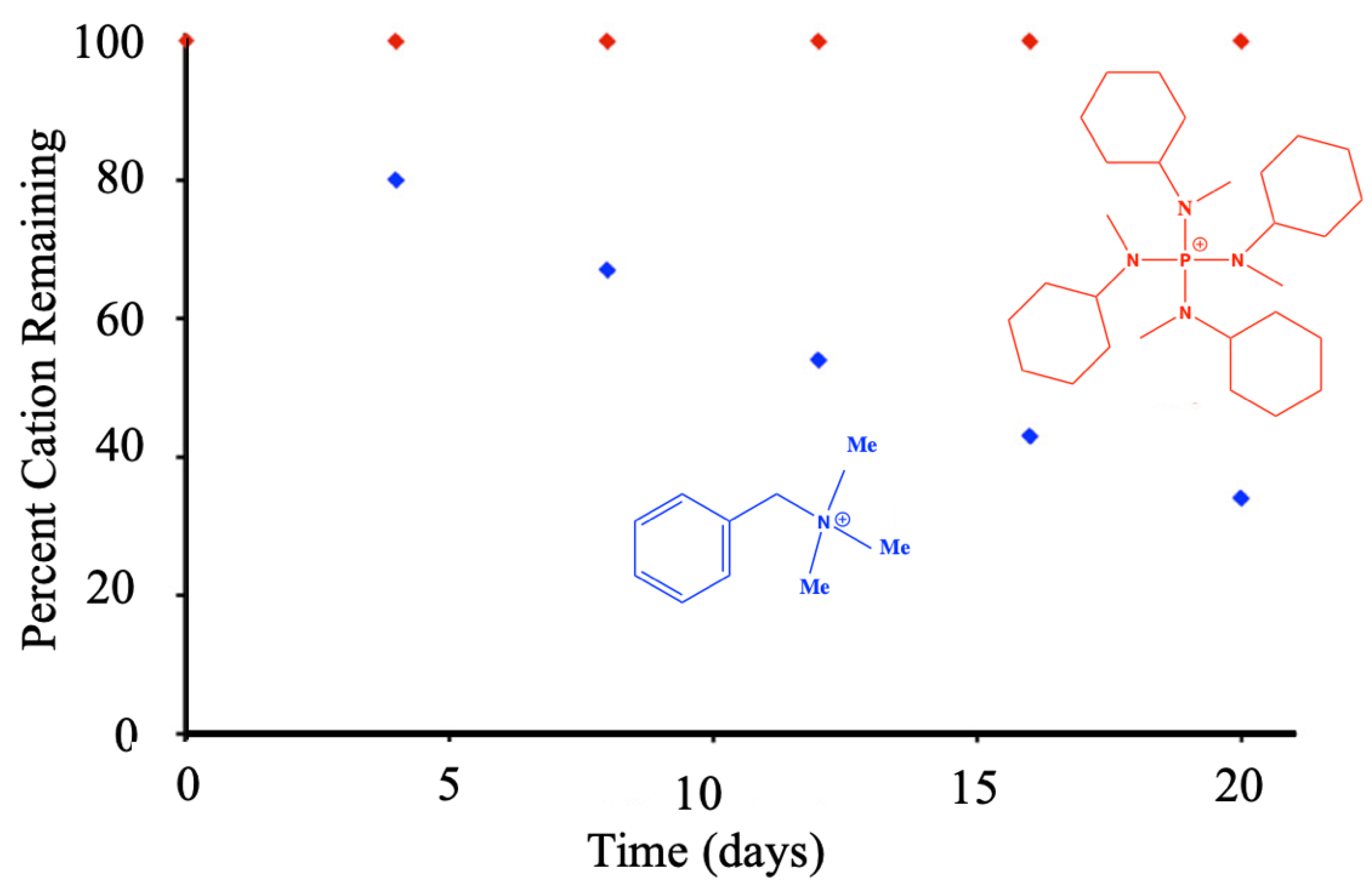
4.5. Sability of Phosphonium PILs
5. Conclusions
Future Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, D.; Ge-Zhang, S.; Zhang, Z.; Tang, H.; Xu, Y.; Gao, F.; Xu, X.; Liu, S.; Zhou, J.; Wang, Z.; et al. Three-Dimensional Honeycomb-Like Carbon as Sulfur Host for Sodium-Sulfur Batteries without the Shuttle Effect. ACS Appl. Mater. Interfaces 2022, 14, 54662–54669. [Google Scholar] [CrossRef] [PubMed]
- Musah, J.D.; Ilyas, A.M.; Venkatesh, S.; Mensah, S.; Kwofie, S.; Roy, V.A.; Wu, C.M.L. Isovalent substitution in metal chalcogenide materials for improving thermoelectric power generation—A critical review. Nano Res. Energy 2022, 1, 9120034. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef]
- Badi, N.; Theodore, A.M.; Alghamdi, S.A.; Al-Aoh, H.A.; Lakhouit, A.; Singh, P.K.; Norrrahim, M.N.F.; Nath, G. The Impact of Polymer Electrolyte Properties on Lithium-Ion Batteries. Polymers 2022, 14, 3101. [Google Scholar] [CrossRef] [PubMed]
- Misenan, M.S.M.; Khiar, A.S.A.; Eren, T. Polyurethane based Polymer Electrolyte for Lithium-Ion Batteries: A Review. Polym. Int. 2022, 71, 751–769. [Google Scholar] [CrossRef]
- Ramström, O. Scientific Background on the Nobel Prize in Chemistry 2019 Lithium-Ion Batteries. 2019. Available online: https://www.nobelprize.org/prizes/chemistry/2019/press-release/ (accessed on 9 June 2023).
- Yang, G.; Song, Y.; Wang, Q.; Zhang, L.; Deng, L. Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries. Mater. Des. 2020, 190, 108563. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Source 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Misenan, M.S.M.; Isa, M.I.N.; Khiar, A.S.A. Electrical and structural studies of polymer electrolyte based on chitosan/methyl cellulose blend doped with BMIMTFSI. Mater. Res. Express 2018, 5, 055304. [Google Scholar] [CrossRef]
- Misenan, M.S.M.; Khiar, A.S.A. Structural Studies and Ionic Transport Properties of Solid Biopolymer Electrolytes Based on Chitosan/Methyl Cellulose Blend Doped with BMIMTFSI. Solid State Phenom. 2020, 307, 119–124. [Google Scholar] [CrossRef]
- Misenan, M.; Khiar, A. Conductivity, Dielectric and Modulus Studies of Methylcellulose-NH 4 TF Polymer. Eurasian J. Biol. Chem. Sci. J. 2018, 1, 59–62. [Google Scholar]
- Shaffie, A.H.; Misenan, M.S.M.; Isa, M.I.N.; Khiar, A.S.A. Effect of Ionic Liquid Bmimno3 to Chitosan-Starch Blend Biopolymer Electrolyte System; SSP: Mount Laurel, NJ, USA, 2019; Volume 290. [Google Scholar]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Chen, M.; White, B.T.; Kasprzak, C.R.; Long, T.E. Advances in phosphonium-based ionic liquids and poly(ionic liquid)s as conductive materials. Eur. Polym. J. 2018, 108, 28–37. [Google Scholar] [CrossRef]
- Wilkes, J.S. A short history of ionic liquids—From molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80. [Google Scholar] [CrossRef]
- Misenan, M.S.M.; Shaffie, A.H.; Khiar, A.S.A. Effect of BMITFSI to the electrical properties of chitosan/methylcellulose based polymer electrolyte. AIP Conf. Proc. 2018, 1972, 030001. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Yan, K.; Zheng, G.; Liang, Z.; Chiang, Y.M.; Cui, Y. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 2015, 6, 7436. [Google Scholar] [CrossRef]
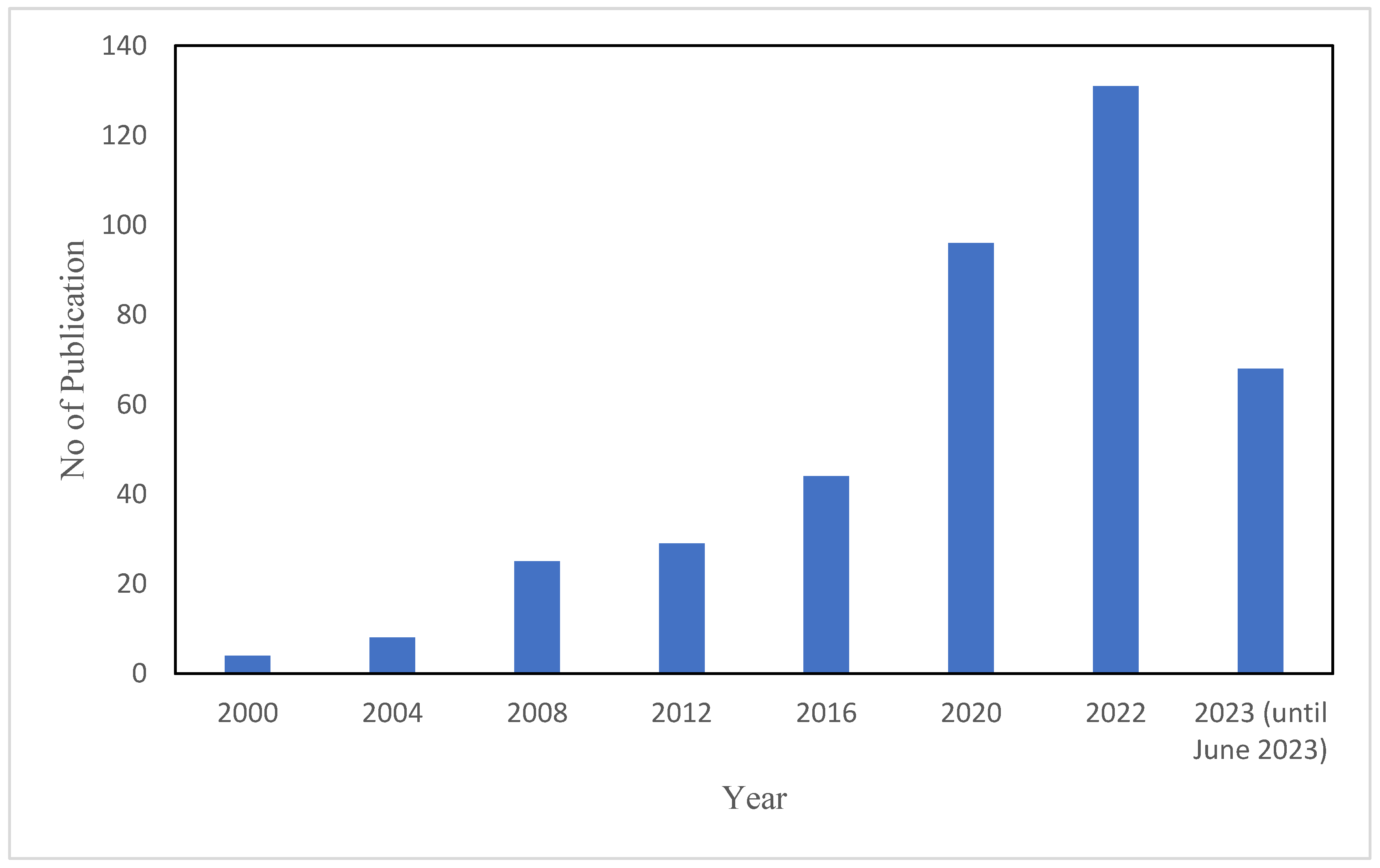
- Scopus. Available online: https://www.scopus.com/results/results.uri?sort=plff&src=s&st1=polymeric+ionic+liquid&sid=75c909220001add0f86bbeac3817fdb9&sot=b&sdt=b&sl=37&s=TITLE-ABSKEY%28polymeric+ionic+liquid%29&origin=searchbasic&editSaveSearch=&yearFrom=Before+1960&yearTo=Presen (accessed on 9 June 2023).
- Lodge, T.P. A Unique Platform for Materials Design. Science 2008, 321, 50–51. [Google Scholar] [CrossRef]
- Hall, C.C.; Zhou, C.; Danielsen, S.P.O.; Lodge, T.P. Formation of Multicompartment Ion Gels by Stepwise Self-Assembly of a Thermoresponsive ABC Triblock Terpolymer in an Ionic Liquid. Macromolecules 2016, 49, 2298–2306. [Google Scholar] [CrossRef]
- Lodge, T.P.; Ueki, T. Mechanically Tunable, Readily Processable Ion Gels by Self-Assembly of Block Copolymers in Ionic Liquids. Acc. Chem. Res. 2016, 49, 2107–2114. [Google Scholar] [CrossRef]
- Moon, H.C.; Kim, C.-H.; Lodge, T.P.; Frisbie, C.D. Multicolored, Low-Power, Flexible Electrochromic Devices Based on Ion Gels. ACS Appl. Mater. Interfaces 2016, 8, 6252–6260. [Google Scholar] [CrossRef]
- Obadia, M.M.; Jourdain, A.; Serghei, A.; Ikeda, T.; Drockenmuller, E. Cationic and dicationic 1,2,3-triazolium-based poly(ethylene glycol ionic liquid)s. Polym. Chem. 2017, 8, 910–917. [Google Scholar] [CrossRef]
- Green, M.D.; Salas-de la Cruz, D.; Ye, Y.; Layman, J.M.; Elabd, Y.A.; Winey, K.I.; Long, T.E. Alkyl-substituted N-vinylimidazolium polymerized ionic liquids: Thermal properties and ionic conductivities. Macromol. Chem. Phys. 2011, 212, 2522–2528. [Google Scholar] [CrossRef]
- Cowan, M.G.; Masuda, M.; McDanel, W.M.; Kohno, Y.; Gin, D.L.; Noble, R.D. Phosphonium-based poly(Ionic liquid) membranes: The effect of cation alkyl chain length on light gas separation properties and Ionic conductivity. J. Memb. Sci. 2016, 498, 408–413. [Google Scholar] [CrossRef]
- Hofmann, A.; Rauber, D.; Wang, T.M.; Hempelmann, R.; Kay, C.W.M.; Hanemann, T. Novel Phosphonium-Based Ionic Liquid Electrolytes for Battery Applications. Molecules 2022, 27, 4729. [Google Scholar] [CrossRef] [PubMed]
- Hemp, S.T.; Zhang, M.; Allen, M.H.; Cheng, S.; Moore, R.B.; Long, T.E. Comparing Ammonium and Phosphonium Polymerized Ionic Liquids: Thermal Analysis, Conductivity, and Morphology. Macromol. Chem. Phys. 2013, 214, 2099–2107. [Google Scholar] [CrossRef]
- Tsunashima, K.; Niwa, E.; Kodama, S.; Sugiya, M.; Ono, Y. Thermal and Transport Properties of Ionic Liquids Based on Benzyl-Substituted Phosphonium Cations. J. Phys. Chem. B 2009, 113, 15870–15874. [Google Scholar] [CrossRef]
- Noonan, K.J.T.; Hugar, K.M.; Kostalik, H.A.; Lobkovsky, E.B.; Abruña, H.D.; Coates, G.W. Phosphonium-Functionalized Polyethylene: A New Class of Base-Stable Alkaline Anion Exchange Membranes. J. Am. Chem. Soc. 2012, 134, 18161–18164. [Google Scholar] [CrossRef]
- Wang, A.; Xu, H.; Liu, X.; Gao, R.; Wang, S.; Zhou, Q.; Chen, J.; Liu, X.; Zhang, L. The synthesis of a hyperbranched star polymeric ionic liquid and its application in a polymer electrolyte. Polym. Chem. 2017, 8, 3177–3185. [Google Scholar] [CrossRef]
- Working Group Phosphorus Chemistry. Gesellschaft Deutscher Chemiker e.V. Available online: www.gdch.de/phosphorchemie (accessed on 9 November 2022).
- Robles, H. Phosphorus; Academic Press: Oxford, UK, 2014; pp. 920–921. [Google Scholar]
- Pradel, M.; Aissani, L. Environmental impacts of phosphorus recovery from a ‘product’ Life Cycle Assessment perspective: Allocating burdens of wastewater treatment in the production of sludge-based phosphate fertilizers. Sci. Total Environ. 2019, 656, 55–69. [Google Scholar] [CrossRef]
- Bradford-Hartke, Z.; Lane, J.; Lant, P.; Leslie, G. Environmental Benefits and Burdens of Phosphorus Recovery from Municipal Wastewater. Environ. Sci. Technol. 2015, 49, 8611–8622. [Google Scholar] [CrossRef]
- Johansson, K.; Perzon, M.; Fröling, M.; Mossakowska, A.; Svanström, M. Sewage sludge handling with phosphorus utilization—Life cycle assessment of four alternatives. J. Clean. Prod. 2008, 16, 135–151. [Google Scholar] [CrossRef]
- Pound, B.G.; Cox, P.; Mortelmans, K.E. The Use of Quaternary Phosphonium Compounds as Antibacterial Corrosion Inhibitors for Low-Alloy Steel. Corrosion 2018, 74, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-Y.; Chen, Y.; Luo, D.I.; Li, J.X.; Chen, P.L.; Lee, S.; Fang, Z.; Li, W.Q.; Zhang, Y.Y.; Li, M.; et al. Black Phosphorus Mid-Infrared Light-Emitting Diodes Integrated with Silicon Photonic Waveguides. Nano Lett. 2020, 20, 6824–6830. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.F.; Diehl, H. A phosphorus pentoxide desiccant employing exfoliated vermiculite as carrier. Talanta 1962, 9, 84–85. [Google Scholar] [CrossRef]
- Kanat, M.; Eren, T. Synthesis of phosphorus-containing flame retardants and investigation of their flame retardant behavior in textile applications. J. Appl. Polym. Sci. 2019, 136, 47935. [Google Scholar] [CrossRef]
- Tuominen, M.; Karp, H.J.; Itkonen, S.T. Phosphorus-Containing Food Additives in the Food Supply—An Audit of Products on Supermarket Shelves. J. Ren. Nutr. 2022, 32, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pan, S.; Zhang, P.; Fan, H.; Chen, Y.; Yan, J. Synthesis and Application of Phosphorus-containing Flame Retardant Plasticizer for Polyvinyl Chloride. Fibers Polym. 2018, 19, 1057–1063. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, X.; Wei, X.; Kai, Z.; Xu, Y. Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication. Sci. Rep. 2021, 11, 23015. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Monge, S.; David, G. Phosphorus-Based Polymers; The Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Scharte, B. Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Rose, V.L.; Mastrotto, F.; Mantovani, G. Phosphonium polymers for gene delivery. Polym. Chem. 2017, 8, 353–360. [Google Scholar] [CrossRef]
- Suer, C.; Demir, C.; Unubol, N.A.; Yalcin, O.; Kocagoz, T.; Eren, T. Antimicrobial Activities of Phosphonium Containing. RSC Adv. 2017, 6, 86151–86157. [Google Scholar] [CrossRef]
- Moghadam, F.; Kamio, E.; Yoshioka, T.; Matsuyama, H. New approach for the fabrication of double-network ion-gel membranes with high CO2/N2 separation performance based on facilitated transport. J. Memb. Sci. 2017, 530, 166–175. [Google Scholar] [CrossRef]
- Hapiot, P.; Lagrost, C. Electrochemical Reactivity in Room-Temperature Ionic Liquids. Chem. Rev. 2008, 108, 2238–2264. [Google Scholar] [CrossRef] [PubMed]
- Sashmitha, K.; Rani, M.U. A Comprehensive Review of Polymer Electrolyte for Lithium-Ion Battery; Springer: Berlin/Heidelberg, Germany, 2022; p. 0123456789. [Google Scholar]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef]
- Xue, X.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Vincent, C.A. Applications of Electroactive Polymers; Scrosati, B., Ed.; Chapman and Hall: London, UK, 1993; p. 354. [Google Scholar] [CrossRef]
- Wang, W.M. Study on All Solid-State Composite Polymer Electrolyte. Adv. Mater. Res. 2012, 571, 13–16. [Google Scholar] [CrossRef]
- Qiao, B.; Leverick, G.M.; Zhao, W.; Flood, A.H.; Johnson, J.A.; Shao-Horn, Y. Supramolecular Regulation of Anions Enhances Conductivity and Transference Number of Lithium in Liquid Electrolytes. J. Am. Chem. Soc. 2018, 140, 10932–10936. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. The importance of the lithium ion transference number in lithium/polymer cells. Electrochim. Acta 1994, 39, 2073–2081. [Google Scholar] [CrossRef]
- Watanabe, M.; Nagano, S.; Sanui, K.; Ogata, N. Estimation of Li+ transport number in polymer electrolytes by the combination of complex impedance and potentiostatic polarization measurements. Solid State Ion. 1988, 28, 911–917. [Google Scholar] [CrossRef]
- Dudley, J.T.; Wilkinson, D.P.; Thomas, G.; LeVae, R.; Woo, S.; Blom, H.; Horvath, C.; Juzkow, M.W.; Denis, B.; Juric, P.; et al. Conductivity of electrolytes for rechargeable lithium batteries. J. Power Source 1991, 35, 59–82. [Google Scholar] [CrossRef]
- Aurbach, D.; Zaban, A.; Schechter, A.; Ein-Eli, Y.; Zinigrad, E.; Markovsky, B. The Study of Electrolyte Solutions Based on Ethylene and Diethyl Carbonates for Rechargeable Li Batteries: I. Li Metal Anodes. J. Electrochem. Soc. 1995, 142, 2873. [Google Scholar] [CrossRef]
- Huang, M.; Feng, S.; Zhang, W.; Giordano, L.; Chen, M.; Amanchukwu, C.V.; Anandakathir, R.; Shao-Horn, Y.; Johnson, J.A. Fluorinated Aryl Sulfonimide Tagged (FAST) salts: Modular synthesis and structure–property relationships for battery applications. Energy Environ. Sci. 2018, 11, 1326–1334. [Google Scholar] [CrossRef]
- Mathews, K.L.; Budgin, A.M.; Beeram, S.; Joenathan, A.T.; Stein, B.D.; Werner-Zwanziger, U.; Pink, M.; Baker, L.A.; Mahmoud, W.E.; Carini, J.P.; et al. Solid polymer electrolytes which contain tricoordinate boron for enhanced conductivity and transference numbers. J. Mater. Chem. A 2013, 1, 1108–1116. [Google Scholar] [CrossRef]
- Savoie, B.M.; Webb, M.A.; Miller, T.F. III Enhancing Cation Diffusion and Suppressing Anion Diffusion via Lewis-Acidic Polymer Electrolytes. J. Phys. Chem. Lett. 2017, 8, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C.; Peng, G.; Chen, X.; Yao, X.; Bai, Y.; Wu, F.; Chen, S.; Xu, X. A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries. J. Power Source 2016, 301, 47–53. [Google Scholar] [CrossRef]
- Safa, M.; Chamaani, A.; Chawla, N.; El-Zahab, B. Polymeric Ionic Liquid Gel Electrolyte for Room Temperature Lithium Battery Applications. Electrochim. Acta 2016, 213, 587–593. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Ren, Y.Y.; Hu, Y.; Li, L.; Yan, F. Ionic Liquid/Poly(ionic liquid)-based Semi-solid State Electrolytes for Lithium-ion Batteries. Chinese J. Polym. Sci. 2020, 38, 506–513. [Google Scholar] [CrossRef]
- Leclère, M.; Bernard, L.; Livi, S.; Bardet, M.; Guillermo, A.; Picard, L.; Duchet-Rumeau, J. Gelled electrolyte containing phosphonium ionic liquids for lithium-ion batteries. Nanomaterials 2018, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Shalu; Balo, L.; Singh, V.K.; Chaurasia, S.K.; Singh, R.K. Effect of phosphonium based ionic liquid on structural, electrochemical and thermal behaviour of polymer poly(ethylene oxide) containing salt lithium bis (trifluoromethylsulfonyl) imide. RSC Adv. 2016, 6, 87878–87887. [Google Scholar] [CrossRef]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent Progress of the Solid-State Electrolytes for High-Energy Metal-Based Batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Li, H.; Wu, H.B. Recent Progress of Hybrid Solid-State Electrolytes for Lithium Batteries. Chem. A Eur. J. 2018, 24, 18293–18306. [Google Scholar] [CrossRef]
- Cai, T.J.; Lo, Y.H.; Wu, J.J. Porous sulfide scaffolded solvent-free PEG-Ti hybrid polymer: All-solution-processed thin film composite polymer electrolytes directly on electrodes for lithium-ion batteries. Mater. Today Energy 2019, 13, 119–124. [Google Scholar] [CrossRef]
- Wu, N.; Chien, P.H.; Li, Y.; Dolocan, A.; Xu, H.; Xu, B.; Grundish, N.S.; Jin, H.; Hu, Y.-Y.; Goodenough, J.B. Fast Li+ Conduction Mechanism and Interfacial Chemistry of a NASICON/Polymer Composite Electrolyte. J. Am. Chem. Soc. 2020, 142, 2497–2505. [Google Scholar] [CrossRef]
- Bi, J.; Mu, D.; Wu, B.; Fu, J.; Yang, H.; Mu, G.; Zhang, L.; Wu, F. A hybrid solid electrolyte Li0.33La0.557TiO3/poly(acylonitrile) membrane infiltrated with a succinonitrile-based electrolyte for solid state lithium-ion batteries. J. Mater. Chem. A 2020, 8, 706–713. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Z.; An, X.; Yoshida, A.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Bifunctional ionic liquid and conducting ceramic co-assisted solid polymer electrolyte membrane for quasi-solid-state lithium metal batteries. J. Memb. Sci. 2019, 586, 122–129. [Google Scholar] [CrossRef]
- Matsuura, S.; Shibata, M.; Han, J.; Fujii, K. Polymer Gel Electrolyte Prepared by ‘Salting-In’ Poly(ethylene glycol) into a Phosphonium-Based Ionic Liquid with a Lithium Salt. ACS Appl. Polym. Mater. 2020, 2, 1276–1282. [Google Scholar] [CrossRef]
- Yunis, R.; Girard, G.M.; Wang, X.; Zhu, H.; Bhattacharyya, A.J.; Howlett, P.; MacFarlane, D.R.; Forsyth, M. The anion effect in ternary electrolyte systems using poly(diallyldimethylammonium) and phosphonium-based ionic liquid with high lithium salt concentration. Solid State Ion. 2018, 327, 83–92. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Marceneiro, S.; Johansen, H.D.; Barsan, M.M.; Brett, C.M.A.; de Sousa, H.C. Phosphonium ionic liquids as greener electrolytes for poly(vinyl chloride)-based ionic conducting polymers. RSC Adv. 2016, 6, 88979–88990. [Google Scholar] [CrossRef]
- Muthupradeepa, R.; Sivakumar, M.; Subadevi, R.; Suriyanarayanan, V.; Ramachandran, M.; Rajkumar, P.; Yuvakkumar, R. Physical and electrochemical chattels of phosphonium ionic liquid-based solid and gel-polymer electrolyte for lithium secondary batteries. J. Mater. Sci. Mater. Electron. 2020, 31, 22933–22944. [Google Scholar] [CrossRef]
- Pringle, J.M.; MacFarlane, D.R.; Forsyth, M. Solid state NMR analysis of polypyrrole grown in a phosphonium ionic liquid. Synth. Met. 2005, 155, 684–689. [Google Scholar] [CrossRef]
- Soares, B.G.; Nascimento, M.R.S.; Sena, A.S.; Indrusiak, T.; Souto, L.F.C.; Pontes, K. Polyaniline co-doped with dodecyl benzene sulfonic acid and zwitterionic-based ionic liquids prepared by inverse emulsion polymerization. Synth. Met. 2020, 266, 116435. [Google Scholar] [CrossRef]
- Soares, B.G.; Silva, A.A.; Pereira, J.; Livi, S. Preparation of epoxy/jeffamine networks modified with phosphonium based ionic liquids. Macromol. Mater. Eng. 2015, 300, 312–319. [Google Scholar] [CrossRef]
- Tsuchida, Y.; Matsumiya, M.; Tsunashima, K. Preparation of polymer electrolytes using ionic liquids and evaluation of physicochemical properties. J. Mol. Liq. 2019, 274, 204–208. [Google Scholar] [CrossRef]
- Eftekhari, A.; Saito, T. Synthesis and properties of polymerized ionic liquids. Eur. Polym. J. 2017, 90, 245–272. [Google Scholar] [CrossRef]
- Lee, S.; Ringstrand, B.S.; Stone, D.A.; Firestone, M.A. Electrochemical Activity of Glucose Oxidase on a Poly(ionic liquid)–Au Nanoparticle Composite. ACS Appl. Mater. Interfaces 2012, 4, 2311–2317. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Y.-N.; Luo, J.-S.; Luo, Z.-H. Poly(ionic liquid)s-based nanocomposite polyelectrolytes with tunable ionic conductivity prepared via SI-ATRP. Polym. Chem. 2014, 5, 882–891. [Google Scholar] [CrossRef]
- Szczęsna-Chrzan, A.; Marczewski, M.; Syzdek, J.; Kochaniec, M.K.; Smoliński, M.; Marcinek, M. Lithium polymer electrolytes for novel batteries application: The review perspective. Appl. Phys. A Mater. Sci. Process. 2023, 129, 37. [Google Scholar] [CrossRef]
- Hatakeyama, E.S.; Ju, H.; Gabriel, C.J.; Lohr, J.L.; Bara, J.E.; Noble, R.D.; Freeman, B.D.; Gin, D.L. New protein-resistant coatings for water filtration membranes based on quaternary ammonium and phosphonium polymers. J. Memb. Sci. 2009, 330, 104–116. [Google Scholar] [CrossRef]
- Parent, J.S.; Penciu, A.; Guillén-Castellanos, S.A.; Liskova, A.; Whitney, R.A. Synthesis and characterization of isobutylene-based ammonium and phosphonium bromide ionomers. Macromolecules 2004, 37, 7477–7483. [Google Scholar] [CrossRef]
- Sanoja, G.E.; Popere, B.C.; Beckingham, B.S.; Evans, C.M.; Lynd, N.A.; Segalman, R.A. Structure–Conductivity Relationships of Block Copolymer Membranes Based on Hydrated Protic Polymerized Ionic Liquids: Effect of Domain Spacing. Macromolecules 2016, 49, 2216–2223. [Google Scholar] [CrossRef]
- Wang, S.-W.; Liu, W.; Colby, R.H. Counterion Dynamics in Polyurethane-Carboxylate Ionomers with Ionic Liquid Counterions. Chem. Mater. 2011, 23, 1862–1873. [Google Scholar] [CrossRef]
- Bamford, D.; Dlubek, G.; Lüpke, T.; Kilburn, D.; Stejny, J.; Menke, T.J.; Alam, M.A. Free volume, glass transition and degree of branching in ethylene/α-olefin copolymers: Positron lifetime, differential scanning calorimetry, wide-angle X-ray scattering, and density studies. Macromol. Chem. Phys. 2006, 207, 492–502. [Google Scholar] [CrossRef]
- Kusuma, V.A.; Freeman, B.D.; Borns, M.A.; Kalika, D.S. Influence of chemical structure of short chain pendant groups on gas transport properties of cross-linked poly(ethylene oxide) copolymers. J. Memb. Sci. 2009, 327, 195–207. [Google Scholar] [CrossRef]
- Kusuma, V.A.; Freeman, B.D.; Smith, S.L.; Heilman, A.L.; Kalika, D.S. Influence of TRIS-based co-monomer on structure and gas transport properties of cross-linked poly(ethylene oxide). J. Memb. Sci. 2010, 359, 25–36. [Google Scholar] [CrossRef]
- La Cruz, D.S.; Green, M.D.; Ye, Y.; Elabd, Y.A.; Long, T.E.; Winey, K.I. Correlating backbone-to-backbone distance to ionic conductivity in amorphous polymerized ionic liquids. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 338–346. [Google Scholar] [CrossRef]
- Isik, M.; Porcarelli, L.; Lago, N.; Zhu, H.; Forsyth, M.; Mecerreyes, D. Proton Conducting Membranes Based on Poly(Ionic Liquids) Having Phosphonium Counter-Cations. Macromol. Rapid Commun. 2018, 39, 1–7. [Google Scholar] [CrossRef]
- Schultz, A.R.; Lambert, P.M.; Chartrain, N.A.; Ruohoniemi, D.M.; Zhang, Z.; Jangu, C.; Zhang, M.; Williams, C.B.; Long, T.E. 3D printing phosphonium ionic liquid networks with mask projection microstereolithography. ACS Macro Lett. 2014, 3, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Li, D.; Cui, X.; Wang, L.; Li, L.; Wang, K. Electrochemical Impedance Spectroscopy: A New Chapter in the Fast and Accurate Estimation of the State of Health for Lithium-Ion Batteries. Energies 2023, 16, 1599. [Google Scholar] [CrossRef]
- Fan, F.; Wang, W.; Holt, A.P.; Feng, H.; Uhrig, D.; Lu, X.; Hong, T.; Wang, Y.; Kang, N.-G.; Mays, J.; et al. Effect of Molecular Weight on the Ion Transport Mechanism in Polymerized Ionic Liquids. Macromolecules 2016, 49, 4557–4570. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, F.; Agapov, A.L.; Yu, X.; Hong, K.; Mays, J.; Sokolov, A.P. Design of superionic polymers—New insights from Walden plot analysis. Solid State Ion. 2014, 262, 782–784. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Huang, A.-C.; Tang, Y.; Yang, Y.-P.; Liu, Y.-C.; Li, Z.-P.; Zhou, H.-L.; Huang, C.-F.; Xing, Z.-X.; Shu, C.-M.; et al. Thermal Effect and Mechanism Analysis of Flame-Retardant Modified Polymer Electrolyte for Lithium-Ion Battery. Polymers 2021, 13, 1675. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, F.; Sun, N.; Wu, A.; Pan, W.; Zheng, L. Single lithium-ion polymer electrolytes based on poly(ionic liquid)s for lithium-ion batteries. Soft Matter 2018, 14, 6313–6319. [Google Scholar] [CrossRef]
- Naudin, C.; Bruneel, J.L.; Chami, M.; Desbat, B.; Grondin, J.; Lassègues, J.C.; Servant, L. Characterization of the lithium surface by infrared and Raman spectroscopies. J. Power Source 2003, 124, 518–525. [Google Scholar] [CrossRef]
- Liang, Z.; Xiang, Y.; Wang, K.; Zhu, J.; Jin, Y.; Wang, H.; Zheng, B.; Chen, Z.; Tao, M.; Liu, X.; et al. Understanding the failure process of sulfide-based all-solid-state lithium batteries via operando nuclear magnetic resonance spectroscopy. Nat. Commun. 2023, 14, 259. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, C.; Wang, S.; Wang, J.; Liu, M.; Ganapathy, S.; Bai, X.; Li, B.; Wagemaker, M. Clarifying the Relationship between the Lithium Deposition Coverage and Microstructure in Lithium Metal Batteries. J. Am. Chem. Soc. 2022, 144, 21961–21971. [Google Scholar] [CrossRef]
- Ruan, Z.; Du, Y.; Pan, H.; Zhang, R.; Zhang, F.; Tang, H.; Zhang, H. Incorporation of Poly(Ionic Liquid) with PVDF-HFP-Based Polymer Electrolyte for All-Solid-State Lithium-Ion Batteries. Polymers 2022, 14, 1950. [Google Scholar] [CrossRef]
- Liao, C. Batteries. In Materials Principles and Characterization Methods; IOP Publishing: Bristol, UK, 2021. [Google Scholar] [CrossRef]
- Maia, B.A.; Magalhães, N.; Cunha, E.; Braga, M.H.; Santos, R.M.; Correia, N. Designing Versatile Polymers for Lithium-Ion Battery Applications: A Review. Polymers 2022, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Zhang, Y.; Dong, S.; Chen, Y.; Zhang, S. Poly(ionic liquid)s Containing Alkoxy Chains and Bis(trifluoromethanesulfonyl)imide Anions as Highly Adhesive Materials. Adv. Mater. 2021, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Lee, Y.M. Anion exchange polyelectrolytes for membranes and ionomers. Prog. Polym. Sci. 2021, 113, 101345. [Google Scholar] [CrossRef]
- Tokuda, H.; Ishii, K.; Susan, M.A.B.H.; Tsuzuki, S.; Hayamizu, K.; Watanabe, M. Physicochemical properties and structures of room-temperature ionic liquids. 3. Variation of cationic structures J. Phys. Chem. B 2006, 110, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Conrad, O.; Vankelecom, I.F.J. Physicochemical properties of phosphonium-based and ammonium-based protic ionic liquids. J. Mater. Chem. 2012, 22, 20574–20579. [Google Scholar] [CrossRef]
- Liang, S.; O’Reilly, M.V.; Choi, U.H.; Shiau, H.S.; Bartels, J.; Chen, Q.; Runt, J.; Winey, K.I.; Colby, R.H. High ion content siloxane phosphonium ionomers with very low Tg. Macromolecules 2014, 47, 4428–4437. [Google Scholar] [CrossRef]
- Choi, U.H.; Mittal, A.; Price, T.L.; Gibson, H.W.; Runt, J.; Colby, R.H. Polymerized ionic liquids with enhanced static dielectric constants. Macromolecules 2013, 46, 1175–1186. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, M.; Wu, T.; Hemp, S.T.; Mather, B.D.; Moore, R.B.; Long, T.E. Ionic aggregation in random copolymers containing phosphonium ionic liquid monomers. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 166–173. [Google Scholar] [CrossRef]
- Schultz, A.R.; Fahs, G.B.; Jangu, C.; Chen, M.; Moore, R.B.; Long, T.E. Phosphonium-containing diblock copolymers from living anionic polymerization of 4-diphenylphosphino styrene. Chem. Commun. 2016, 52, 950–953. [Google Scholar] [CrossRef]
- Miralles-Comins, S.; Zanatta, M.; Sans, V. Advanced Formulations Based on Poly(ionic liquid) Materials for Additive Manufacturing. Polymers 2022, 14, 5121. [Google Scholar] [CrossRef]
- Kesküla, A.; Peikolainen, A.L.; Kilmartin, P.A.; Kiefer, R. Solvent effect in imidazole-based poly(Ionic liquid) membranes: Energy storage and sensing. Polymers 2021, 13, 3466. [Google Scholar] [CrossRef] [PubMed]
- Ramnial, T.; Taylor, S.A.; Bender, M.L.; Gorodetsky, B.; Lee, P.T.; Dickie, D.A.; McCollum, B.M.; Pye, C.C.; Walsby, C.J.; Clyburne, J.A.C. Carbon-Centered Strong Bases in Phosphonium Ionic Liquids. J. Org. Chem. 2008, 73, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Zanger, M.; Werf, C.A.V.; McEwen, W.E. Kinetic study of the decomposition of quaternary phosphonium hydroxides. J. Am. Chem. Soc. 1959, 81, 3806–3807. [Google Scholar] [CrossRef]
- Sanoja, G.E.; Schauser, N.S.; Bartels, J.M.; Evans, C.M.; Helgeson, M.E.; Seshadri, R.; Segalman, R.A. Ion Transport in Dynamic Polymer Networks Based on Metal–Ligand Coordination: Effect of Cross-Linker Concentration. Macromolecules 2018, 51, 2017–2026. [Google Scholar] [CrossRef]
- RMorco, P.; Joseph, J.M.; Wren, J.C. The chemical stability of phosphonium-based ionic liquids under gamma irradiation. RSC Adv. 2015, 5, 28570–28581. [Google Scholar] [CrossRef]
- Yuan, C.; Sheldon, B.W.; Xu, J. Heterogeneous Reinforcements to Mitigate Li Penetration through Solid Electrolytes in All-Solid-State Batteries. Adv. Energy Mater. 2022, 12, 2201804. [Google Scholar] [CrossRef]
- Doan Tran, H.; Kim, C.; Chen, L.; Chandrasekaran, A.; Batra, R.; Venkatram, S.; Kamal, D.; Lightstone, J.P.; Gurnani, R.; Shetty, P.; et al. Machine-learning predictions of polymer properties with Polymer Genome. J. Appl. Phys. 2020, 128, 171104. [Google Scholar] [CrossRef]
- Liu, M.; Clement, C.; Liu, K.; Wang, X.; Sparks, T.D. A data science approach for advanced solid polymer electrolyte design. Comput. Mater. Sci. 2021, 187, 110108. [Google Scholar] [CrossRef]
- Hukari, S.; Hermann, L.; Nättorp, A. From wastewater to fertilisers—Technical overview and critical review of European legislation governing phosphorus recycling. Sci. Total Environ. 2016, 542, 1127–1135. [Google Scholar] [CrossRef]
- Manzardo, A.; Marson, A.; Roso, M.; Boaretti, C.; Modesti, M.; Scipioni, A.; Lorenzetti, A. Life Cycle Assessment Framework to Support the Design of Biobased Rigid Polyurethane Foams. ACS Omega 2019, 4, 14114–14123. [Google Scholar] [CrossRef]

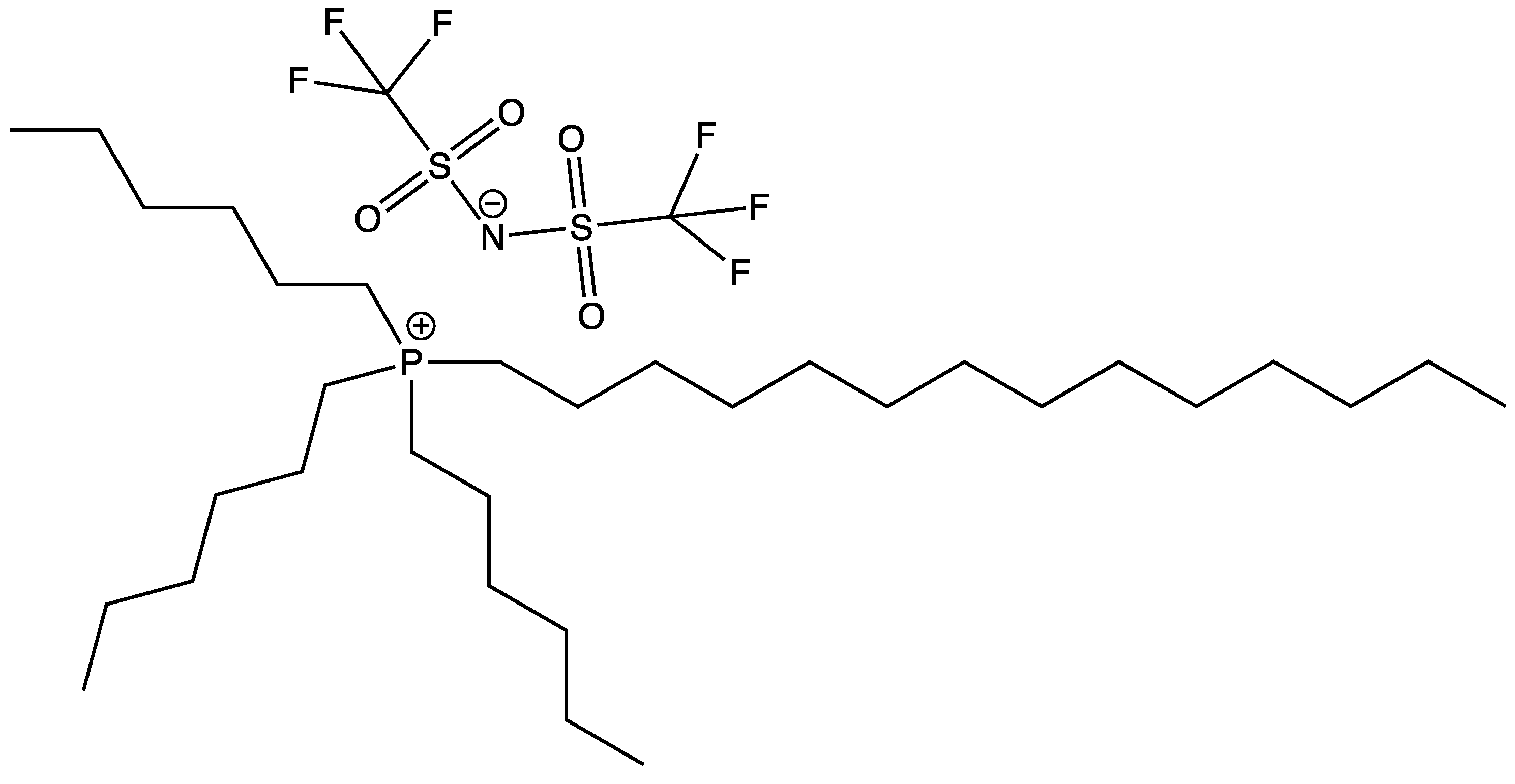
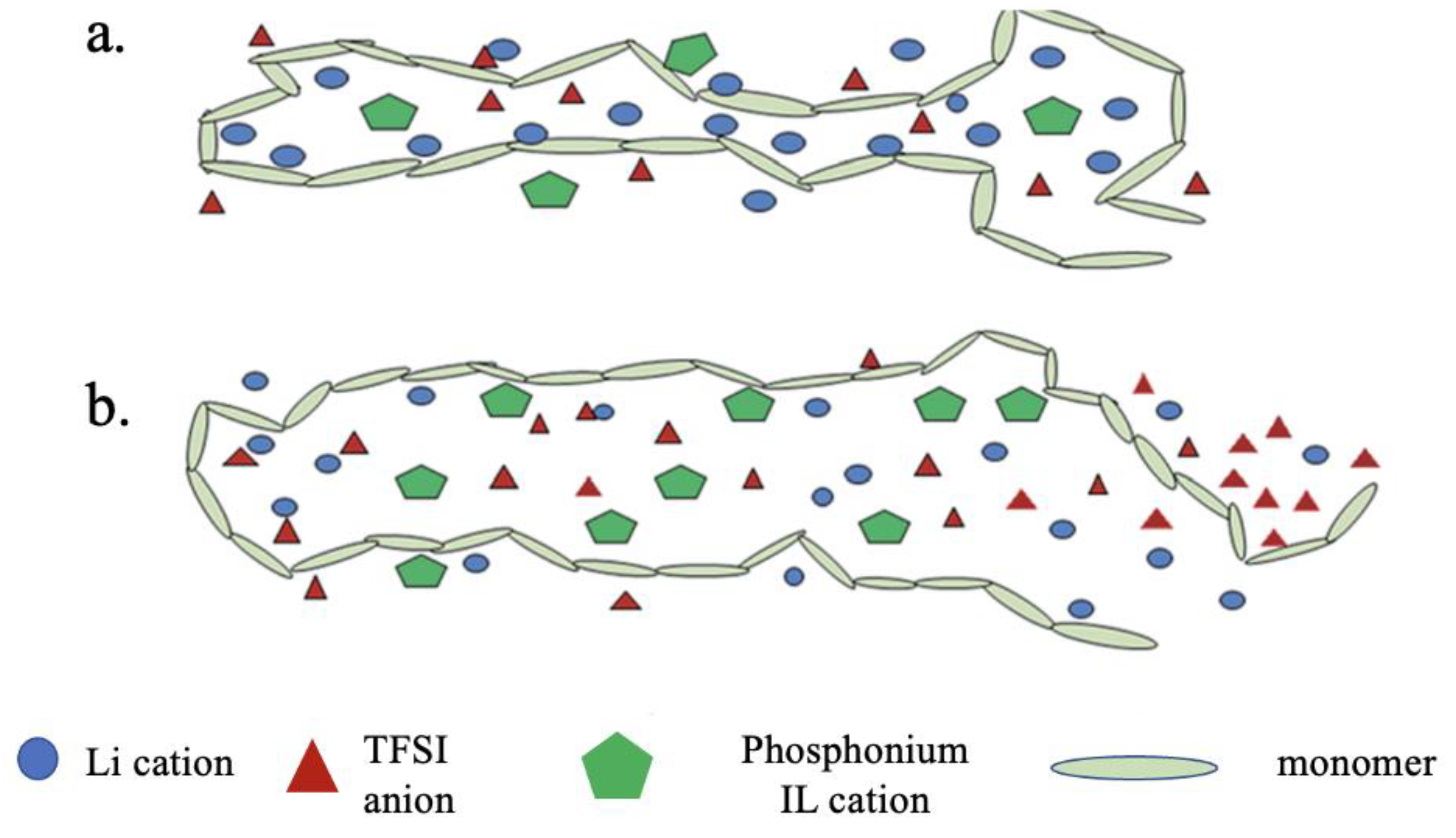
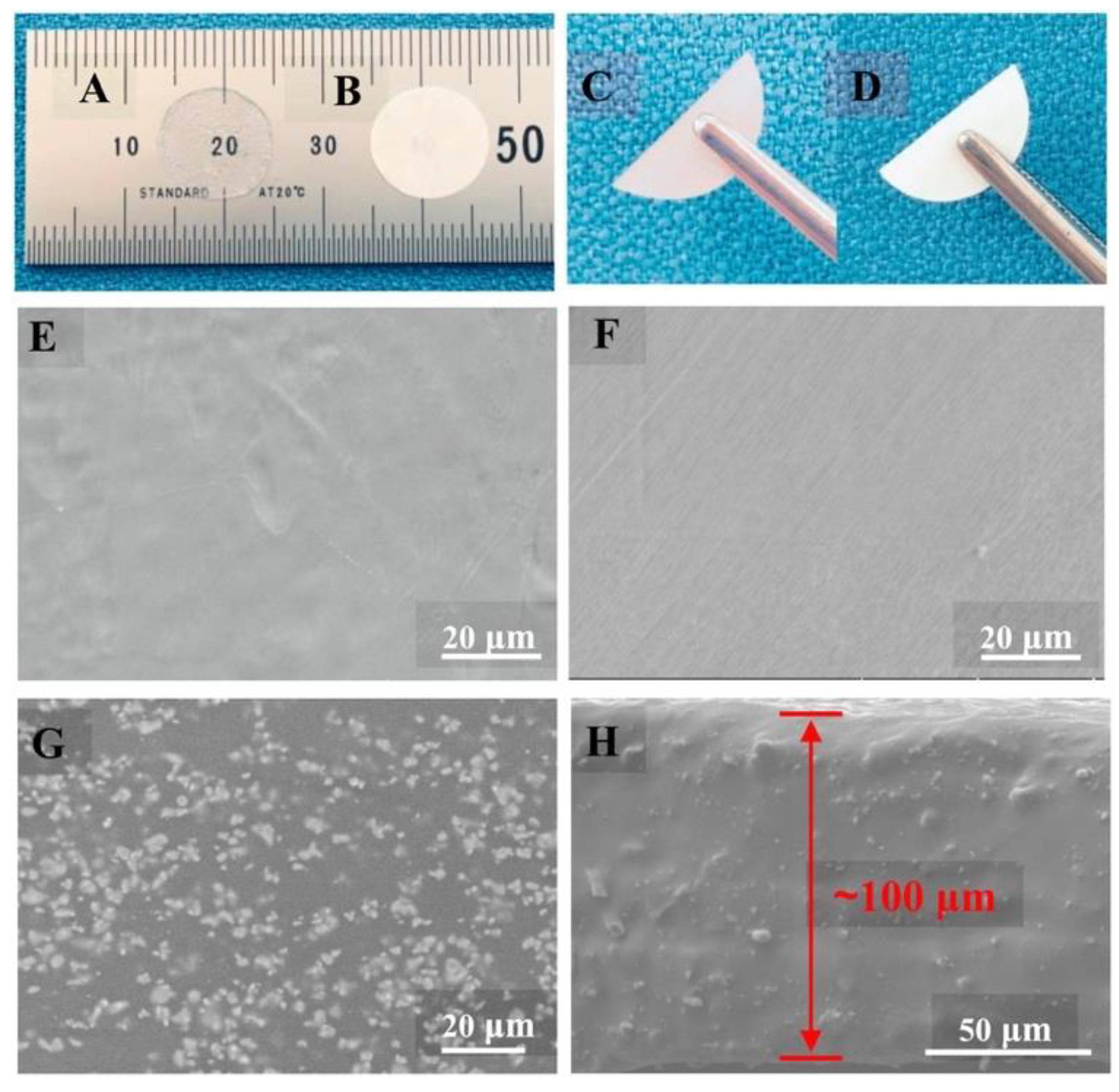
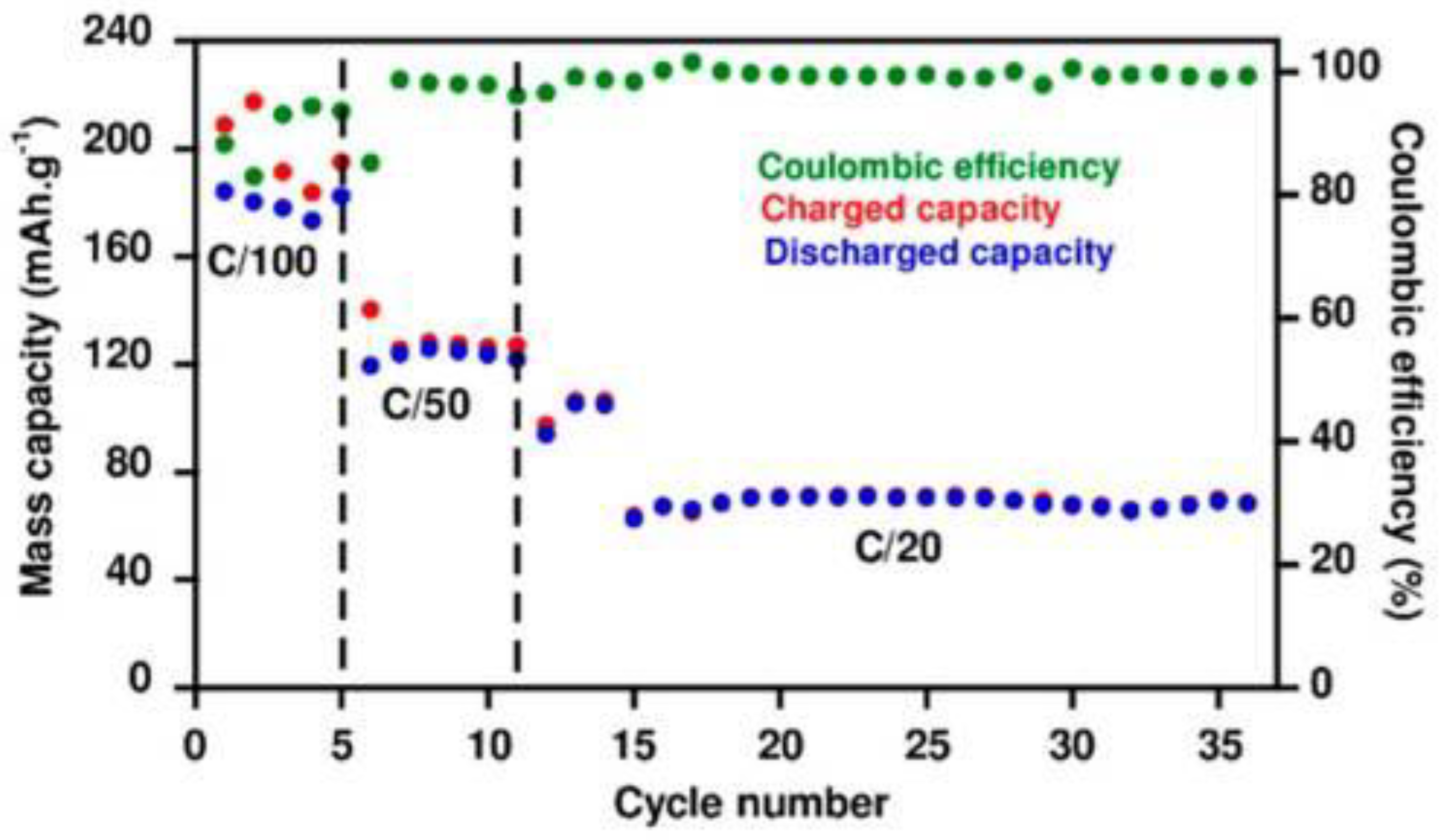
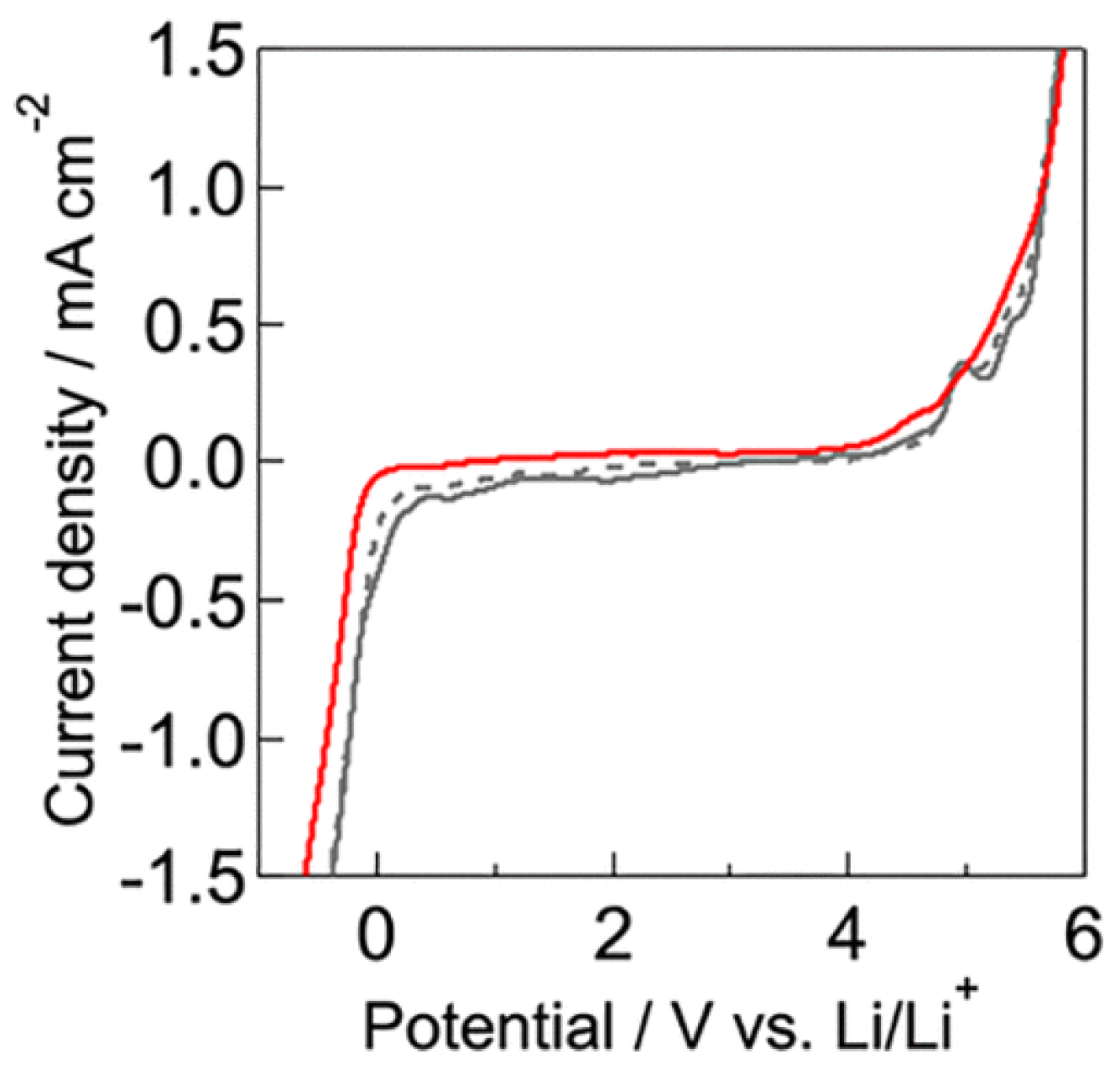

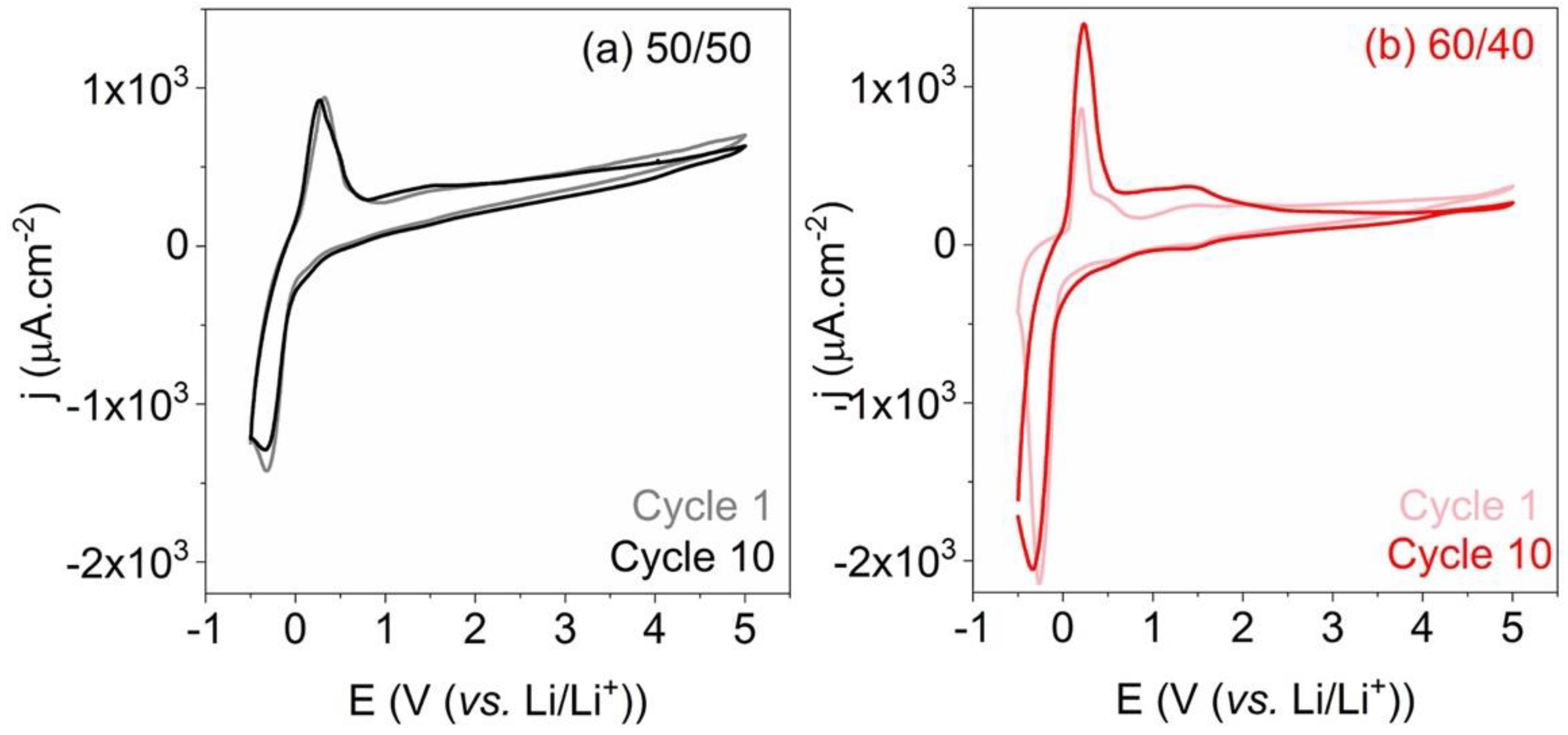
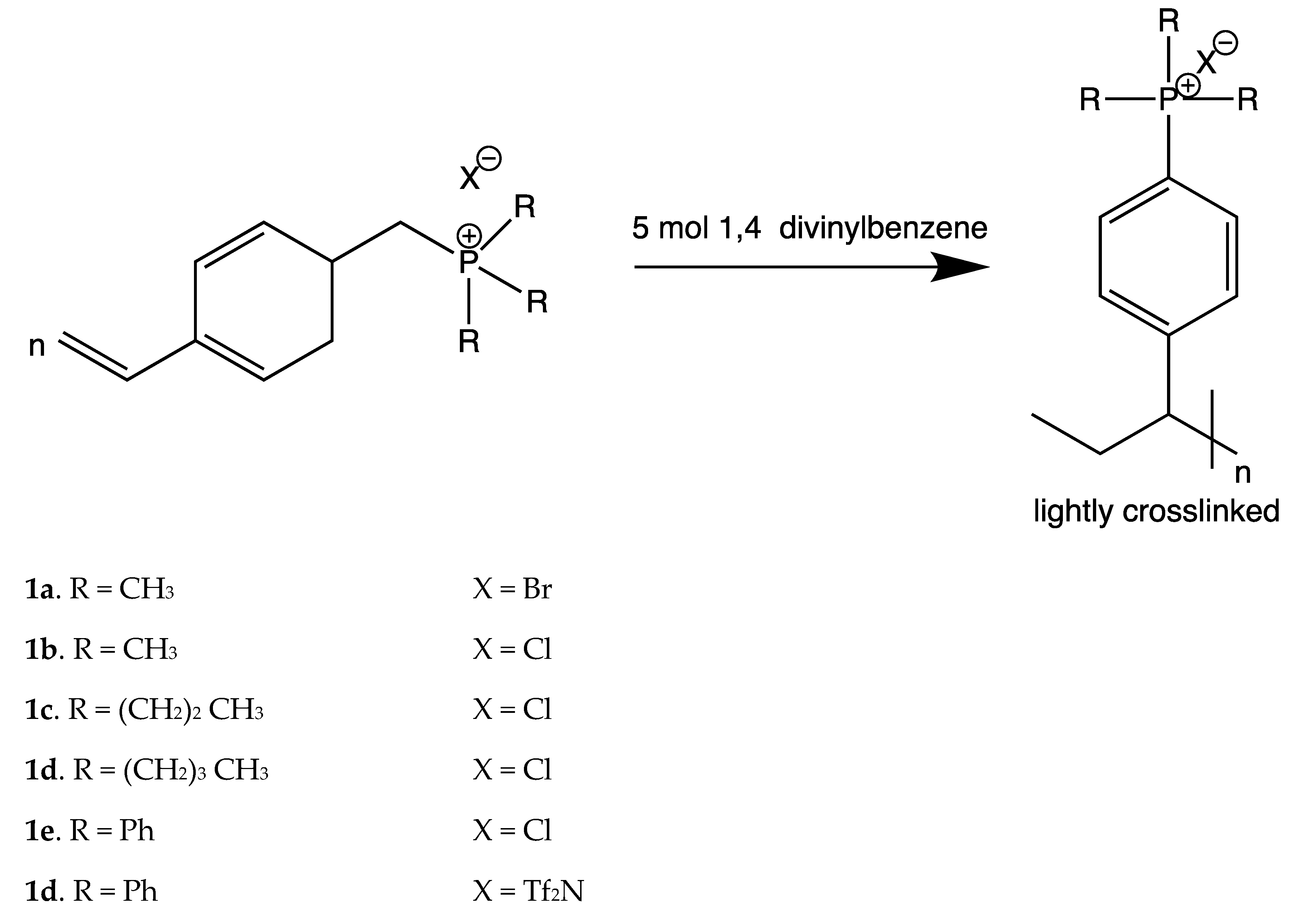

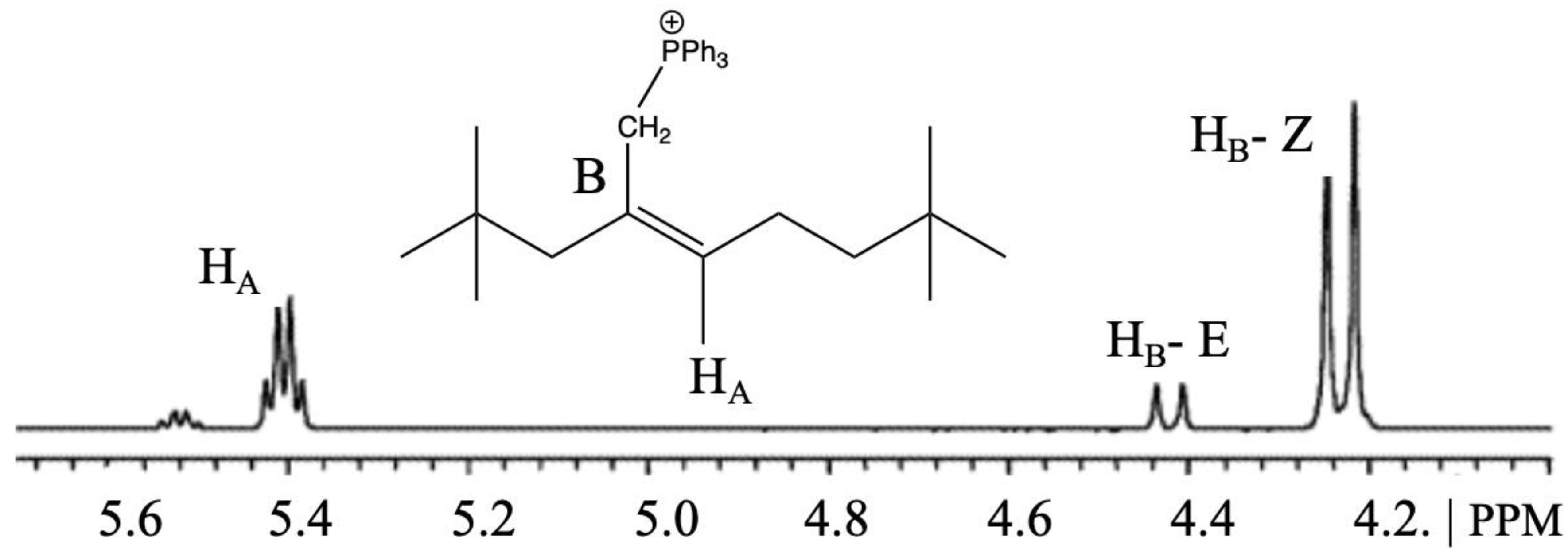
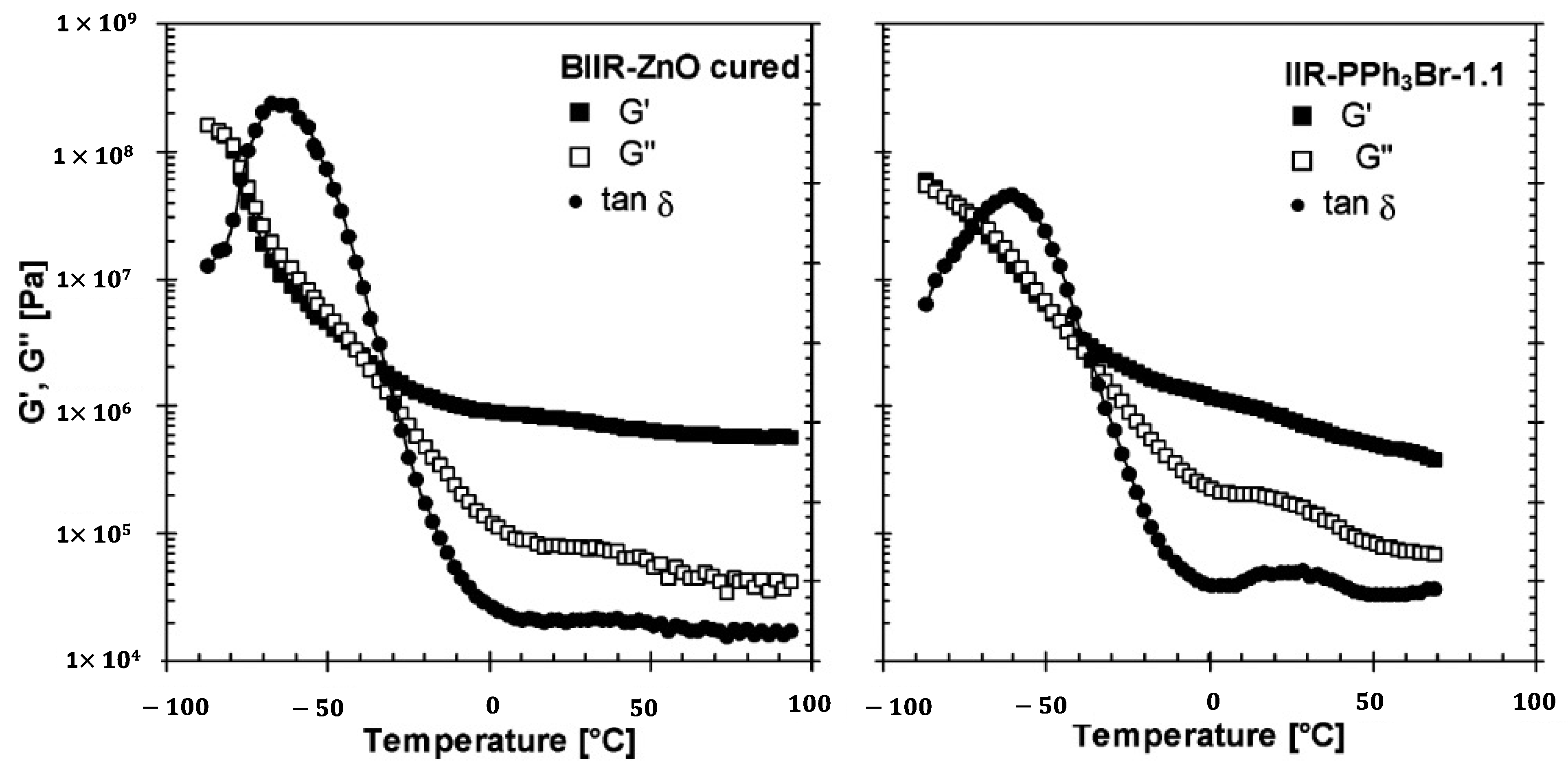
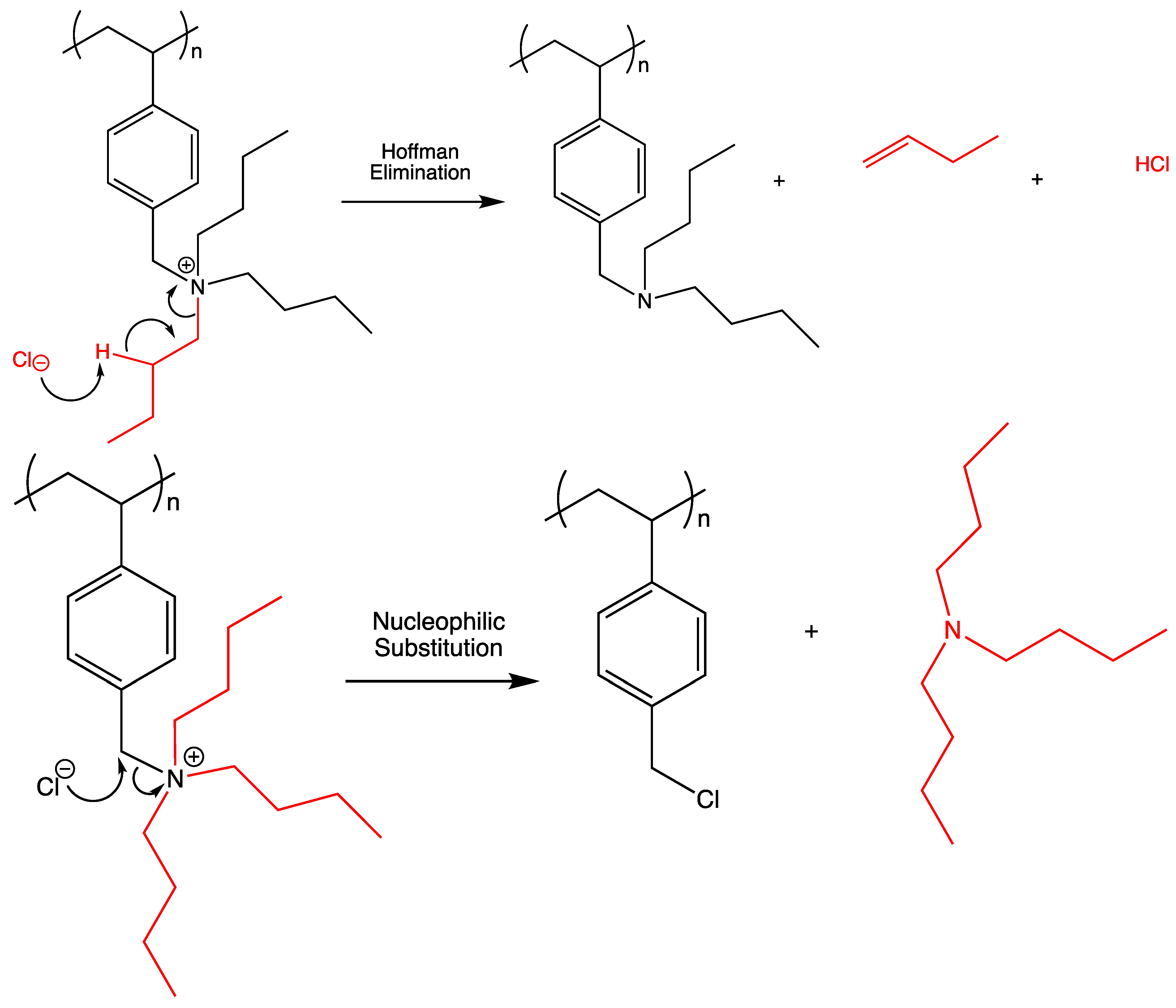



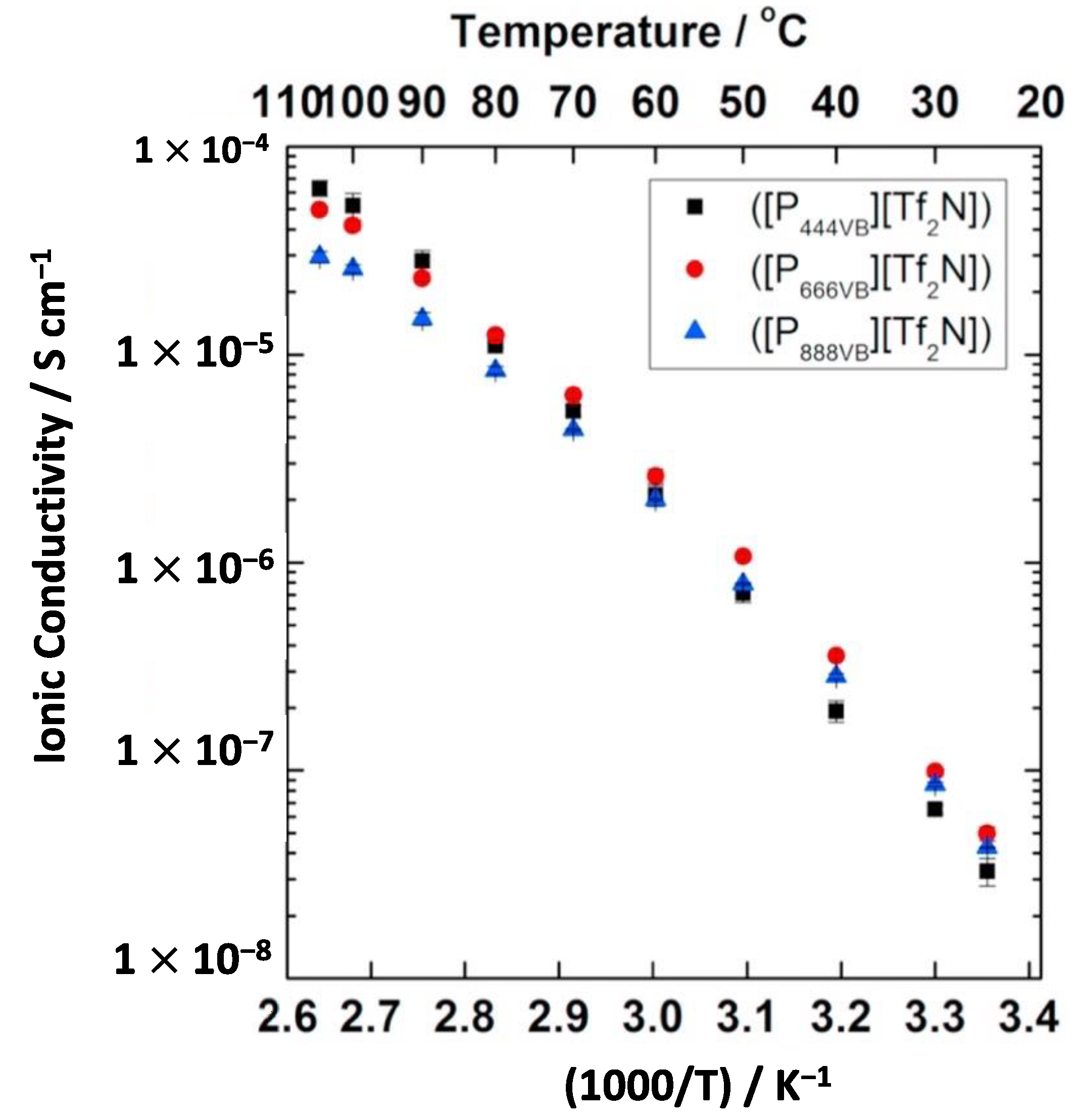
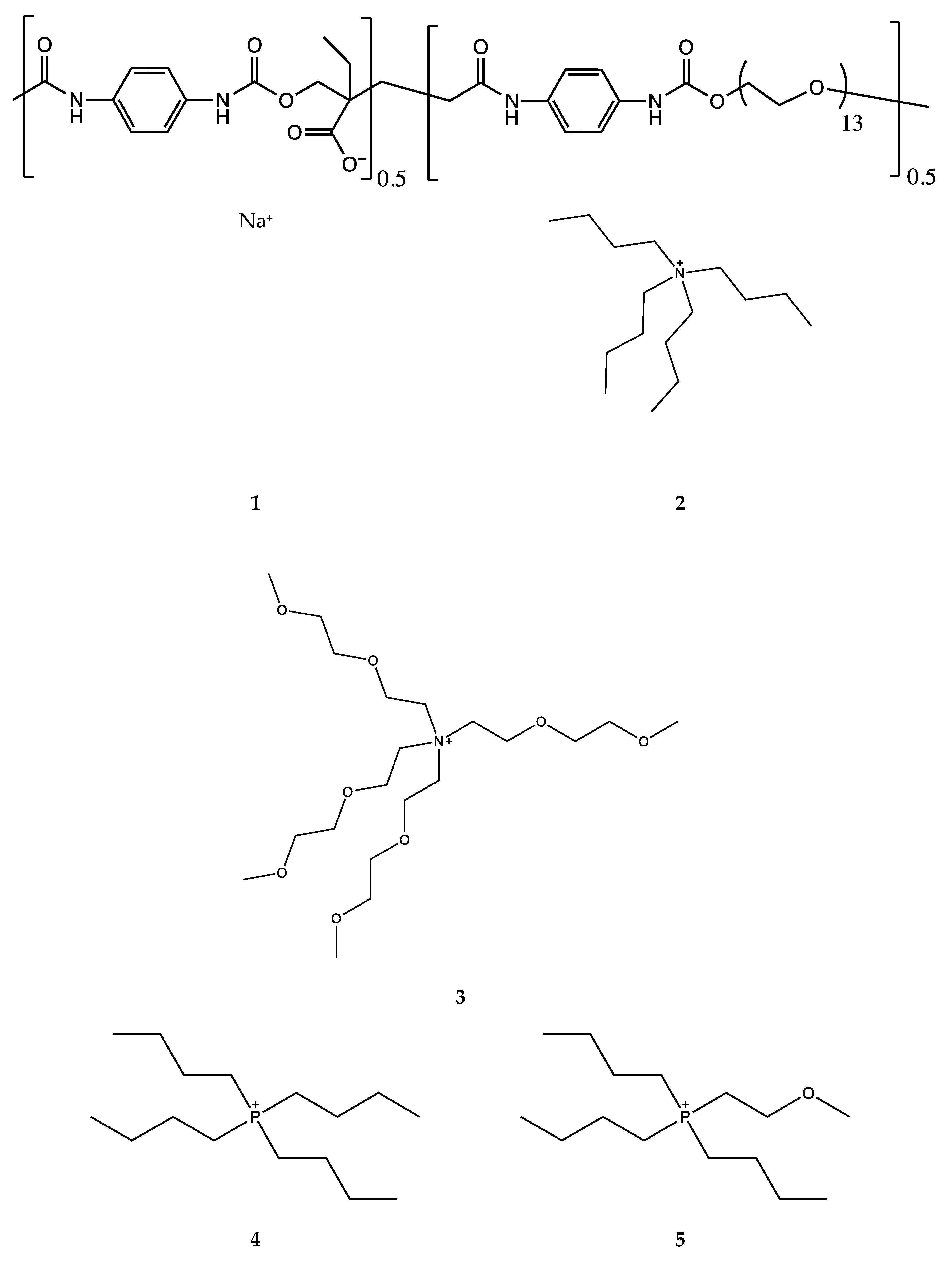
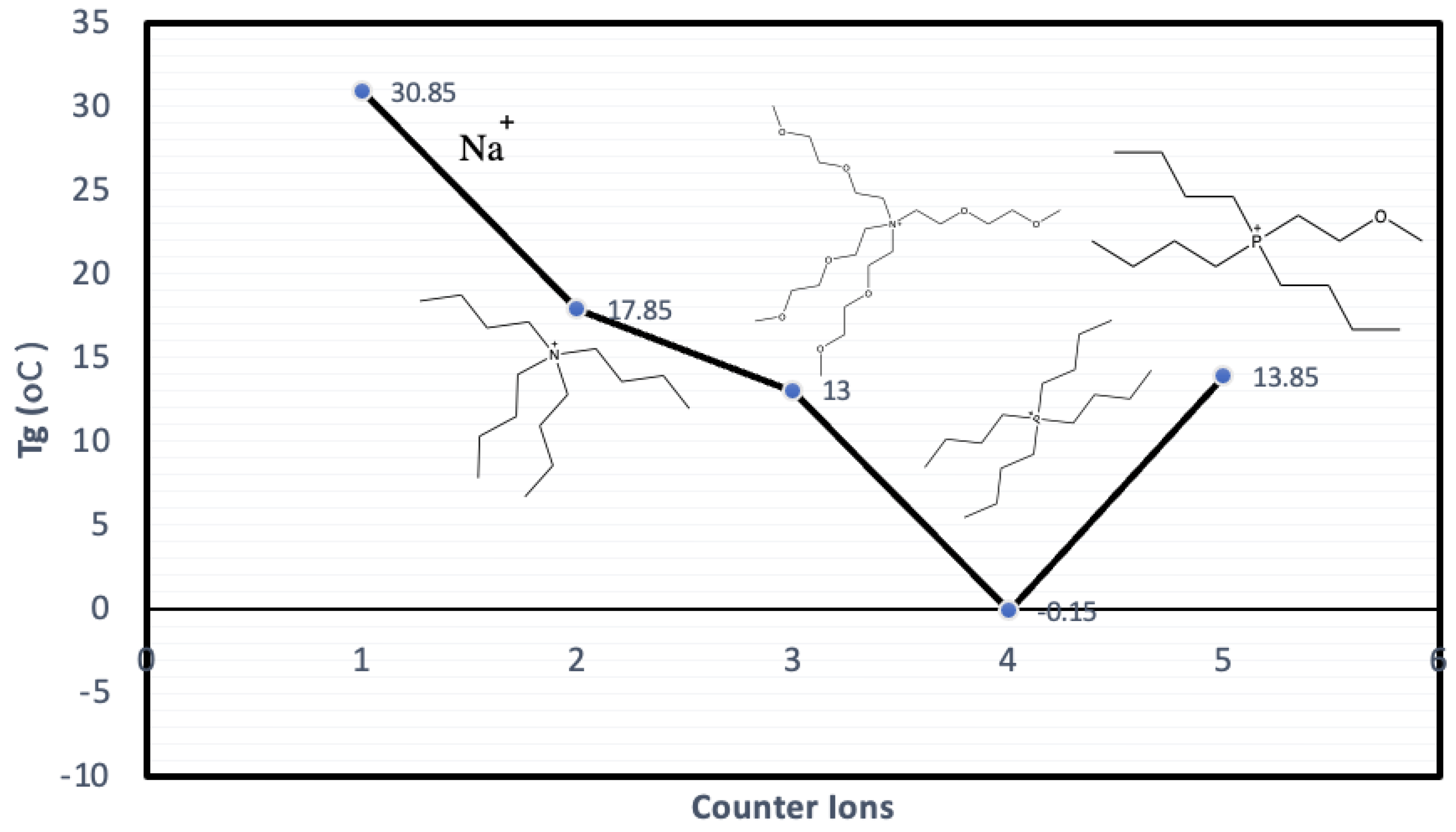
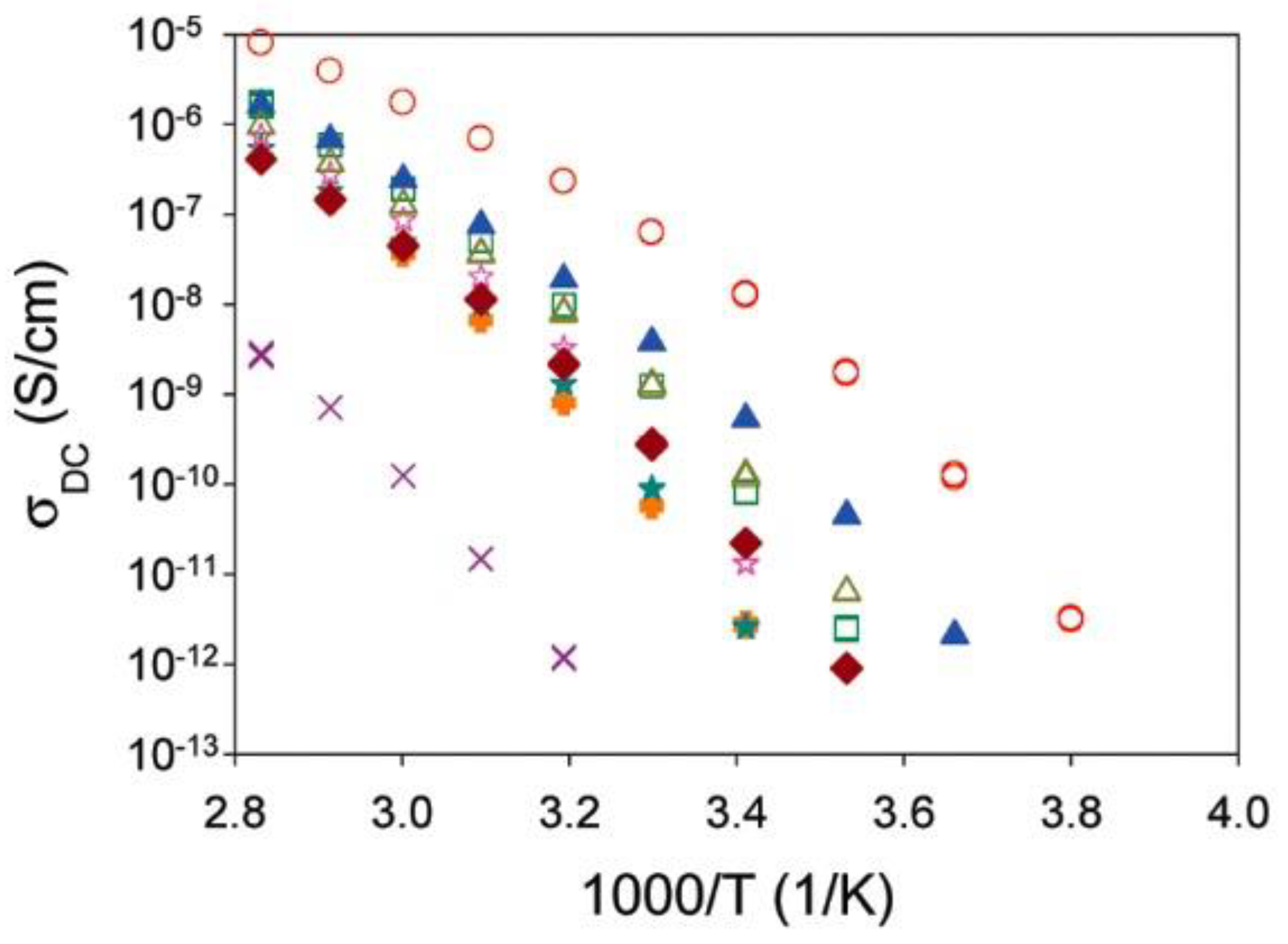

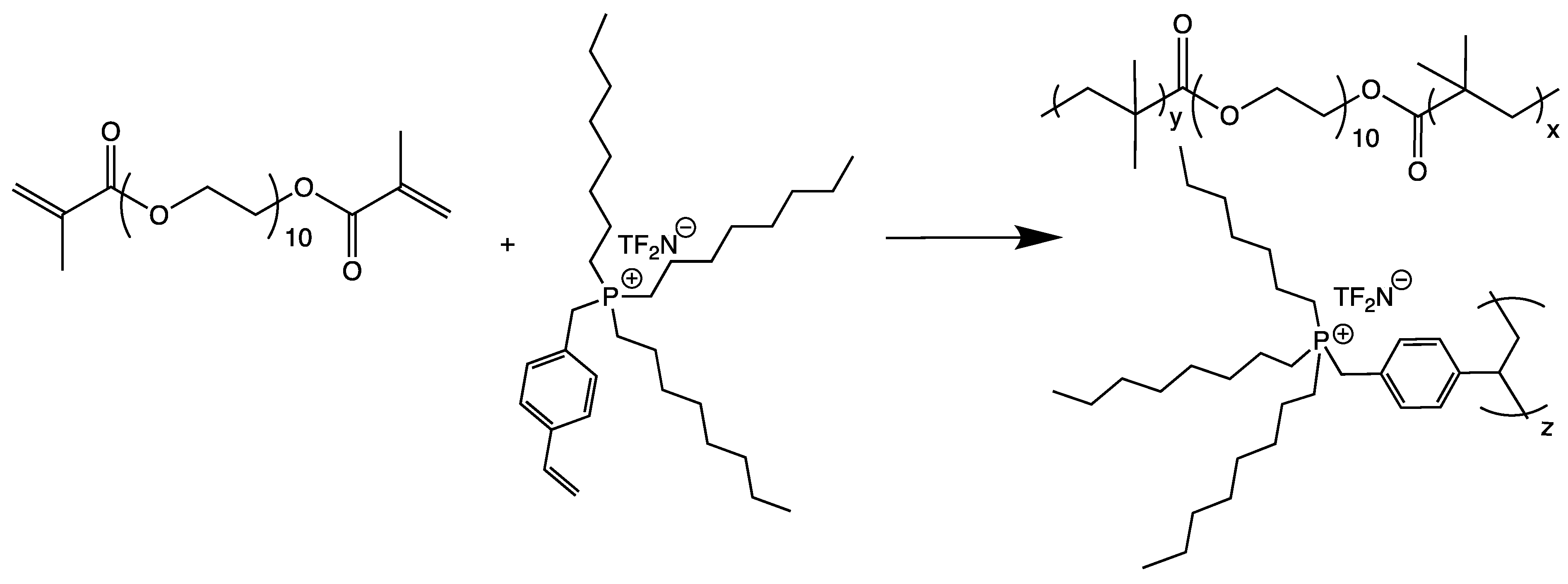
| Doped Phosphonium Ionic Liquid | Polymer Host | Conductivity Value (S cm−1) | Electrochemical Performance (V vs. Li0/Li+) | Ref. |
|---|---|---|---|---|
| trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)imide, (P14,6,6,6)(Tf2N) | Polypropylene glycol (PPO) | 0.13 × 10−3 at 100 °C | 3.95 | [69] |
| trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)imide, (P14,6,6,6)(Tf2N) | Polyethylene oxide (PEO) | 4.2 ×10−5 at ambient | 3.34 | [70] |
| trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)imide, (P14,6,6,6)(Tf2N) | Poly(vinyl chloride) (PVC) | 2.4 × 10−6 at ambient | N.A | [79] |
| trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl) amide (P14,6,6,6)(Tf2N) | Poly(vinylidenefluoride-co-hexafluoropropylene) P(VdF-co-HFP) | 3.2 × 10−6 at ambient | 3.4 | [80] |
| trimethylisobutylphosphonium bis(fluorosulfonyl)imide (P111i4FSI) | Poly(diallyldimethylammonium) (PDADMA) | 0.49 × 10 −3 at 40 °C | 5.0 | [78] |
| trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl) amide (P14,6,6,6) (Tf2N) | Polypyrrole | 0.6 at ambient | - | [81] |
| triphenylphosphonium-butyl sulfonate (PPh3.ZIL)/dodecylbenzenesulfonic acid | Polyaniline (PANI) | 4.9 at ambient | - | [82] |
| triethylpentylphosphonium bis(trifluoromethanesulfonyl)amide, (P2225) (Tf2N)) | Tetra-arm poly (ethylene glycol) (TetraPEG) | 2.78 × 10−1 at ambient | 4.2 | [77] |
| trihexyltetradecylphosphonium dicyanamide (P14,6,6,6)(DCA) | Diglycidyl ether of bisphenol A (DGEBA)-based epoxy | ~10−6 at room temperature | - | [83] |
| [P4441][Tf2N] | Polymethylmethacrylate (PMMA) | 7.94 × 10−5 at 30 °C | - | [84] |
| [P2225][Tf2N] | Polymethylmethacrylate (PMMA) | 1.46 × 10−4 at 30 °C | - | [84] |
| Used Phosphonium Polymeric Ionic Liquid | Polymer Matrix | Conductivity (S cm−1) | Ref. |
|---|---|---|---|
| PAMPS HP+444 | 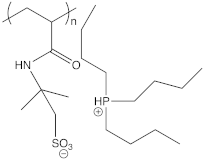 | 5 × 10−6 at 30 °C | [97] |
| [P(N(Me)Cy)4]+ polyethylene |  | 2.2 ×10−2 at 22 °C | [29] |
| [Bu4P+]polyurethane | 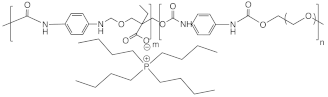 | ~10−6 at 100 °C | [93] |
| [Me3(MOE)P+] polyurethane |  | ~10−6 at 100 °C | [93] |
| 1,4bis(di-phenyl phosphine) butane 1,12 di-trifluoro sulfonyl imide dodecane | 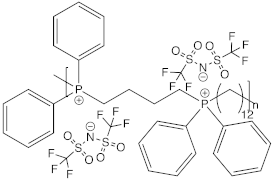 | 7 × 10−6 | [98] |
| PEO-grafted Siloxane Phosphonium Ionomers |  | 9 × 10−2 at ambient temperature. | [99] |
| PIL | Td (°C) | Tg (°C) | Conductivity (S cm−1) |
|---|---|---|---|
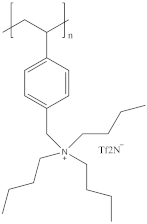 | 330 | 62 | 5.01 × 10−5 |
 | 437 | 66 | 1.6 × 10−4 |
| Center (P or N) | α—CH2 | β—CH2 | |
|---|---|---|---|
Bu4P+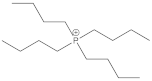 | 1.1 | −0.2 | 0 |
Bu4N+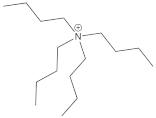 | −0.5 | 0.3 | 0 |
BuMeIm+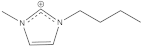 | −0.3, −0.3 | 0.4 | 0 |
| Samples | Anion | n | Tg (°C) | Conductivity at 30 °C (S cm−1) |
|---|---|---|---|---|
| PSPE-5Br(3) | Br− | 5 | −83 | 5.60 × 10−7 |
| PSPE-8Br(3) | 8 | −82 | 7.50 × 10−7 | |
| PSPE-11Br(3) | 11 | −80 | 6.88 × 10−7 | |
| PSPE-22Br(3) | 22 | −86 | 1.44 × 10−6 | |
| PSPE-5TFSI(3) | TFSI− | 5 | −81 | 1.09 × 10−5 |
| PSPE-8TFSI(3) | 8 | −81 | 3.12 × 10−5 | |
| PSPE-11TFSI(3) | 11 | −80 | 2.12 × 10−5 | |
| PSPE-5F(3) | F− | 5 | −80 | 1.9 × 10−7 |
| PSPE-8F(3) | 8 | −83 | 2.00 × 10−7 | |
| PSPE-11F(3) | 11 | −82 | 1.7 × 10−7 | |
| PSPE-22F(3) | 22 | −73 | 7.4 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misenan, M.S.M.; Hempelmann, R.; Gallei, M.; Eren, T. Phosphonium-Based Polyelectrolytes: Preparation, Properties, and Usage in Lithium-Ion Batteries. Polymers 2023, 15, 2920. https://doi.org/10.3390/polym15132920
Misenan MSM, Hempelmann R, Gallei M, Eren T. Phosphonium-Based Polyelectrolytes: Preparation, Properties, and Usage in Lithium-Ion Batteries. Polymers. 2023; 15(13):2920. https://doi.org/10.3390/polym15132920
Chicago/Turabian StyleMisenan, Muhammad Syukri Mohamad, Rolf Hempelmann, Markus Gallei, and Tarik Eren. 2023. "Phosphonium-Based Polyelectrolytes: Preparation, Properties, and Usage in Lithium-Ion Batteries" Polymers 15, no. 13: 2920. https://doi.org/10.3390/polym15132920
APA StyleMisenan, M. S. M., Hempelmann, R., Gallei, M., & Eren, T. (2023). Phosphonium-Based Polyelectrolytes: Preparation, Properties, and Usage in Lithium-Ion Batteries. Polymers, 15(13), 2920. https://doi.org/10.3390/polym15132920