Abstract
Pharmaceuticals are acknowledged as emerging contaminants in water resources. The concentration of pharmaceutical compounds in the environment has increased due to the rapid development of the pharmaceutical industry, the increasing use of human and veterinary drugs, and the ineffectiveness of conventional technologies to remove pharmaceutical compounds from water. The application of biomaterials derived from renewable resources in emerging pollutant removal techniques constitutes a new research direction in the field. In this context, the article reviews the literature on pharmaceutical removal from water sources using microbial biomass and natural polymers in biosorption or biodegradation processes. Microorganisms, in their active or inactive form, natural polymers and biocomposites based on inorganic materials, as well as microbial biomass immobilized or encapsulated in polymer matrix, were analyzed in this work. The review examines the benefits, limitations, and drawbacks of employing these biomaterials, as well as the prospects for future research and industrial implementation. From these points of view, current trends in the field are clearly reviewed. Finally, this study demonstrated how biocomposites made of natural polymers and microbial biomass suggest a viable adsorbent biomaterial for reducing environmental pollution that is also efficient, inexpensive, and sustainable.
1. Introduction
The ecosystem and all forms of life on Earth are currently in peril due to the unregulated discharge of a variety of contaminants into water, air, or soil. The rapid industrialization and global population increase during the last century determined the release of a vast number of harmful substances into the environment. The majority of these organic and inorganic contaminants were found in surface water, groundwater, soil, and drinking water [1,2,3,4]. The removal methods of these pollutants have been extensively researched in recent decades, but they remains a hot topic in the global scientific community [2,4,5,6].
Water use has expanded dramatically over the last century, resulting in the depletion of natural water resources, the deterioration of fauna, and certain aspects of life quality. An estimated 4 billion people worldwide do not have or have limited access to safe drinking water, and millions die each year from debilitating diseases caused by contaminated water [2,7,8]. This issue, together with rising energy usage, has prompted the development of novel technology for producing drinking water with little energy consumption. As a result, one of the primary global issues today is the development of innovative, environmentally friendly, low-cost, and high-efficiency water treatment technologies [5,9,10]. The negative impacts of organic and inorganic pollutants (dyes, pharmaceuticals, toxic metals, metalloids, radionuclides, etc.) on ecosystems and their health dangers have been demonstrated over time, necessitating the use of increasingly complicated pollutant detection methods [4,6,11]. The release of pharmaceutical compounds, organic dyes, and heavy metal ions into the environment by companies such as the pharmaceutical, textile, pulp and paper, and food industries endangers human health and ecosystems [12,13,14].
Pharmaceuticals represent an important part of the non-biodegradable or hardly biodegradable compounds found in wastewater and wastewater treatment plant effluents (WWTP). They have been extensively studied in the last 15 years, both in terms of quantifying their presence in different environmental matrices, toxic effects (Table 1), and removal methods [15].

Table 1.
The toxic effects of some different therapeutic group of pharmaceuticals.
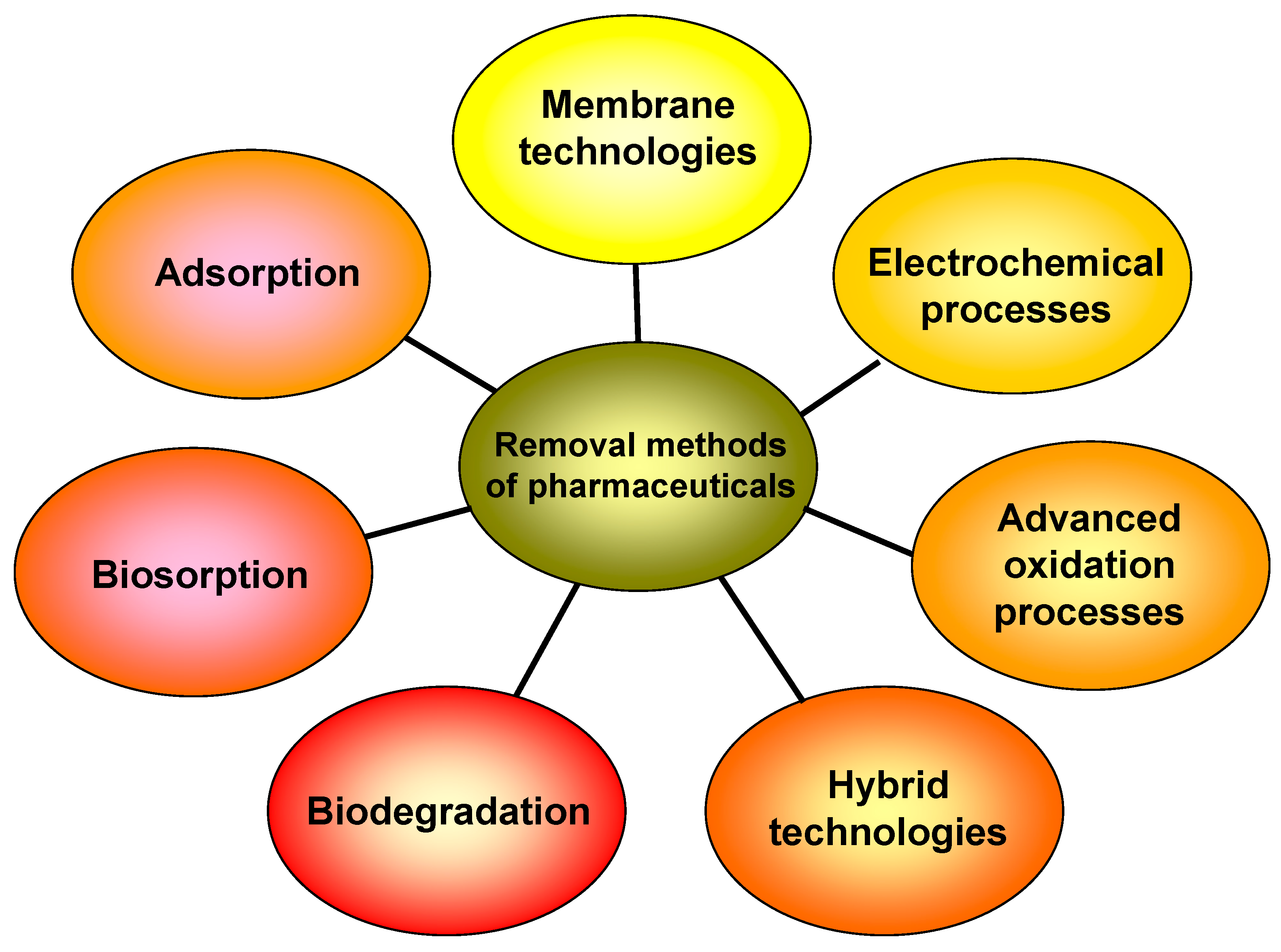
The global annual production of drugs has been estimated to be in the thousands of tons [2,6,21,22]. A wide range of methods (Figure 1), such as membrane separation, ozonation, flocculation, advanced oxidation, photocatalysis, microbial degradation, electrochemical processes, and adsorption, have been utilized to remove pharmaceuticals from aqueous matrices [3,7,8,23,24,25,26,27]. Most of these procedures involve the transfer of pollutants between different phases, the employment of additional chemicals, or the use of huge quantities of energy.
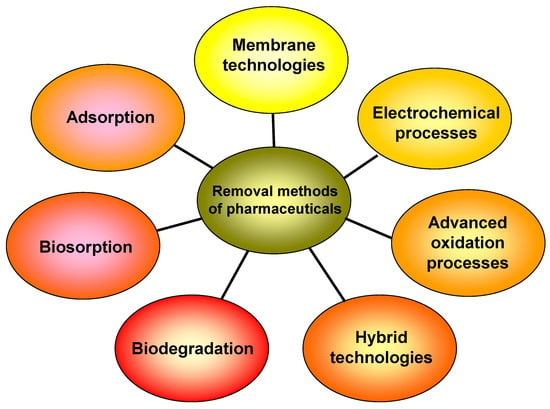
Figure 1.
Various methods for removal of pharmaceuticals from aqueous matrices.
Furthermore, several of them generate trash and byproducts that must be treated in the following phases. Some of them, such as biological methods used in treatment plants, are ineffective at removing a variety of organic pollutants, such as contaminants of emerging concern, personal care products, pharmaceuticals, pesticides, endocrine disrupting compounds, dyes, and so on. These substances are stable and difficult to degrade, which has led to their accumulation in the environment [3,20,28].
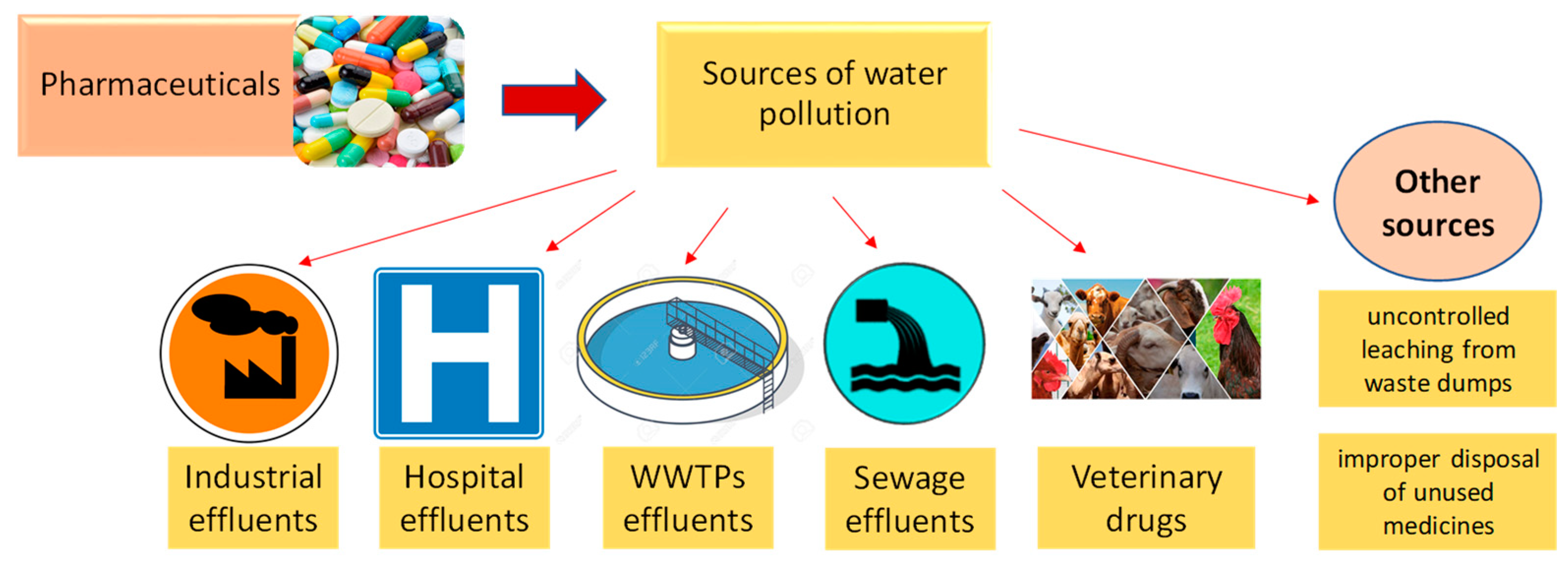
Pharmaceutical products and their transformation products can pollute surface water, underground water, and implicitly drinking water from a variety of sources (Figure 2), including wastewater treatment plant effluents, uncontrolled leaching from waste dumps, the pharmaceutical industry, hospitals, animal feed, improper disposal of unused medicines, etc. [2,6,21,22].
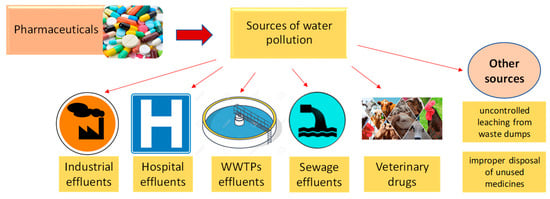
Figure 2.
The main sources of water pollution with pharmaceuticals.
The wastewater treatment plant effluents are regarded as the primary source since, in most cases, these compounds are not eliminated and are detected in the treated water [6,21,29,30].
Researchers reviewed the occurrence and availability of pharmaceutical compounds in the environment, especially in various aqueous matrices [4,30,31,32,33,34]. Javaid Akhtar et al. [3] analyzed the presence of commonly detected pharmaceuticals in different water sources, such as hospital effluents, wastewater treatment plant influents, industrial effluents, river effluents, urban effluents, and surface water. It is evident from the examination of the data that a variety of pharmaceuticals are present, including antibiotics, anti-inflammatory drugs, lipid regulators, beta blockers, anticonvulsants, and contrast agents in concentrations ranging from 2.9 ng/L to 1.1 × 105 ng/L.
Deblonde et al. [4] reported six classes of pharmaceutical compounds, including over 50 drugs, in WWTP influents and effluents ranging from 0.079 to 56.63 g/L. It has been demonstrated that pharmaceuticals are removed in WWTP in varying percentages, ranging from 1.4% for antiepileptics (trimethoprim) to 95.1% for antibiotics (tetracycline); however, it was found that for the majority of studied compounds, the rate of removal is lowered.
Vasilachi et al. [20] conducted a study on the environmental and health risks associated with emerging pollutants, in which they also made specific references to pharmaceutical compounds. The authors reported the toxicity of 35 residues of pharmaceutical compounds from 12 therapeutic groups in aquatic organisms and plants. The toxic effects of endocrine disruptors on human health were also presented, among which we mention: problems in the cardiovascular system, abnormal neural behaviors linked to obesity, altered endogenous steroid levels, etc., diabetes, and an altered reproductively relevant, sexually dimorphic neuroendocrine system.
Antibiotic-resistant bacterial strains have emerged as a result of persistent sublethal levels of antibiotic residues in aquatic environments [35]. The presence of these pollutants in natural water constitutes a serious risk, as stated by existing legislation [2,36], and several of them have already been designated as priority substances in water protection plans.
To assure the removal of pharmaceutical compounds from water, novel water treatment technologies must be developed. Recent research has concentrated on a number of techniques to accomplish this goal, but some of them come with the drawback that the breakdown of organic molecules can lead to new products with toxicity levels that are often even higher than the original chemicals [5,20,27].
Recent reviews mention the use of adsorption/biosorption processes for the removal of emergent contaminants from water and wastewater [10,11,21,37,38], but they are not focused on microbial biomass, its potential for drug removal, or the benefits and drawbacks of its use. Biosorption has emerged as a technology with considerable potential for the removal of these compounds from aqueous matrices in recent years, necessitating a linkage of information on biomaterials and the biosorption processes in which they might be used.
The purpose of this study is to provide an overview of current advances in pharmaceutical compound biosorption processes, with a special emphasis on microbial biomass and natural polymers as biosorbent materials, as well as the impact and obstacles connected with these investigations. This review also raises awareness about the importance of biomaterials in achieving a sustainable future. The biosorption potential of several biomaterials is explored, including microbial biomass, residual microbial biomass, natural polymers, biocomposites based on diverse inorganic compounds, and microbial biomass immobilized in natural polymers.
2. Bibliographic Research Methodology
The selection of studies for this review was completed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement published in 2020 (with the checklists, explanation and elaboration, and flow diagram) [39].
The literature research strategy was developed in accordance with the PICO (Problem, Intervention, Comparison, Outcome(s)) framework, which is utilized to divide a topic into searchable components (Table 2).

Table 2.
PICO strategy applied in the present review.
The PICO strategy was used to conduct the research, and the following exclusion and inclusion criteria were established.
Inclusion criteria:
I1. Research articles published from 2010 to the present, full text;
I2. Removal of emerging pollutants—for automated screening, only the term “pollutant” was used;
I3. Removal of pharmaceutical compounds—for automated screening, only the term “pharmaceutical” was used;
I4. Evaluation of the application of microbial biomass (microbial cells, residual microbial biomass) and natural polymers for the removal of pharmaceutical compounds—manual screening;
I5. Relevance to the subject of the review (new information provided);
I6. Articles published or available in English.
Exclusion criteria:
E1. Articles published before 2010;
E2. Book or book chapters;
E3. Conference papers, notes, letters, short surveys, errata, or conference reviews;
E4. Articles published in languages other than English;
E5. Articles presenting the removal of pollutants monitored in routine studies (such as dyes and heavy metals, intensively studied pollutants).
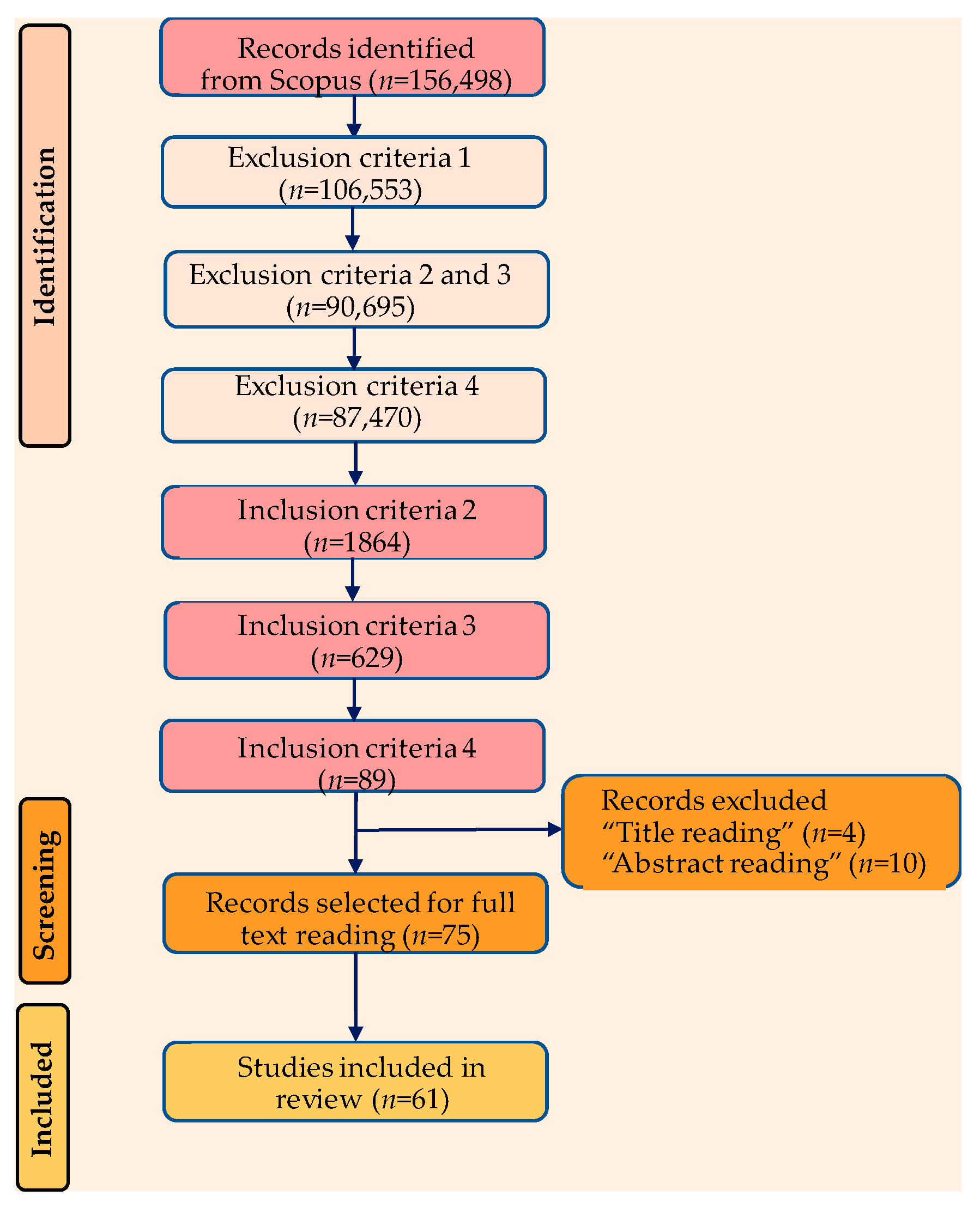
Using “biomaterial” as the primary search keyword, a literature search was conducted using the Scopus database (a comprehensive bibliographic database). Based on the aforementioned inclusion (I1 ÷ I3) and exclusion (E1 ÷ E4) criteria, papers were automatically chosen, and the decision to include them in the current review was made after carefully reading each manuscript (Figure 3).
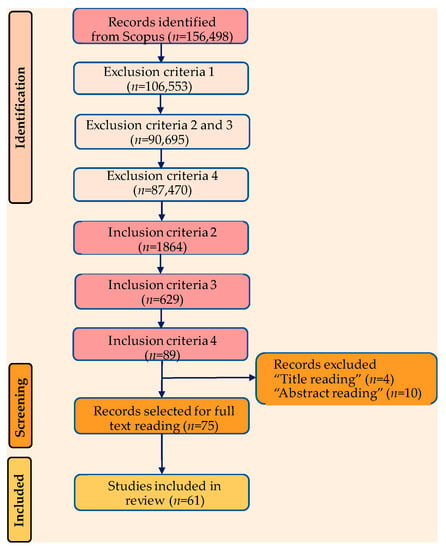
Figure 3.
PRISMA flow chart of article selection process.
A total of 61 articles covering the removal of pharmaceuticals using microbial biomass and natural polymers were chosen for inclusion in the present review after applying the full set of exclusion/inclusion criteria, including title, abstract, and full-text reading (Figure 3). Additional work has been added to the selected items in order to provide the required context. These articles were retrieved manually by using a “search and find” strategy and certain keywords (such as “biosorption”, “pharmaceutical removal”, etc.) in the Scopus database. Studies revealing the removal of dyes and heavy metals on the one hand and other biomaterials that are not based on microbial biomass and natural polymers on the other hand were not taken into consideration for inclusion in the current study.
The papers selected for this review present applications of microbial biomass and natural polymers for the removal of pharmaceutical compounds from aqueous matrices.
3. Biosorption—Concept and Current Perspectives
The remarkable capacity of microorganisms to remove organic and inorganic pollutants has been exploited in the development of biological methods for environmental depollution for a long time [11]. The concept of “biosorption” refers to a multifaceted process that depends on a number of factors, including the availability of various mechanisms, the type of bio-sorbent being employed, process parameters, and the presence or absence of metabolic activities in living organisms.
According to Fomina M. and Gadd G. M. [11], one of the significant properties of active and inactive microorganisms (and their components) is their ability to bind pollutants, which is utilized for the remediation of contaminants by biosorption and biodegradation. Their use, as well as the use of residual microbial biomass, microalgae, and agro-industrial waste in biosorption processes has produced impressive results for the removal of persistent, inorganic, and organic pollutants in low to medium concentrations in aqueous effluents [21,40,41,42].
Due to its ease of use (operation that is similar to that of traditional ion exchange technology), effectiveness in removing pollutants, and accessibility of biomass and residual biomass, biosorption has been regarded as a promising biotechnology for the removal and/or recovery of pollutants from aqueous solutions since the beginning of studies [11,21,41]. Great efforts have been made since the initial biosorption investigations to produce efficient, cheap, and adaptable biomaterials for wastewater treatment. Initially, studies were concentrated on the removal of metals and associated chemicals, but further biosorption research moved into additional areas of potential use, including pharmaceuticals [11,21,37,38,42]. Due to its dependence on the physical-chemical and biological properties of components (both pollutant and biosorbent), as well as the lack of clarity regarding the underlying mechanisms, biosorption is a complex process, as demonstrated by decades of research [11,41].
Most of the time, biosorption is considered a passive physico-chemical process, with mechanisms including: adsorption, ion exchange, and complexation. In reality, depending on the biosorbent-pollutant system and the given biosorption conditions, this can be an extremely complex process from the point of view of the mechanism [3,11]. In most cases, the term” biosorption” is assigned generically without regard to the mechanism, even if the process involves biodegradation or bioaccumulation.
Understanding the mechanism of interaction of target pharmaceutical compounds with microbial biomass-based biosorbents is mainly determined by the type of microbial cells involved, whether they are active or inactive. Thus, it can be said that in the case of active cells, the compound is removed by biodegradation and in the case of inactive cells by biosorption. Considering both the chemical structure of the pharmaceutical compounds in which different functional groups are present (i.e., phenolic, carboxylic, amide, amino, hydroxyl, alkoxide groups, etc.) or bonds (i.e., C=C aromatic) and the characteristics of the biosorbent, possible interactions that could describe the mechanism can be evaluated.
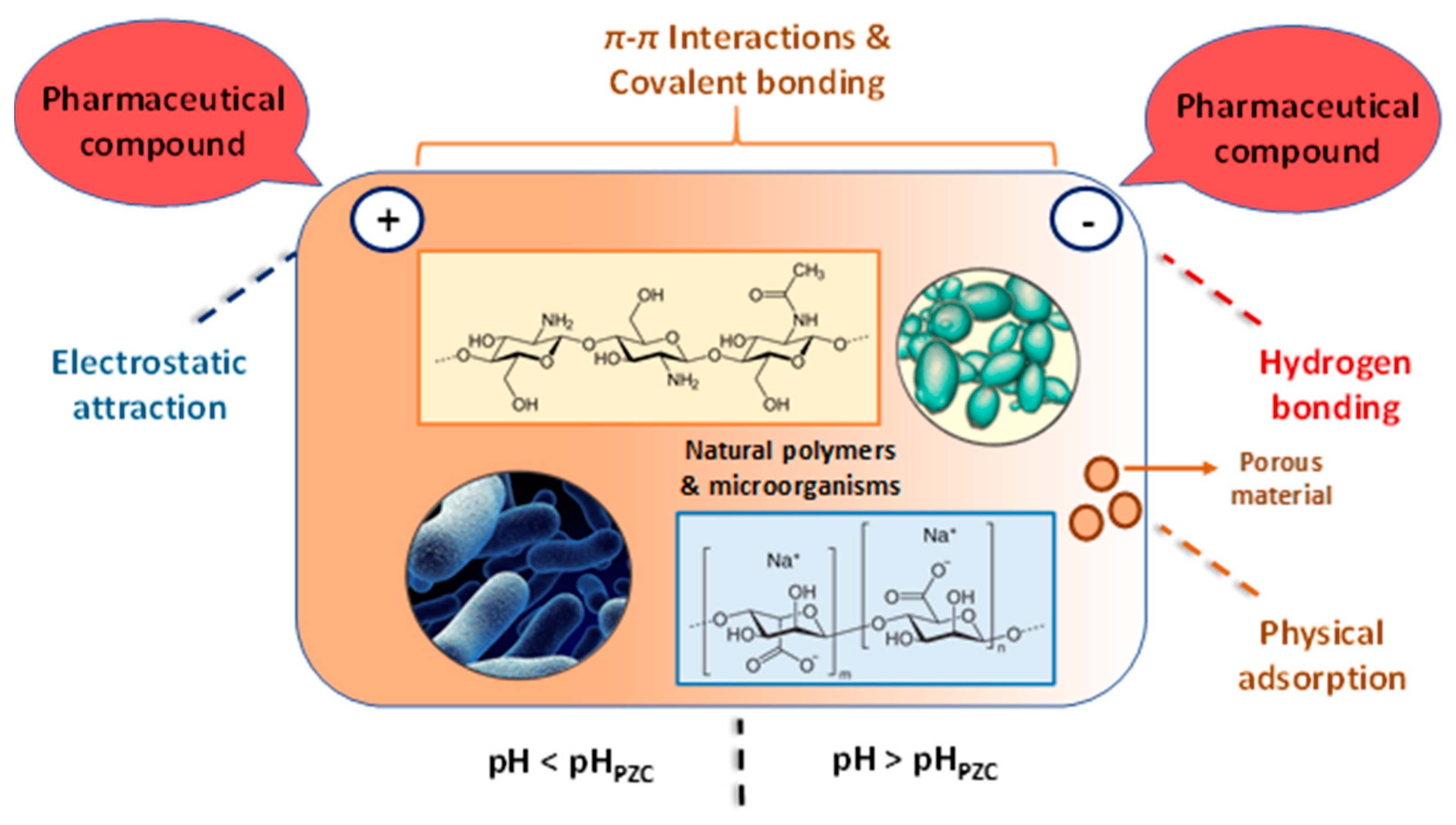
As shown in Figure 4, four dominant mechanisms can be taken into account in the biosorption of pharmaceutical compounds on biosobents based on natural polymers and microbial biomass (inactive cells): (i) electrostatic attraction between the bio-sorbent and drug; (ii) π–π interaction between the surface of biosorbents and the pharmaceutical compound; (iii) hydrogen bonding interaction; (iiii) physical adsorption in the pores of the biosorbent. The pH value of the pollutant solution plays an important role in the biosorption process. Thus, at pH < pHPZC, the electrostatic attraction is established between the negatively charged pharmaceutical compounds and the positive charge present on the surface of the biosorbent, and for pH values > pHPZC the surface of the biosorbent has a negative charge, and H bonds (hydrogen bonds) can be made between the pharmaceutical compounds and it.
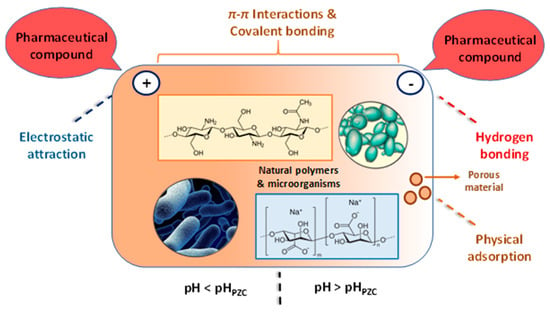
Figure 4.
Schematic representation of the biosorption mechanism of pharmaceutical compounds (The figure was created taking into account the mechanism descriptions presented by Rashtbari et al. [43] (adapted with Elsevier permission, license 5578630226919, june 30, 2023); Grisales-Cifuentes et al. [44]; Samarghandi et al. [45]; Al-Gheethi et al. [46]; Yu et al. [47]; Akhtar et al. [3]; Rusu et al. [48]; Rusu et al. [49]).
Samarghandi et al. [45] highlight the role of pH in the biosorption of amoxicillin (AMX) from aqueous solutions using Saccharomyces cerevisiae. The ionization of the substance in solution is influenced by pH, which also affects the adsorbent’s surface charge and biosorption capacity. The key components of AMX biosorption are functional groups on the biosorbent cell wall and active sites. As a result, the amines are proteinized, and their electrostatic charge changes to positive, allowing AMX to bind to the absorbent. The efficiency of AMX removal is significantly impacted by changes in the initial pH value. At a pH of 5, the removal efficiency was at its highest (81%).
The potential of living microorganisms to biodegrade pharmaceutical compounds underlies many of their removal processes.
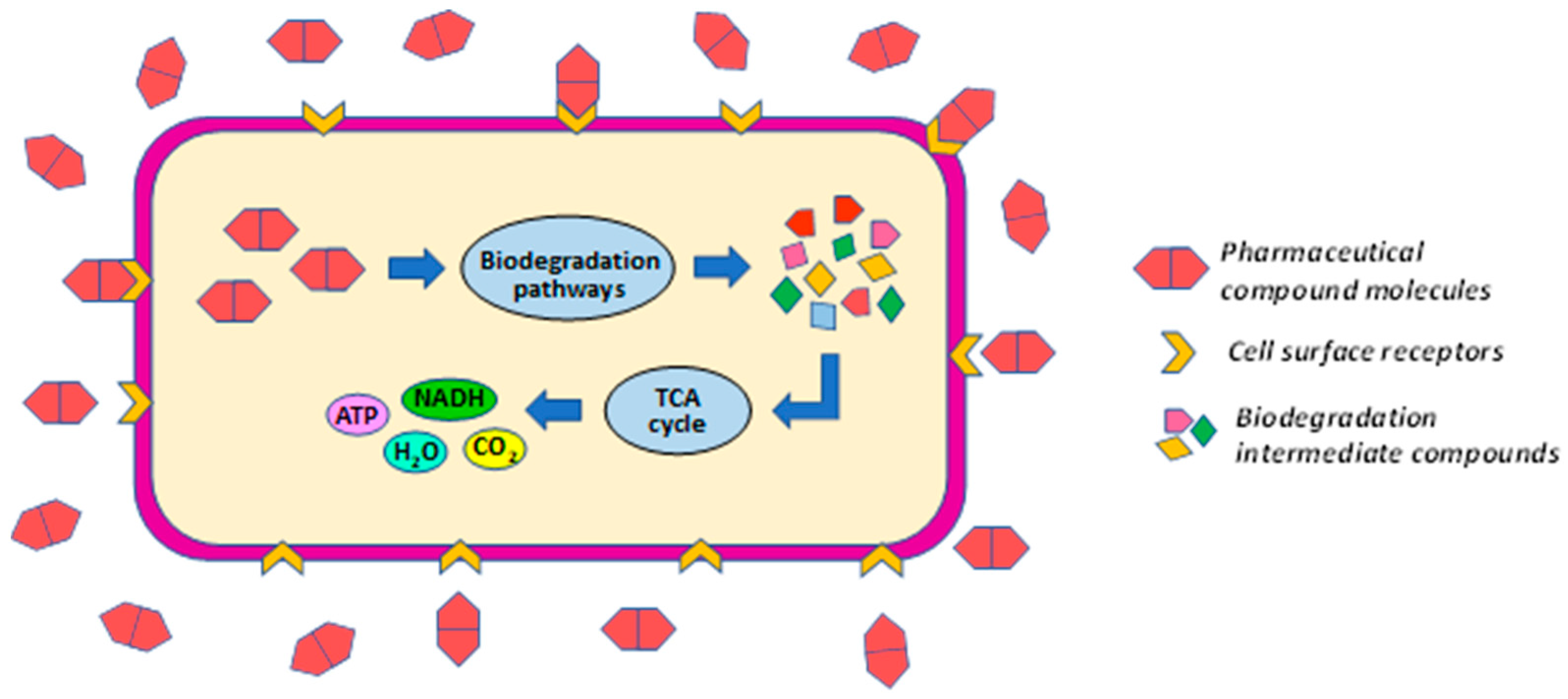
In the case of biodegradation, a primary degradation (Figure 5) can take place in which the pollutant in question is used as an energy source and is directly metabolized by the microbial strain to obtain energy. This type of degradation process takes place inside the cell, and the pollutant must be able to travel through the microbial cell wall to the cytoplasm. However, in many cases, due to the complexity of metabolic activities, complete metabolism of the pollutant is not achieved, and hazardous biodegradation products may result.
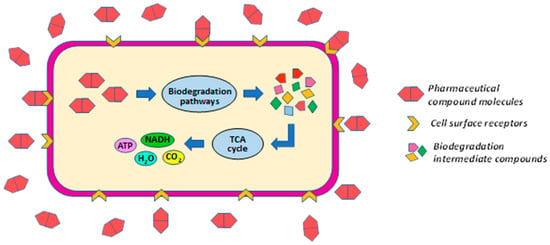
Figure 5.
Predicted pharmaceutical compounds biodegradation mechanism. The biochemical degradation pathways represented here are created based on studies by Poddar et al. [16]; Adel et al. [50]; Wang et al. [51].
The Pseudomonas PrS10 strain of bacteria uses paracetamol as an energy source, according to an estimate of the biodegradation mechanism provided by Poddar et al. [16]. This is supported by a GC-MS study that reveals an insignificant presence of metabolites in the biodegradation broth.
Since studies so far have primarily concentrated on the removal of a single pollutant under static operating conditions and the use of biosorbents that are not viable for treating large volumes of water, biosorption has not yet achieved commercial success in its traditional direction as a low-cost and environmentally friendly depollution process [11,41]. Recently, there has been an increase in interest in the study of biosorption processes for binary or multiple solute systems, which are more typical of real-world wastewater and waste valorization problems [12]. The immobilization of biomass, which provides easy handling of biosorbents with good mechanical characteristics and easy separation, has markedly advanced the field of biosorbents domain [15,52].
In this context, it’s important to keep in mind the present trend of employing renewable bioresources to produce biosorbents [49,53]. On the other hand, both pragmatic market and cost justification should be considered when directing future research, so attention should be directed to alternative applications such as the recovery of pharmaceuticals, valuable metals, and elements, the manufacture of enriched feed and fertilizers, and the detoxification of food.
4. Biosorbents—Types and Applications in the Removal of Pharmaceutical Residues
Biosorbents are based on low-value products or wastes that are readily accessible in sufficient quantities, non-hazardous, cheap to acquire, and easy to use [11,12,37,40,41]. Analyzing the studies conducted so far (more than 13,000 scientific papers have been published in peer-reviewed journals to date), it appears that practically all biological materials have an affinity for organic and inorganic pollutants, indicating the enormous potential for biosorption [3,11,20,37,54,55].
As research has concentrated on identifying efficient and economical biosorbents as well as new opportunities for pollution control, a wide range of microbial and plant biomasses, as well as derived products, have been studied in various forms and in connection to various types of pollutants. As a result, the various biologically derived materials that have been extensively investigated to develop biosorbents include: microbial biomass (bacteria, cyanobacteria, filamentous fungi, yeasts, microalgae), marine algae (macroalgae), industrial waste (fermentation waste, food waste, activated sludge, sludge recovered from anaerobic fermentations, etc.), agricultural waste (fruit/vegetable waste, rice straw, wheat bran, sugar beet pulp, soybean husks, etc.), natural residues (residues of plants, sawdust, tree bark, weeds, peat), and other materials (chitosan, cellulose, seashell waste, etc.) [13,14,41,54,56,57,58].
Biosorbents as biological materials can be obtained using polymeric materials (alginate, chitosan, etc.) but also using microbial biomass, either free or immobilized. The microbial cells easily grown or available in large quantities in nature (bacteria, yeast, filamentous fungi) can be used in the pollutant’s removal process as active cells, when they are able to grow and reproduce in the polluted medium, but also in their inactive form, as dead cells, when the functional groups from the cell membrane or cell wall can still bind pollutants, but the cells do not need conditions compatible with life. In industrial activities (biotechnology: production of organic acids, amino acids, antibiotics, etc.), at the end of the biosynthetic process, the biomass is considered a by-product (industrial waste) that needs to be either valorized or disposed of. This is considered residual biomass (usually in inactive form) and can be used for the production of biosorbents. Most studies on pharmaceutical elimination use inactive microbial cells as biosorbents as the preferred approach to reduce complexity, as a microbial biosorbent that has been autoclaved performs better in biosorption as a result of the degradation of the cell wall, which leads to the appearance of additional binding sites. Active biosorbents have other advantages, as they could, through an active metabolism, modify the structure of the pollutant. Additionally, results on active biomass should not be disregarded because they have been utilized successfully to remove toxic metals and residues of persistent organic pollutants like dyes and pharmaceuticals [50,59].
The biosorptive capacities of different types of biomass have been reported in thousands of research papers and quantitatively compared in many reviews, from which it is evident that they can vary considerably and depend to a large extent on the experimental conditions and possible pretreatments applied [10,11,21,37,41,42,55,60]. The selection of the most promising biosorbents from a wide range of affordable and easily accessible biomaterials has been and continues to be the key problem. The goal is to obtain or choose biosorbents that are appropriate for industrial application for the greatest variety of large-scale persistent organic pollutants.
From this perspective, it could be based on (i) industrial waste (by-products from fermentative processes), which could be available for free or at a reduced cost; (ii) organisms that are readily obtainable in vast quantities in nature; and (iii) organisms that are easily cultivable.
4.1. Microorganisms and Residual Microbial Biomass
The use of microorganisms in biosorption processes began with the ability of biomass composed of active and inactive cells to form complexes with metal ions and was later expanded to include additional substances such as dyes and pharmaceutical contaminants. The inactive biomass has advantages such as low cost, low toxicity, ease of regeneration, ion exchange capacity, and adaptation to diverse pH and temperature values.
Biomass action is influenced not just by its chemical composition but also by external physico-chemical variables. Chelation, complexation, adsorption, ion exchange, degradation, electrostatic interaction, microprecipitation, coordination, and donor-acceptor interaction are frequently cited as mechanisms for micropollutant removal utilizing biomass [10,11,21,38,41]. Different types of microorganisms and residual microbial biomass have been used in the removal of pharmaceutical compounds by biosorption or biodegradation (Table 3).
Among the microorganisms used in biosorption/biodegradation processes are bacteria (Pseudomonas, Enterobacter, Streptomonas, Aeromonas, Acinetobacter, Klebsiella, Bacillus) and fungi (Myceliophthora thermophile, Trametes versicolor, Phanerochaete chrysosporium, Ganoderma lucidum), etc.
Numerous studies show that they can remove various pharmaceutical micropollutants from contaminated environments, e.g., ciprofloxacin, sulfamethoxazole, lomefloxacin, ofloxacin, norfloxacin, paracetamol, amoxicillin, sulfapyridine, sulfamethazine, diclofenac, ibuprofen, naproxen, iopromide, venflaxin, caffeine, and metoprolol [61,62,63,64,65,66,67].


Table 3.
Various microorganisms and microbial biomass used for removal of several pharmaceuticals residues from aqueous matrices.
Table 3.
Various microorganisms and microbial biomass used for removal of several pharmaceuticals residues from aqueous matrices.
| Biosorbent | Therapeutic Group/ Pharmaceutical Compound | Process Parameters | Obtained Results | Ref. |
|---|---|---|---|---|
| BACTERIAL BIOMASS | ||||
| Antibiotics | ||||
| Bacillus subtilis 1156WTNCC strain (active cells) | Amoxicillin | pH = 6.5; temperature = 35 °C; time = 12 days; initial concentration of pharmaceutical compound = 0.2 ÷ 5.0 mg/mL; aerobic conditions; batch system | Maximum biodegradation efficiency 25.03% for C0 = 1 mg/mL | [68] |
| Ampicillin | Maximum biodegradation efficiency 15.59% for C0 = 0.8 mg/mL | |||
| Cephalexin | Maximum biodegradation efficiency 22.59% for C0 = 1.0 mg/mL | |||
| Cefuroxime | Maximum biodegradation efficiency 10.62% for C0 = 1.0 mg/mL | |||
| Ciprofloxacin | Maximum biodegradation efficiency 2.45% for C0 = 0.6 mg/mL | |||
| Bacterial community composed of Desulfovibrio, Enterococcus and Peptostreeptococcus spp. (active cells) | Ciprofloxacin | sulfate-reducing conditions; temperature = 25 ± 2 °C in the dark; time = 6 days; initial concentration of pharmaceutical compound = 1.0 mg/L; anaerobic conditions; batch system | Biodegradation efficiency was 85%, after 6 days | [64] |
| Bacterial community composed of Comamonas, Arcobacter, Dysgonomonas, Macellibacteroides and Actinomyces, genera (active cells) | Ciprofloxacin | nitrate-reducing conditions; temperature = 25 ± 2 °C in the dark; time = 6 days; initial concentration of pharmaceutical compound = 1.0 mg/L; anaerobic conditions; batch system | Biodegradation efficiency was 83%, after 6 days | [64] |
| Acinetobacter sp. (active cells) | Sulfamethoxazole | pH = 7.0; temperature = 25 °C in the dark; time = 6 days; initial concentration of pharmaceutical compound = 30.0 mg/L; in a shaker at 150 rpm; batch system | Biodegradation efficiency was 98.8%, after 10 h | [51] |
| Bradyrhizobium sp. GLC_01 strain (active cells) | Ciprofloxacin | biodegradation via cometabolism with another carbon substrate (glucose and sodium acetate); temperature = 25 °C; time = 8 days; initial concentration of pharmaceutical compound = 0.05 ÷ 10 mg/L; in a rotatory shaker at 150 rpm; batch system | Over 70% biodegradation was achieved at 0.05 mg/L whereas decreased to 26% at 10 mg/L | [69] |
| Bacillus subtilis strain (active cells) | Cephalexin | pH = 6.5; temperature = 35 °C; time = 12 days; initial concentration of pharmaceutical compound = 1.0 g/L; batch system | Biodegradation potential was 27, 22 and 21% in the presence of Ni2+, Cu2+, Zn2+ ions in solution at 10 mg/L concentration | [50] |
| Bacterial consortium composed of Acinetobacter lwoffii ACRH76, Bacillus pumulis C2A1, and Acinetobacter sp. HN3) (active cells immobilized or in suspension) | Ciprofloxacin | environmental conditions; temperature = 30 ± 2 °C; time = 20 days; initial concentration of pharmaceutical compound = 100.00 mg/L; floating treatment wetland strategy (FTWs) | Maximum biodegradation was 97% in the FTWs having immobilized bacteria | [70] |
| Achromobacter sp. JL9 with in-situ generated biogenic manganese oxides (active cells) | Sulfamethoxazole | pH = 7.0; temperature = 30 ± 1 °C; time = 84 h; initial concentration of pharmaceutical compound = 5.0 mg/L; in a shaker at 125 rpm; batch system | Maximum biodegradation was 97.43% for the Mn (II) concentration of 2 mg/L | [71] |
| Microbial community including Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria and Armatimonadetes (active cells) | Chlortetracycline | pH = 7.2; temperature = 5 ÷ 45 °C; time = 28 days; initial con-centration of pharmaceutical compound = 100 µg/L; aerobic conditions; in a shaker at 120 rpm; batch system | Biodegradation rates of 48.7% and 84.9% were achieved by acclimated microbial populations in one and four weeks, respectively for the initial chlortetracycline level of 100 µg/L | [72] |
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Sulfamethoxazole | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic conditions | Removal rates by biodegradation of 73.2 ± 21.3% were achieved by simultaneous removal of drugs | [73] |
| Pseudomonas sp. CE22 strain isolated from activated sludge (active cells) | Cephalexin | temperature =26 °C; time = 10 h; initial concentration of pharmaceutical compound = 10 mg/L; in a shaker at 200 rpm; batch system | Biodegradation over 90% after incubation for 10 h | [74] |
| Activated sludge bacteria (inactivated biomass) | Ofloxacin | pH = 7.0; temperature =25 °C; time = 48 h; initial concentration of pharmaceutical compounds = 100 ÷ 700 ng/mL; in an orbital shaker at 120 rpm; batch system | Removal efficiency 45% for C0 = 100 ng/mL and 21% for C0 = 700 ng/mL; maximum biosorbtion capacity 1.5 ± 0.03 mg/g TSS | [75] |
| Norfloxacin | Removal efficiency 50% for C0 = 100 ng/mL and 39% for C0 = 700 ng/mL; maximum biosorbtion capacity 3.24 ± 0.05 mg/g TSS | |||
| Ciprofloxacin | Removal efficiency 59% for C0 = 100 ng/mL and 43% for C0 = 700 ng/mL; maximum biosorbtion capacity 3.39 ± 0.06 mg/g TSS | |||
| Bacterial consortium of Burkholderia cepacia, Chrysomonas luteola, Pseudomonas fluorescens, Bacillus subtilis, Bacillus megaterium, Bacillus sterothermophilus, Citrobacter freundii, Kluyvera (active and inactive cells) | Cephalexin | pH = 6.0; temperature =25 °C; time = 90 min; initial concentration of pharmaceutical compound = 0.2 ÷ 5.0 mg/L; in an orbital shaker at 125 rpm; batch system | Maximum biosorption efficiency (94.73% vs. 92.98% for living and dead cells respectively) was recorded at C0 = 0.4 mg/L, while for C0 = 5 mg/L dead cells exhibited more efficiency compared with living cells (82.36% vs. 46.66% respectively) | [46] |
| Antipyretics and analgesics | ||||
| Pseudomonas PrS10 strain (active cells) | Paracetamol | pH = 7.2 ÷ 7.4; temperature =30 °C; time = 4–7 days; initial concentration of pharmaceutical compound = 3 g/L; in an orbital shaker at 140 rpm; batch system | Maximum biodegradation efficiency 96.37%, with 4.8 g/L of carbohydrate added, after 7 days | [16] |
| Anti-inflammatories | ||||
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Ibuprofen | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic conditions | Removal rates by biodeg-radation of 24.2 ± 14.6% were achieved by simultaneous removal of drugs | [73] |
| Nitrifying bacteria isolated from activated sludge (active cells) | Ibuprofen | pH at approximately 7.5–8.0 during the incubation period; temperature =25 °C; initial concentration of pharmaceutical compounds = 25 ÷ 200 µg/L; in an orbital shaker at 80 rpm; batch system | Complete biodegradation (100%) at the lower concentration levels (25–100 µg/L) in 24 h | [76] |
| Ketoprofen | Complete biodegradation (100%) at the lower concentration levels (25–100 µg/L) in 150 h | |||
| Activated sludge bacteria (active cells) | Ibuprofen | pH = 7.0; initial concentration of pharmaceutical compound = 50 ÷ 300 mg/L; temperature =30 °C; in a shaker at 100 rpm in the dark; aerobic conditions; batch system | Biodegradation to undetectable concentrations within 4 days for C0 = 250 mg/L | [77] |
| Diclofenac | Biodegradation rate of 75% within 3 weeks for C0 = 300 mg/L | |||
| Brevibacterium sp. D4 strain (active cells) | Diclofenac | temperature =25 °C; initial concentration of pharmaceutical compounds = 10 mg/L; time = 30 days; in an orbital shaker at 150 rpm; batch system | Biodegradation was 35% for C0 = 10 mg/L of drug as a sole carbon source; Periodic feeding with acetate as a supplementary carbon source increased biodegradation by up to 90% | [78] |
| Anti-epileptics | ||||
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Carbamazepine | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic conditions | Removal rates by bio-degradation of 4.2 ± 2.3% were achieved by simultaneous removal of drugs | [73] |
| Starkeya sp. C11 strain and Rhizobium sp. C12 strain (active cells) | Carbamazepine | temperature =25 °C; initial concentration of pharmaceutical compounds = 10 mg/L; time = 30 days; in an orbital shaker at 150 rpm; batch system | Biodegradation was 30% for C0 = 10 mg/L of drug as a sole carbon source | [78] |
| Antidepressants | ||||
| Labrys portucalensis F11 strain (active cells) | Fluoxetine (racemic mixture) and its enantiomers FLX | temperature = 25 °C; initial concentration of pharmaceutical compounds = 2 µM ÷ 21 µM; time = 56 days; in an orbital shaker at 130 rpm; protected from light; batch system | Complete biodegradation of both enantiomers at C0 = 2 µM for FLX as sole carbon source was achieved in 30 days; The enantiomers were partially degraded at initial concentrations of 4 and 9 µM. Complete biodegradation of the two enantiomers occurred in the presence of acetate as an additional carbon source at 4, 9, and 21 µM | [79] |
| Antiseptics | ||||
| Enterobacter hormaechei ssp. Xiangfangensis KG216S strain (active cells) | Basic fuchsine | temperature = 37 ± 2 °C; initial concentration of pharmaceutical compounds = 20 ÷ 100 mg/L; time = 72 h; in an orbital shaker at 100 rpm; batch system | Maximum biosorption capacity was 140.54 mg/g for C0 = 20 mg/L, with 4 g/L of sucrose added | [80] |
| Histamine-2 blockers | ||||
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Ranitidine | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic conditions | Removal rates by biodegradation and biosorption of 60.8 ± 15.0% were achieved by simultaneous removal of drugs | [73] |
| Hormones | ||||
| Bacterial community composed of Comamonas, Arcobacter, Dysgonomonas, Macellibacteroides and Actinomyces, genera (active cells) | 17β-estradiol | nitrate-reducing conditions; temperature = 25 ± 2 °C in the dark; time = 6 days; initial concentration of pharmaceutical compounds = 1.0 mg/L; anaerobic conditions; batch system | Biodegradation efficiency was 84%, after 6 days | [64] |
| Psycho-stimulants | ||||
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Caffeine | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic condition | Removal rates by biodegradation and biosorption of 5.3 ± 4.4% were achieved by simultaneous removal of drugs | [73] |
| FUNGAL BIOMASS | ||||
| Antibiotics | ||||
| Saccharomyces cerevisiae (active cells) | Amoxicillin | pH = 2 ÷ 8, initial concentration of pharmaceutical compound = 5 ÷ 5 mg/L, the amount of biosorbent = 0.1 ÷ 1.5 g/L; contact time = 10 ÷ 240 min | The highest removal efficiency, 93%, was obtained for C0 = 5 mg/L, bioadsorbent dose 0.75 g/L, pH = 5, time = 120 min; the highest value of adsorption capacity was 12 mg/g in the same conditions | [45] |
| Trametes versicolor ATCC 42530 strain (active cells) | Sulfapyridine | pH = 4.5 ± 0.3; temperature = 25 °C; biomass dose 1.8 g/L (measured as dry weight); tank bioreactor with mechanical agitation at 115 rpm; 5 g/L of glucose added; aeration condition; batch model | Complete biodegradation (100%) for C0 = 21.4 ng/g within 26 days | [66] |
| Sulfathiazole | Removal efficiency by biodegradation was 85.9% for C0 = 143.0 ng/g within 26 days | |||
| Anti-inflammatories | ||||
| Trametes versicolor ATCC 7731 strain (living cells and chemically inactivated cells) | Naproxen | pH = 4.0; temperature = 25 °C; initial concentration of pharmaceutical compounds = 50–100 μg/L; biomass dose 0.4 g/L (measured as freshly grown fungus culture); time = 24 h; rotary shaker at 70 rpm; batch model | Biodegradation efficiency was over 60% with living cells and biosorption efficiency was 14 ± 5% with inactivated cells | [81] |
| Ibuprofen | Biodegradation efficiency was over 60% with living cells and biosorption efficiency was 32 ± 1% with inactivated cells | |||
| Trametes versicolor Ganoderma lucidum (active cells) | Diclofenac | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | The overall removal (100%) of diclofenac and ibuprofen after 7 days of incubation were achieved by both strains, T. versicolor and G. lucidum, by abiotic, biosorption and biodegradation | [82] |
| Ibuprofen | ||||
| Trametes versicolor Irpex lacteus Trichoderma reesei (active cells) | Diclofenac | pH = 5.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 2.5 ÷ 5 mg/L; time = 3 h ÷ 14 days; shaking incubator (150 rpm); batch model and fungal biofilm | T. versicolor and I. lacteus was able to completely (>99.9%) remove diclofenac after 7 days, by both mechanisms: enzyme activity and biosorption | [83] |
| Fusarium solani Pleurotus ostreatus (active cells) | Diclofenac | F. solani indicated a maximum reduction of 90% of diclofenac after 21 days; P. ostreatus removed the diclofenac >99.9% after 14 days; The combination of F. solani and P. ostreatus showed >80% removal of diclofenac after 14 days | [83] | |
| Trametes versicolor (active cells) | Ketoprofen | Only T. versicolor was able to reduce more than 80% of ketoprofen after 21 days of incubation | [83] | |
| Phanerochaete chrysosporium (active cells) | Naproxen | pH = 3.2 ÷ 4.5; temperature = 30 °C; initial concentration of pharmaceutical compound = 1.0 mg/L; continuous aerating mode; time = 28 days; batch system | Removal efficiency was 80.55 ± 3.26 on day 7; A removal higher than 95% was achieved after the addition of 8.25% sodium hypochlorite for inhibiting contamination in the reactor, on day 21; More than 90% naproxen C0 = 10 mg/L) was removed by the crude enzyme in the first two days | [84] |
| Ganoderma lucidum (FP-58537-Sp strain) (active cells) | Diclofenac | pH = 4.5 ± 0.5; temperature = 25 °C; initial concentration of pharmaceutical compound = 47 ÷ 184 μg/L; time = 6–26 days; first batch system, orbital shaking (135 rpm), dark conditions | Total removal was 98 ± 15% of which 58 ± 8% by biodegradation and 40 ± 6%by biosorption | [63] |
| Anti-epileptics | ||||
| Trametes versicolor Ganoderma lucidum (active cells) | Carbamazepine | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | Maximum removal was 32%, achieved by biosorption, using the combined fungal system | [82] |
| Trametes versicolor (active cells) | Carbamazepine | pH = 7.5; temperature = 34–37 °C; initial concentration of pharmaceutical compound = 1 ÷ 20 mg/L; time = 5 h–7 d; first batch operation, then a continuous mode | Around 80% was eliminated when the diluted synthetic medium was applied as feeding. An effective elimination was achieved in ~100 days continuous operation, if sufficient nutrients were supplied | [85] |
| Phanerochaete chrysosporium (active cells) | Carbamazepine | pH = 3.2 ÷ 4.5; temperature = 30 °C; initial concentration of pharmaceutical compound = 1.0 mg/L; time = 28 days; aerating mode; batch system | Removal efficiency was 32.55 ± 1.22% on day 7 | [84] |
| Stropharia rugosoannulata (FBCC 475 strain) (active cells) | Carbamazepine | pH = 4.5 ± 0.5; temperature = 25 °C; initial concentration of pharmaceutical compound = 47 ÷ 184 μg/L; time = 6–26 days; first batch system, orbital shaking (135 rpm), dark conditions | Total removal was 86 ± 7%, of which 84 ± 7% by biodegradation and 2% by biosorption | [63] |
| Ganoderma lucidum (FP-58537-Sp strain) (active cells) | Carbamazepine | Total removal was 36 ± 7%, of which 31 ± 6% by biodegradation and 5 ± 1% by biosorption | [63] | |
| Antidepressants | ||||
| Trametes versicolor (ATCC #42,530 strain) (active cells) | Venlafaxine | pH = 4.5 ± 0.5; temperature = 25 °C; initial concentration of pharmaceutical compound = 47 ÷ 184 μg/L; time = 6–26 days; first batch system, orbital shaking (135 rpm), dark conditions | Total removal was 55 ± 8%, of which 53 ± 8% by biodegradation and 2% by biosorption | [63] |
| Lipid regulators | ||||
| Trametes versicolor Ganoderma lucidum (active cells) | Gemfibrozil | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | Complete removal (100%) is attributed to high intracellular oxidative biological pathway | [82] |
| Clofibric acid | Removal efficiency was 14%, achieved by biosorption with T. versicolor strain and 41% with both strains simultaneously | |||
| Hormones | ||||
| Trametes versicolor Ganoderma lucidum (active cells) | Progesterone | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | Total removal (100%) of progesterone was achieved by both strains, T. versicolor and G. lucidum, predominantly through biodegradation | [82] |
| Histamine-2 blockers | ||||
| Trametes versicolor Ganoderma lucidum (active cells) | Ranitidine | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | Total removal (100%) of ranitidine was mainly attributed to biological removal of the live fungal biomass by intra- or extracellular oxidative mechanisms | [82] |
The characteristics of bacterial cell walls (type, nature, and number of active sites; acidity and basicity; chemical composition; morphology, etc.) influence biosorption, with the main responsible mechanisms being extracellular ones, biodegradation through co-metabolism, biodegradation through substrate consumption, and adsorption [11,64,68,73,74,79]. In the case of fungi, the cell wall is responsible for these microorganisms’ ability to remove pharmaceutical contaminants. Their application is particularly widespread due to the enhanced availability afforded by the possibility of large-scale cultivation with high yields as well as the numerous genetic alterations they can undergo, among other things.
As can be observed from the information in Table 3, pH, temperature, biomass type and nature, the presence or absence of oxygen, the addition of an additional carbon source, light, and initial pollutant concentration are the primary variables that affect the biodegradation process.
The process is influenced by factors such as biomass dose, biomass type and nature, pH, ionic strength, and initial pollutant concentration. Because of the small size of the microbial cells, it is necessary to apply hydrostatic pressure in the treatment of polluted water, which might cause cell disintegration. However, when the biomass is immobilized using various techniques, very good results are obtained [45,63,66,81,83,86,87].
4.2. Biosorbents Based on Natural Polymers
Natural polymers have become increasingly used in wastewater treatment for pharmaceutical removal during the last few years, as can be seen from Table 4. Superior values are obtained for the removal of pharmaceuticals using alginate-based biosorbents compared with chitosan in the case of tetracycline and ibuprofen.
According to numerous studies on the subject, one of the key factors contributing to biopolymers’ perceived benefits in wastewater treatment is their potential to be both environmentally and economically sustainable [88]. Natural polymers are also renewable, sustainable, biodegradable, cost-effective, eco-friendly, non-toxic, biocompatible, and hydrophilic [89,90]. They also have a range of functional groups, such as hydroxyls (-OH) and amines (-NH2), to which contaminants might bind via chemisorption or physical adsorption during the water purification process [91].


Table 4.
Various biosorbent based on natural polymers designed for removal of several pharmaceuticals residues from aqueous matrices.
Table 4.
Various biosorbent based on natural polymers designed for removal of several pharmaceuticals residues from aqueous matrices.
| Biosorbent | Therapeutic Group/ Pharmaceutical Compound | Process Parameters | Obtained Results | Ref. |
|---|---|---|---|---|
| ALGINATE | ||||
| Antibiotics | ||||
| Alginate-graphene-ZIF67 aerogel (AG-ZIF) Alginate-graphene-Co aerogel (AG-Co) | Tetracycline | pH = 6.0; room temperature; time = 720 min; initial concentration of pharmaceutical compound = 100 mg/L; adsorbent dose = 1 g/L; shaker at 150 rpm; batch system | Maximum adsorption capacities for AG-ZIF and AG-Co were 456.62 and 105.49 mg/g, respectively | [92] |
| Ga-based metal-organic gel/sodium alginate composite beads | Chlortetracycline hydrochloride | pH = 4.0 ÷ 8.0; temperature = 25 °C; initial concentration of each pharmaceutical compound = 20 mg/L; adsorbent dose = 1.00 g/L (measured as dry weight); time = 72 h; shaken conditions (150 rpm); batch model; dark environment | Maximum adsorption capacity was 1085.19 mg/g | [93] |
| Ciprofloxacin hydrochloride | Maximum adsorption capacity was 862.07 mg/g | [93] | ||
| Polyvinyl alcohol-copper alginate gel beads | Tetracycline | pH = 3 ÷ 11; temperature = 20–45 °C; initial concentration of each pharmaceutical compound = 20 mg/L; adsorbent dose = 0.2 ÷ 2 g/L, time = 24 h; shaker at 150 rpm; batch system | Removal efficiency was 97.8% and the maximum adsorption capacity was 231.431 mg/g, when the dose of adsorbent is 2 g/L and temperature = 45 °C | [94] |
| Anti-inflammatories | ||||
| Alginate/ Carbon-based films | Diclofenac | pH = 3 ÷ 11; temperature = 30–68 °C; initial concentration of each pharmaceutical compound = 10 ÷ 50 mg/L; adsorbent dose = 0.25÷2.0 g/L, time = 6 h; batch system under stirring of 400 rpm | Maximum DCF adsorption of 29.9 mg/g was obtained at pH = 3 and 30 °C | [95] |
| Alginate/polypyrrole/ ZnFe2O4 beads | Ibuprofen | pH = 5 ÷ 7; initial concentration of the pharmaceutical compound = 50–350 mg/L; beads dosage = 0.2–1 g/L (dry weight); temperature = 25–55 °C; contact time = 3 h; orbital shaking 150 rpm; batch system | Maximum adsorption capacity was 108.2 mg/g, that increase with 12% under an external magnetic field (EMF); C0 = 350 mg/L; adsorbent dose = 0.2 g/L, pH = 7.0, temperature = 25 °C; stirring 100 rpm | [96] |
| Antipyretics and analgesics | ||||
| Calcium alginate/activated hydrochar composite beads | Paracetamol | pH = 6.5; initial concentration of the pharmaceutical compound = 100–250 mg/L; temperature = 15–35 °C; time = 10 h; shaker 150 rpm; batch system | Maximum adsorption capacity was 165.94 mg/g, for C0 = 250 mg/L, after 4 h at temperature = 25 °C | [97] |
| Alginate/polypyrrole/ ZnFe2O4 beads | Paracetamol | pH = 5 ÷ 7; initial concentration of the pharmaceutical compound = 50–350 mg/L; beads dosage = 0.2–1 g/L (dry weight); temperature = 25–55 °C; contact time = 3 h; orbital shaking 150 rpm; batch system | Maximum adsorption capacity was 106.7 mg/g, that increase with 14% under an EMF; C0 = 350 mg/L; adsorbent dose 0.2 g/L, pH = 7.0, temperature = 25 °C; stirring 100 rpm | [96] |
| Biomarkers | ||||
| Alginate-graphene composites | Rhodamine B | initial concentration of the pharmaceutical compound = 5 mg/L; contact time = 24 h; darkness and gentle stirring; batch system | Maximum adsorption capacity of 178 mg/g was achieved for reduced graphene oxide-based beads | [98] |
| Antiretroviral | ||||
| Activated carbon encapsulated in sodium alginate | Tenofovir disoproxil fumarate | pH = 4, an initial TDF concentration = 0.1 mM; temperature = 25 °C; adsorbent dose = 1 g of wet beads to 10 mL of TDF solution, the beads were whirled at 350 rpm | Maximum removal efficiency by adsorption was 92.68% after a contact time of 120 min | [99] |
| CHITOSAN | ||||
| Antibiotics | ||||
| Chitosan-alginate-bentonite composites | Tetracycline | pH = 5.5; temperature = 25–50 °C; initial concentration of each pharmaceutical compound = 10–550 mg/L; adsorbent dose = 1–4 g/L; time = 240 min; batch system under continuous agitation | Adsorption efficiency was 97.7% for C0 = 10 mg/L at 50 °C after 30 min | [100] |
| Chitosan | Rifampicin | pH = 6.7; temperature = 20–45 °C; initial concentration of each pharmaceutical compound = 20–200 mg/L; adsorbent dose = 0.5–10 g/L; time = 240 min; orbital shaker (100 rpm); batch technique | Maximum adsorption capacity was 66.91 mg/g for C0 = 30 mg/L and adsorbent dose = 1.5 g/L | [101] |
| Streptomycin | Maximum adsorption capacity was 11.00 mg/g for C0 = 30 mg/L and adsorbent dose = 1.5 g/L | [101] | ||
| Chitosan-based magnetic composite | Tetracycline hydrochloride | pH = 4 ÷ 12; temperature = 15–35 °C; initial concentration of pharmaceutical compound = 20–200 mg/L; adsorbent dose = 0.5 g (dry weight); time = 12 h; shaking speed of 140 rpm; dark environment; batch system | Maximum adsorption capacity was 50.2 mg/g for C0 = 100 mg/L at pH = 10 and temperature = 25 °C | [102] |
| Metal and clay embedded cross-linked chitosan | Tetracycline | pH = 2 ÷ 12; temperature = 25–45 °C; initial concentration of pharmaceutical compound = 20 mg/L; adsorbent dose = 0.5 g (dry weight); time = 24 h; orbital shaker 140 rpm; dark environment; batch system | Maximum adsorption capacity was 104.17 mg/g using zirconium loaded chitosan modified by perlite (Zr/Cht/Pt) composite at pH = 4; temperature = 25 °C | [103] |
| CuCoFe2O4—Chitosan magnetic nanohybrid | Tetracycline | pH = 3.5 ÷ 11.5; temperature = 25–40 °C; initial concentration of pharmaceutical compound = 5–30 mg/L; adsorbent dose = 0.2–1 g/L; time = 30 min; batch system | Highest adsorption efficiency was 93.07% for C0 = 5 mg/L, pH = 3.5, contact time of 20 min, the dose of 0.4 g/ L, and temperature of 25 °C | [104] |
| Chitosan-curdlan composite magnetized by zinc ferrite | Tetracycline | pH = 1.0 ÷ 11.0; temperature = 10–65 °C; initial concentration of pharmaceutical compound = 20–160 mg/L; adsorbent dose = 0.25–0.85 g/L; time = 120 min; batch system | Maximum adsorption capacity was 371.42 mg/g at 55 °C, C0 = 160 mg/L, 0.65 g/L dosage of adsorbent and pH = 6 | [105] |
| Chitosan-carbon black waste composite beads | Amoxicillin | pH = 6.5 ÷ 8.5; temperature = 22 °C; initial concentration of pharmaceutical compound = 25–50 mg/L; composite beads dose = 10–20 g/L; time = 24 h; batch system | Maximum adsorption capacity was 12 mg/g for C0 = 25 mg/L (in demineralized water) and 15 mg/g for C0 = 25 mg/L (in tap water); pH = 6.5 ÷ 7.5 | [106] |
| Chitosan-carbon black waste composite beads | Tetracycline | Maximum adsorption capacity was 39 mg/g for C0 = 25 mg/L (in demineralized water) at pH = 7.5 ÷ 8.5 | [106] | |
| Anti-inflammatories | ||||
| Chitosan | Ibuprofen | pH = 6.7; temperature = 20–45 °C; initial concentration of each pharmaceutical compound = 20–200 mg/L; adsorbent dose = 0.5–10 g/L; time = 240 min; orbital shaker (100 rpm); batch system | Maximum adsorption capacity was 24.21 mg/g for C0 = 30 mg/L and adsorbent dose = 1.5 g/L | [101] |
| Chitosan-based magnetic composite | Diclofenac sodium | pH = 4 ÷ 12; temperature = 15–35 °C; initial concentration of pharmaceutical compound = 20–200 mg/L; adsorbent dose = 0.5 g (dry weight); time = 12 h; shaking speed of 140 rpm; dark environment; batch system | Maximum adsorption capacity was 123 mg/g for C0 = 100 mg/L at pH = 6 and temperature = 25 °C | [102] |
| Chitosan/Zr-MOF (UiO-66) composite foams | Ketoprofen | pH = 2 ÷ 9; initial concentration of pharmaceutical compound = 5–50 mg/L; adsorbent dose = 0.2 g/L; time = 10 h; shaking speed of 180 rpm; batch system | Maximum adsorption capacity of 209.7 mg/g was achieved for C0 = 50 mg/L at pH 4 | [107] |
| Magnetic Fe/Cu–alginate nanocomposite beads | Naproxen | pH = 5.0; temperature = 25 °C; initial concentration of each pharmaceutical compound = 25 mg/L; adsorbent dose = 25 mg/30 mL of drug solutions; mixed at 250 rpm for 10 min; batch system | Removal efficiency was 84% for C0 = 25 mg/L and adsorbent dose = 25 mg/50 mL | [108] |
| Magnetic Fe/Cu-chitosan nanocomposite beads | Diclofenac sodium | Removal efficiency was 92% for C0 = 25 mg/L and adsorbent dose = 25 mg/50 mL | [108] | |
| Psycho-stimulants | ||||
| Chitosan/activated carbon composite beads | Caffeine | natural pH; temperature = 25 °C; initial concentration of each pharmaceutical compound = 50–800 mg/L; adsorbent dose = 1 g/L; time = 48 h; orbital shaker at 150 rpm; batch system | Maximum adsorption capacity was 391.00 mg/g for C0 = 10 mg/L | [109] |
| Non-ergot dopamine agonists | ||||
| Chitosan grafted with sulfonic acid | Pramipexole | pH = 10; temperature = 25 °C; initial concentration of the pharmaceutical compound = 0–500 mg/L; adsorbent dose = 1 g/L; orbital shaker at 160 rpm; time = 24 h; batch system | Maximum adsorption capacity was 339 mg/g for C0 = 10 mg/L in the presence of 20 mg/L humic acid | [110] |
| Hormones | ||||
| Chitosan nanoparticles | Estrogen | pH = 7.3; estrogen initial concentration = 3.5 ÷ 11.5 mg/L; adsorbent dosage = 1.45 g/L; time = 300 min. and magnetic stirrer at 600 rpm; batch system | Removal efficiency was 92.50% for C0 = 5.7 mg/L and contact time of 220 min | [111] |
| OTHER NATURAL POLYMERS | ||||
| Anti-epileptics | ||||
| Ball-Milled Silk Fibroin Films | Carbamazepine | pH = 2–12; temperature = 15–45 °C; initial concentration of the pharmaceutical compound = 250 μg/L; time = 180 min; agitation speed of 100 rpm; batch system | Removal efficiency was 53% and adsorption capacity = 281 μg/g at pH = 12 | [112] |
| Antibiotics | ||||
| Heterogeneous natural polymer-based on dialdehyde inulin and laccase | Ofloxacin | pH = 4.5; temperature = 40 °C; initial concentration of the pharmaceutical compound = 25 mM; adsorbent dose = 8.5 mg/mL of the immobilized laccase; time = 60 h; stirring; batch system | Removal efficiency by biodegradation was 63% after 60 h of incubation | [113] |
| Biomarkers | ||||
| Gelatin/activated carbon composite | Rhodamine B | pH = 2–11; temperature = 30–60 °C; initial concentration of the pharmaceutical compound = 50–50 mg/L; adsorbent dose = 3 g/L; time = 42 h; agitation speed of 100 rpm; batch system | Maximum adsorption capacity was 256.41 mg/g for C0 = 5.7 mg/L; pH = 4; temperature = 30 °C; time = 27 h | [114,115] |
| Poly(lactic acid)/activated carbon | Rhodamine B | pH = 2–12; temperature = 30–60 °C; initial concentration of the pharmaceutical compound = 100 mg/L; adsorbent dose = 2 ÷ 10 g/L; time = 54 h; agitation speed of 100 rpm; batch system | Removal efficiency was 88.99%; a highest adsorption capacity was 149.57 mg/g at pH = 4 and temperature = 60 °C | [116] |
Alginate is a linear and anionic polysaccharide obtained from brown seaweeds such as Laminaria hyperborea, Macrocystis pyrifera, and Ascophyllum nodosum. This biopolymer is composed of alternating blocks of 1,4-L-guluronic acid (G) and 1,4-D-mannuronic acid (M) units and comes in a variety of grades depending on the purity necessary for a certain application [117]. Although it can also be obtained from bacterial sources, the commercial product is acquired in the form of a salt, such as sodium alginate, from algae. Alginate is well known for its biodegradability, low toxicity, and chemical versatility, but its unique property of forming a stable gel in aqueous media by the addition of multivalent cations makes this biopolymer useful in cell immobilization [48,117]. Aside from these, the remarkable cross-linking ability should be emphasized as a vital attribute for creating composite materials.
Chitosan is an amino-polysaccharide formed by N-deacetylation of chitin, which results in the formation of amine groups (-NH2) from acetamide groups (-NHCOCH3) [118,119]. Due to its unique properties, such as polyelectrolyte properties, biocompatibility, hydrophilicity, adhesion properties, biodegradability, and recyclability, chitosan has gained a great deal of interest in a variety of biomedical, water treatment, cosmetics, and food sectors, as demonstrated in numerous papers [42,120,121]. Chitosan has been identified as a promising cationic adsorbent for the removal of anions, heavy metals, toxic organic dyes, aromatic compounds, and pharmaceutical residues in recent studies, as can be seen from Table 4 and more [122,123,124,125,126,127].
This polymer has numerous advantages, including biodegradability, biocompatibility, high reactivity, hydrophilicity, and nontoxicity [122]. Furthermore, chitosan has a linear polyamine structure with a number of free amine groups that can be crosslinked and modified [42,52]. However, chitosan has several drawbacks, including low water resistance, a small specific surface area, incomplete recovery after adsorption, poor mechanical and thermal properties, a high agglomeration tendency, and high acid solubility [42,128]. As a result, several physical and chemical modification/functionalization techniques, such as sulfonation, carboxymethylation, and amination, were used to improve the adsorption characteristics and selectivity to remove pharmaceutical compounds from water as well as other emerging pollutants [129,130,131,132].
4.3. Biosorbents Based on Microbial Biomass Immobilized in Natural Polymers
The use of inactive microorganisms for the biosorption of pharmaceuticals offers a promising approach for the removal of these compounds from wastewater or contaminated solutions. While inactive microorganisms can effectively bind pharmaceutical compounds, it is important to note that the specific biosorption capacity and efficiency depend on several factors, such as the type of microorganism, surface properties, composition of pharmaceutical pollutants, and operating conditions. In addition, it is important to consider the possible release of pharmaceuticals from the biosorbent after use and the proper disposal or treatment of the spent biosorbent to avoid environmental contamination. Inactive microorganisms are commonly used for biosorption to reduce the need for maintenance, growth, and potential contamination risks associated with living organisms. The biosorption process using microorganisms often requires optimization of various parameters, such as pH, temperature, biomass dosage, contact time, and mixing, to maximize the biosorption efficiency [60].
Although the use of microorganisms for biosorption offers several advantages, there are also some disadvantages to consider: low specificity (different microorganisms have different affinities and selectivities, requiring screening for each pharmaceutical compound in order to obtain an effective biosorbent); slow kinetics (binding of pharmaceutical compounds with complex structures to the surface of the microorganism or their diffusion into the microorganism may be slower compared with other adsorbents); insufficient mechanical stability; difficult biosorbent regeneration; difficult separation from the aqueous phase due to the small size of the microbial cells [133].
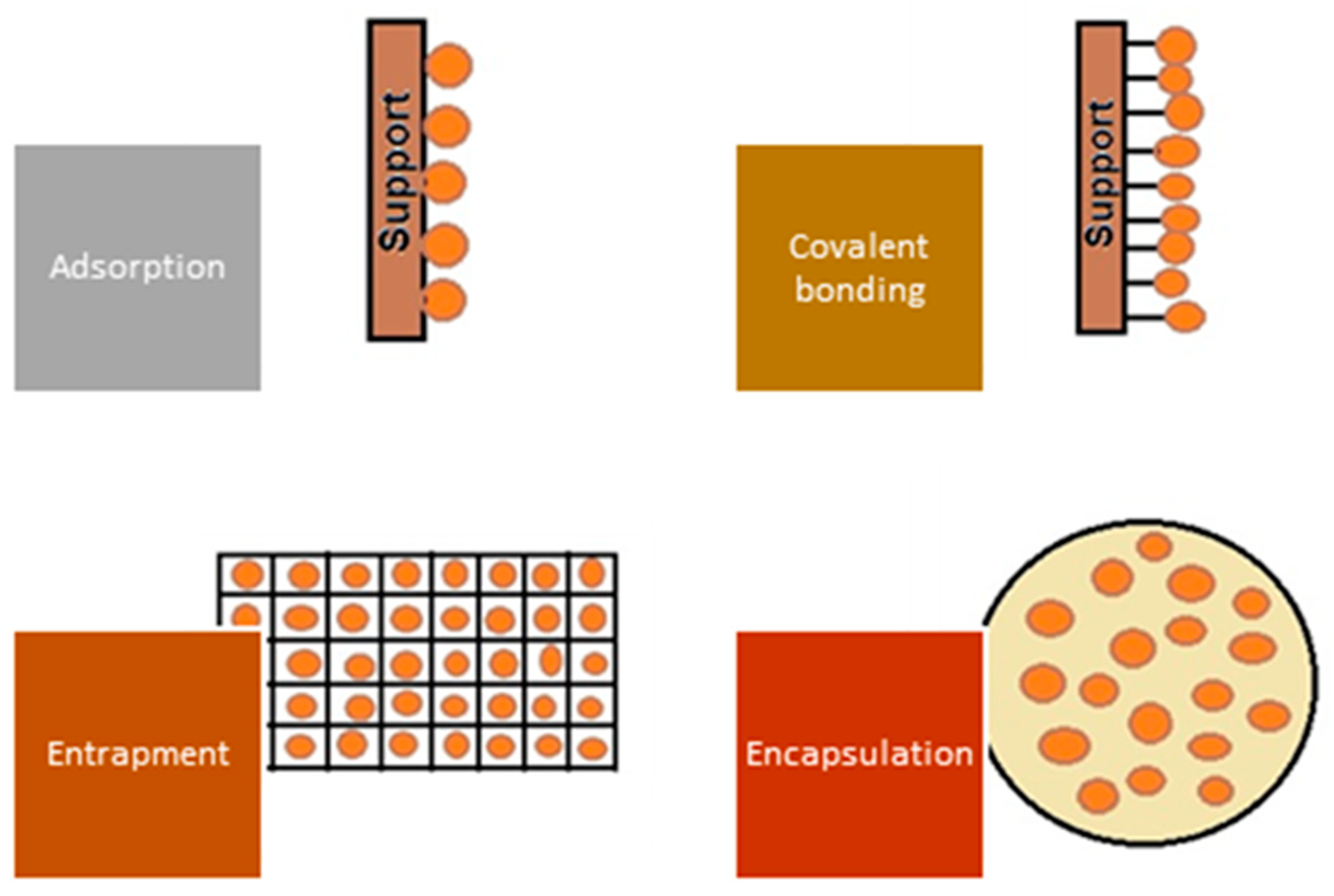
A significant improvement in the biosorption process’s performance is possible by using biosorbents obtained by immobilization (covalent bonding, cross-linking, encapsulation, entrapment in a polymeric matrix) of microorganisms or residual microbial biomass, as illustrated in Figure 6.
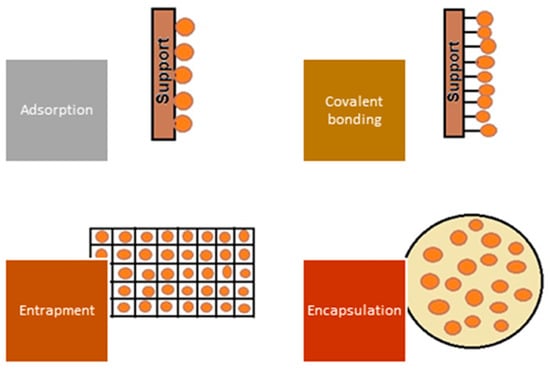
Figure 6.
Representation of main immobilization techniques.
The entrapment consists of capturing cells inside gel-like materials forming capsules (beads with mechanical strength, stability, and durability but with mass transfer limitations (e.g., oxygen, substrate, or nutrients) with an internal structure similar to a network, protecting them from external threats and also from washout of the biomass without affecting its microbial capacity. The encapsulation of microorganisms (microencapsulation) is a kind of cell immobilization that consists of covering, coating, or trapping microorganisms in a very similar way to entrapment, with the difference of using a semi-permeable protective film, which allows for better nutrients and substrate on, variou transfer. Several microorganisms have shown high sorption capacities compared with conventional adsorbents (ion exchange resins and celluloses, activated carbon, and various polymeric materials). However, their use is limited due to physical problems that can be solved by immobilization to obtain particles with good physicochemical stability (higher mechanical strength) [134]. Several aspects need to be considered when choosing an appropriate immobilization technique, as immobilization involves additional costs, diffusion of the pollutant through the carrier can prolong the duration of the process and reduce its efficiency, and the biosorption capacity decreases due to the interaction between the support and the active functional groups of the biosorbent [15].
The term of immobilization refers to the process of binding or entrapping microorganisms in a matrix or support material so that they retain their activity and functionality and facilitate their use in various applications. The aim of immobilization is to produce 0.5 to 3.5 mm particles with good porosity, physical stability, and chemical resistance that can be used in dynamic systems (fixed bed or fluidized bed columns). There are several immobilization techniques that can be used for microorganisms and residual microbial biomass [135,136]:
- – In entrapment, microorganisms are physically entrapped in a porous matrix or gel suitable for different types of cells. They are usually mixed with a gel-forming material such as calcium alginate, agar, or polyacrylamide, which solidifies into beads or capsules and immobilizes the microorganisms, allowing good mass transfer.
- – In adsorption, microorganisms are bound to a solid support material by non-covalent interactions such as electrostatic forces, hydrogen bonds, or hydrophobic interactions. The support material can be in the form of particles, fibers, or a thin layer. Immobilization by adsorption is relatively easy, but the microorganisms may be desorbed over time.
- – In covalent bonding, the microorganisms are chemically bound to the support material using cross-linking agents or functional groups. This method offers stronger and more stable bonding compared with adsorption but requires specific reactive groups on the microorganisms and the support material for successful bonding.
- – In encapsulation, the microorganisms are enclosed in a semi-permeable membrane, or microcapsule. The membrane allows the diffusion of contaminants while protecting the microorganisms from external factors. Common encapsulation materials include polymers such as alginate, chitosan, or various gums. Encapsulation provides good protection for the microorganisms but can restrict mass transfer.
These immobilization techniques offer advantages such as improved stability, better reusability, and better control over the behavior of the microorganisms. The choice of immobilization technique depends on the specific application, the type of microorganism, the desired immobilization properties, and the conditions of use. Various biosorbents based on microbial biomass immobilized on natural polymers or other materials were analyzed for removing pharmaceutical residues from aqueous matrices (Table 5). The maximum biosorption capacity is evaluated under various operating conditions, and there is no standardized method for estimating the dry weight of the biomass and biosorbent used. As a result, studies from different authors can be difficult to compare.

Table 5.
Various biosorbent based on microbial biomass immobilized on natural polymers or other materials designed for removing of several pharmaceuticals residues from aqueous matrices.
The removal of pharmaceuticals from model, natural, and wastewater samples is obviously a capability of microbial biomasses, but the biosorption capacity of any biosorbent depends on its structure, pretreatment, chemical modification, immobilization technique, and also on the contaminant structure and properties. Analyzing the results obtained by Rusu et al., for the biosorption of cephalexin using the same microbial strain: Saccharomyces cerevisiae immobilized in calcium alginate [48] or chitosan [52], very different biosorption capacities were obtained for the two support materials: 93.34 mg/g compared with 22.78 mg/g, thus proving the importance of the polymer used for the immobilization. For the same biosorbent, Phanerochaete chrysosporium immobilized in wood chips, the removal efficiency varies significantly with the structure of the pharmaceutical compounds (Table 5).
Temperature usually increases the biosorption efficiency; pH controls the dissociation of both functional groups from the cell envelopes but also of the pharmaceuticals, providing binding sites. However, the support must be stable at the biosorption optimum conditions; chitosan, for example, is unstable (soluble) at a strong acidic pH. The microbial cells pretreatment methods also influence their efficiency, as through autoclaving or alkaline treatment, the cell wall is degraded and more functional groups are made available for biosorption. Initial pharmaceutical concentration can also have an impact on biosorption; at high concentrations, the amount of pollutant that is biosorbed per unit weight of the biosorbent increases, but removal effectiveness drops.
On the other hand, while the results obtained for free cells are occasionally better than those obtained for immobilized cells, the latter has the advantage of a high mechanical resistance, allowing the biosorbent to be used in a dynamic operating regime.
It is worth noting that the choice of the specific microorganism and the appropriate immobilization technique depends on factors such as the type and concentration of pharmaceuticals, the process conditions, and the objectives of the removal process. Further research and optimization are needed to determine the type of microbial biomass and the most suitable method of its immobilization to ensure efficient biosorption of pharmaceuticals under different conditions.
5. Conclusions and Future Perspectives
Pharmaceutical water pollution is a global issue that has an impact on both the environment and human health, especially when traditional approaches can’t provide an effective and secure solution; a large number of recent papers published underline the topic’s importance. According to this viewpoint, new environmentally friendly methods for removing pharmaceuticals from aqueous matrices must be developed, with the obvious goal of being applied at an industrial level.
The biosorption process can be efficient in removing pharmaceutical residues from various types of wastewater if a suitable biosorbent can be developed. From an ecological point of view, the use of biomaterials from renewable resources (microbial biomass, natural polymers) in biosorption processes has seen a constant increase in popularity in the last ten years.
This review evaluated the use of microbial biomass and natural polymers as biosorbents for the removal of pharmaceuticals from wastewater (simulated, real, or model solutions) by examining recent literature and taking into account the lack of in-depth analyses on this topic. The analyzed articles detailed several preparation and modification processes for biosorbents, demonstrating that microorganisms or natural polymers can provide extremely high biosorption capabilities and can be used to treat organically loaded wastewater in a more economic, efficient, and effective way than conventional wastewater treatment methods due to their high level of tolerance to contaminants. The production of efficient biosorbents (with improved adsorptive capacity and higher porosity) requires more analysis, as according to the literature data, natural polymers are less frequently utilized as single adsorbents and are more frequently used as composites, either with microbial biomass or with inorganic compounds. Therefore, research on microbial biomass or biopolymers is required to enhance the effectiveness of drug removal.
Future research should concentrate on the features of microbial biomass, whether it is active or inert, with the goal of achieving superior qualities and a good cost-benefit ratio acceptable for pharmaceutical removal. The use of microbial biomass in immobilized form could be helpful to future researchers and could serve as the basis for future advances, as it provides the framework for continuous system operation. These systems allow the treatment of large volumes of the liquid phase and should be applied for the study of real systems, i.e., wastewater from various polluted sources loaded with residues of pharmaceutical products. Additionally, it is crucial to optimize the water treatment process in pilot plants, for easy scale up. Biosorption studies focus on laboratory-scale applications, but they provide current knowledge of biosorption that is sufficient to provide a solid basis for expanding its use. The application of biosorption on an industrial scale has not yet been exploited, and this constitutes one of the major weaknesses that biosorption has to face.
Deeper studies covering biosorption on more emerging contaminants, including occur-ring mechanisms, more details on reaction intermediates and related pathways, related environmental aspects expressed in terms of water quality parameters, and more details on the effect of water parameters, are required in order to make biosorption an industrial process. Wherever feasible, biosorption should be incorporated into conventional techniques to create new secondary treatment systems to remove emerging contaminants, thus making these systems more profitable and environmentally friendly.
Author Contributions
Conceptualization, L.R. and D.Ș.; methodology, L.R. and E.-M.S.; validation, L.R., E.-M.S., F.M.N. and D.Ș.; investigation, E.-M.S., L.R., F.M.N. and A.-C.B.; resources, L.R.; writing—original draft preparation, L.R., E.-M.S., A.-C.B. and D.Ș.; writing—review and editing, L.R. and E.-M.S.; supervision, L.R. and D.Ș.; funding acquisition, L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.-H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Ahmad, A.; Priyadarshini, M.; Das, S.; Ghangrekar, M.M. Electrocoagulation as an efficacious technology for the treatment of wastewater containing active pharmaceutical compounds: A review. Sep. Sci. Technol. 2021, 57, 1234–1256. [Google Scholar] [CrossRef]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment—Occurrence and environmental implications. Eur. J. Pharmacol. 2019, 866, 172813. [Google Scholar] [CrossRef]
- Silva, C.P.; Jaria, G.; Otero, M.; Esteves, V.I.; Calisto, V. Waste-based alternative adsorbents for the remediation of pharmaceutical contaminated waters: Has a step forward already been taken? Bioresour. Technol. 2018, 250, 888–901. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Zhang, Y.; Ge, F.; Steel, R.M.; Sun, L. Advances in technologies for pharmaceuticals and personal care products removal. J. Mater. Chem. A 2017, 5, 12001–12014. [Google Scholar] [CrossRef]
- Uribe, I.O.; Mosquera-Corral, A.; Rodicio, J.L.; Esplugas, S. Advanced Technologies for Water Treatment and Reuse; Wiley Online Library: Hoboken, NJ, USA, 2015; Volume 61, pp. 3146–3158. [Google Scholar]
- Fierascu, R.C.; Fierascu, I.; (Brazdis), R.I.M.; Manaila-Maximean, D. Natural and Natural-Based Polymers: Recent Developments in Management of Emerging Pollutants. Polymers 2023, 15, 2063. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Fighir, D.; Paduraru, C. Simultaneous biosorption of micropollutants from aqueous effluents by rapeseed waste. Process. Saf. Environ. Prot. 2019, 132, 231–239. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L. Removal of methylene blue dye from aqueous solution using seashell wastes as biosorbent. Environ. Eng. Manag. J. 2012, 11, 1977–1985. [Google Scholar] [CrossRef]
- Rusu, L.; Harja, M.; Munteanu, C.; Ciobanu, G.; Suteu, D. Red and brown peat use in removing pollutants from municipal and industrial wastewater. J. Environ. Prot. Ecol. 2014, 15, 1690–1699. [Google Scholar]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Blaga, A.-C.; Harja, M. Encapsulation of Saccharomyces pastorianus Residual Biomass in Calcium Alginate Matrix with Insights in Ethacridine Lactate Biosorption. Polymers 2022, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Poddar, K.; Sarkar, D.; Chakraborty, D.; Patil, P.B.; Maity, S.; Sarkar, A. Paracetamol biodegradation by Pseudomonas strain PrS10 isolated from pharmaceutical effluents. Int. Biodeterior. Biodegrad. 2022, 175, 105490. [Google Scholar] [CrossRef]
- Parolini, M.; Binelli, A. Sub-lethal effects induced by a mixture of three non-steroidal anti-inflammatory drugs (NSAIDs) on the freshwater bivalve Dreissena polymorpha. Ecotoxicology 2011, 21, 379–392. [Google Scholar] [CrossRef]
- Ramos, A.; Correia, A.; Antunes, S.; Gonçalves, F.; Nunes, B. Effect of acetaminophen exposure in Oncorhynchus mykiss gills and liver: Detoxification mechanisms, oxidative defence system and peroxidative damage. Environ. Toxicol. Pharmacol. 2014, 37, 1221–1228. [Google Scholar] [CrossRef]
- Basol, N.; Ozmen, C.; Ocakli, S.; Cetin, S. Evaluation of the effects of curcumin, erdosteine, vitamin E and vitamin C on paracetamol toxicity. Medicine 2022, 11, 465. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Adewuyi, A. Chemically Modified Biosorbents and Their Role in the Removal of Emerging Pharmaceutical Waste in the Water System. Water 2020, 12, 1551. [Google Scholar] [CrossRef]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Freitas, O.M. The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: A Review. Water 2019, 11, 1555. [Google Scholar] [CrossRef]
- Adewuyi, A.; Oderinde, R.A. Chemically modified vermiculite clay: A means to remove emerging contaminant from polluted water system in developing nation. Polym. Bull. 2019, 76, 4967–4989. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, C.; Zheng, X.; Liu, S.; Hu, F.; Xu, L.; Xu, G.; Peng, X. Removal of Ciprofloxacin with Aluminum-Pillared Kaolin Sodium Alginate Beads (CA-Al-KABs): Kinetics, Isotherms, and BBD Model. Water 2020, 12, 905. [Google Scholar] [CrossRef]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of Produced Water in the Permian Basin for Hydraulic Fracturing: Comparison of Different Coagulation Processes and Innovative Filter Media. Water 2020, 12, 770. [Google Scholar] [CrossRef]
- Vrinceanu, N.; Hlihor, R.M.; Simion, A.I.; Rusu, L.; Fekete-Kertész, I.; Barka, N.; Favier, L. New Evidence of the Enhanced Elimination of a Persistent Drug Used as a Lipid Absorption Inhibitor by Advanced Oxidation with UV-A and Nanosized Catalysts. Catalysts 2019, 9, 761. [Google Scholar] [CrossRef]
- Favier, L.; Rusu, L.; Simion, A.I.; Hlihor, R.M.; Pacala, M.L.; Augustyniak, A. Efficient degradation of clofibric acid by heterogeneous photocatalytic oxidation process. Environ. Eng. Manag. J. 2019, 18, 1683–1692. [Google Scholar] [CrossRef]
- Fernández-Castro, P.; Vallejo, M.; Román, M.F.S.; Ortiz, I. Insight on the fundamentals of advanced oxidation processes. Role and review of the determination methods of reactive oxygen species. J. Chem. Technol. Biotechnol. 2015, 90, 796–820. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Napierska, D.; Sanseverino, I.; Loos, R.; Cortés, L.G.; Niegowska, M.; Lettieri, T. Modes of action of the current Priority Substances list under the Water Framework Directive and other substances of interest. Publ. Off. Eur. Union. Doi 2018, 10, 226911. [Google Scholar]
- Pal, R.; Megharaj, M.; Kirkbride, K.P.; Naidu, R. Illicit drugs and the environment—A review. Sci. Total Environ. 2013, 463, 1079–1092. [Google Scholar] [CrossRef]
- Ort, C.; Lawrence, M.G.; Rieckermann, J.; Joss, A. Sampling for Pharmaceuticals and Personal Care Products (PPCPs) and Illicit Drugs in Wastewater Systems: Are Your Conclusions Valid? A Critical Review. Environ. Sci. Technol. 2010, 44, 6024–6035. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S. Review: Pharmacological Pollution in Water. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1074–1116. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Urase, T.; Kusakabe, O. Biodegradation Characteristics of Pharmaceutical Substances by Whole Fungal Culture Trametes versicolor and its Laccase. J. Water Environ. Technol. 2010, 8, 125–140. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.; Silva, A.M. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Noman, E.A.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Talip, B.A.; Hossain, S.; Altowayti, W.A.H.; Ismail, N. Sustainable approaches for removal of cephalexin antibiotic from non-clinical environments: A critical review. J. Hazard. Mater. 2021, 417, 126040. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; El-Monaem, E.M.A.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2021, 15, 103543. [Google Scholar] [CrossRef]
- Rashtbari, Y.; Hazrati, S.; Azari, A.; Afshin, S.; Fazlzadeh, M.; Vosoughi, M. A novel, eco-friendly and green synthesis of PPAC-ZnO and PPAC-nZVI nanocomposite using pomegranate peel: Cephalexin adsorption experiments, mechanisms, isotherms and kinetics. Adv. Powder Technol. 2020, 31, 1612–1623. [Google Scholar] [CrossRef]
- Grisales-Cifuentes, C.M.; Galvis EA, S.; Porras, J.; Flórez, E.; Torres-Palma, R.A.; Acelas, N. Kinetics, isotherms, effect of structure, and computational analysis during the removal of three representative pharmaceuticals from water by adsorption using a biochar obtained from oil palm fiber. Bioresour. Technol. 2021, 326, 124753. [Google Scholar] [CrossRef] [PubMed]
- Samarghandi, M.R.; Asgari, G.; Shokoohi, R.; Dargahi, A.; Arabkouhsar, A. Removing amoxicillin antibiotic from aqueous solutions by Saccharomyces cerevisiae yeast bioadsorbent: Kinetic, thermodynamic and isotherm studies. Desalination Water Treat. 2019, 152, 306–315. [Google Scholar] [CrossRef]
- Al-Gheethi, A.; Efaq, A.; Mohamed, R.; Norli, I.; Kadir, M. Potential of bacterial consortium for removal of cephalexin from aqueous solution. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 24, 141–148. [Google Scholar] [CrossRef]
- Yu, J.; Kang, Y.; Yin, W.; Fan, J.; Guo, Z. Removal of Antibiotics from Aqueous Solutions by a Carbon Adsorbent Derived from Protein-Waste-Doped Biomass. ACS Omega 2020, 5, 19187–19193. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.M.; Șuteu, D.; Harja, M. Application of Saccharomyces cerevisiae/Calcium Alginate Composite Beads for Cephalexin Antibiotic Biosorption from Aqueous Solutions. Materials 2021, 14, 4728. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Istrate, B.; Harja, M. Biosorption Potential of Microbial and Residual Biomass of Saccharomyces pastorianus Immobilized in Calcium Alginate Matrix for Pharmaceuticals Removal from Aqueous Solutions. Polymers 2022, 14, 2855. [Google Scholar] [CrossRef]
- Adel, A.; Lalung, J.; Efaq, A.; Ismail, N. Removal of cephalexin antibiotic and heavy metals from pharmaceutical effluents using Bacillus subtilis strain. Expert Opin. Environ. Biol. 2015, 4, 2. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Wang, J. Biodegradation of typical pharmaceutical compounds by a novel strain Acinetobacter sp. J. Environ. Manag. 2018, 217, 240–246. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Suceveanu, E.M.; Simion, A.-I.; Botezatu, A.V.D.; Istrate, B.; Doroftei, I. Eco-Friendly Biosorbents Based on Microbial Biomass and Natural Polymers: Synthesis, Characterization and Application for the Removal of Drugs and Dyes from Aqueous Solutions. Materials 2021, 14, 4810. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Schnakovszky, C.; Favier, L. Investigation into Biosorption of Pharmaceuticals from Aqueous Solutions by Biocomposite Material Based on Microbial Biomass and Natural Polymer: Process Variables Optimization and Kinetic Studies. Polymers 2022, 14, 3388. [Google Scholar] [CrossRef]
- Dhankhar, R.; Hooda, A. Fungal biosorption—An alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 2011, 32, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.; Saravanan, A.; Kumar, P.S.; Yaashikaa, P.; Thamarai, P.; Shaji, A.; Rangasamy, G. A review on algae biosorption for the removal of hazardous pollutants from wastewater: Limiting factors, prospects and recommendations. Environ. Pollut. 2023, 327, 121572. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.-S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Rusu, L.; Harja, M.; Simion, A.I.; Suteu, D.; Ciobanu, G.; Favier, L. Removal of Astrazone Blue from aqueous solutions onto brown peat. Equilibrium and kinetics studies. Korean J. Chem. Eng. 2014, 31, 1008–1015. [Google Scholar] [CrossRef]
- Suteu, D.; Biliuta, G.; Rusu, L.; Coseri, S.; Nacu, G. Cellulose cellets as new type of adsorbent for the removal of dyes from aqueous media. Environ. Eng. Manag. J. 2015, 14, 525–532. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Wang, T. Cadmium biosorption by lactic acid bacteria Weissella viridescens ZY-6. Food Control 2020, 123, 107747. [Google Scholar] [CrossRef]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef]
- Benjedim, S.; Romero-Cano, L.A.; Pérez-Cadenas, A.F.; Bautista-Toledo, M.I.; Lotfi, E.M.; Carrasco-Marín, F. Removal of emerging pollutants present in water using an E-coli biofilm supported onto activated carbons prepared from argan wastes: Adsorption studies in batch and fixed bed. Sci. Total Environ. 2020, 720, 137491. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Yang, M.; Zhang, J.; Yang, B.; Lin, K.; Li, J.; Gan, J. Toxicity, degradation and metabolic fate of ibuprofen on freshwater diatom Navicula sp. J. Hazard. Mater. 2017, 330, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.; Castellet-Rovira, F.; Villagrasa, M.; Badia-Fabregat, M.; Barceló, D.; Vicent, T.; Caminal, G.; Sarrà, M.; Rodríguez-Mozaz, S. The role of sorption processes in the removal of pharmaceuticals by fungal treatment of wastewater. Sci. Total. Environ. 2018, 610, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Sanches, S.; Pereira, I.A. Anaerobic biodegradation of pharmaceutical compounds: New insights into the pharmaceutical-degrading bacteria. J. Hazard. Mater. 2018, 357, 289–297. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.; Feijoo, G.; Moreira, M.; Lema, J. Operation of stirred tank reactors (STRs) and fixed-bed reactors (FBRs) with free and immobilized Phanerochaete chrysosporium for the continuous removal of pharmaceutical compounds. Biochem. Eng. J. 2012, 66, 38–45. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.E.; Barón, E.; Gago-Ferrero, P.; Jelić, A.; Llorca, M.; Farré, M.; Díaz-Cruz, M.S.; Eljarrat, E.; Petrović, M.; Caminal, G.; et al. Removal of pharmaceuticals, polybrominated flame retardants and UV-filters from sludge by the fungus Trametes versicolor in bioslurry reactor. J. Hazard. Mater. 2012, 233, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiang, Z.; Li, Y.; Ben, W. An insight into the removal of fluoroquinolones in activated sludge process: Sorption and biodegradation characteristics. J. Environ. Sci. 2017, 56, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Al-Gheethi, A.A.S.; Ismail, N. Biodegradation of Pharmaceutical Wastes in Treated Sewage Effluents by Bacillus subtilis 1556WTNC. Environ. Process. 2014, 1, 459–481. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Nghiem, L.D.; Oh, S. Aerobic biotransformation of the antibiotic ciprofloxacin by Bradyrhizobium sp. isolated from activated sludge. Chemosphere 2018, 211, 600–607. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Rehman, M.U.; Tauseef, M.; Islam, E.; Hayat, A.; Iqbal, S.; Arslan, M.; Afzal, M. Ciprofloxacin Removal from Aqueous Media Using Floating Treatment Wetlands Supported by Immobilized Bacteria. Sustainability 2022, 14, 14216. [Google Scholar] [CrossRef]
- Liang, D.H.; Hu, Y.; Cheng, J.; Chen, Y. Enhanced performance of sulfamethoxazole degradation using Achromobacter sp. JL9 with in-situ generated biogenic manganese oxides. Bioresour. Technol. 2021, 333, 125089. [Google Scholar] [CrossRef]
- Liao, X.; Zou, R.; Li, B.; Tong, T.; Xie, S.; Yuan, B. Biodegradation of chlortetracycline by acclimated microbiota. Process. Saf. Environ. Prot. 2017, 109, 11–17. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Molina, R.; Martínez, F.; Melero, J.A. Biological removal of pharmaceutical and personal care products by a mixed microbial culture: Sorption, desorption and biodegradation. Biochem. Eng. J. 2013, 81, 108–119. [Google Scholar] [CrossRef]
- Lin, B.; Lyu, J.; Lyu, X.-J.; Yu, H.-Q.; Hu, Z.; Lam, J.C.; Lam, P.K. Characterization of cefalexin degradation capabilities of two Pseudomonas strains isolated from activated sludge. J. Hazard. Mater. 2015, 282, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.R.A.; Amorim, C.L.; Cravo, S.M.; Tiritan, M.E.; Castro, P.M.L.; Afonso, C.M.M. Fluoroquinolones biosorption onto microbial biomass: Activated sludge and aerobic granular sludge. Int. Biodeterior. Biodegrad. 2016, 110, 53–60. [Google Scholar] [CrossRef]
- Dawas-Massalha, A.; Gur-Reznik, S.; Lerman, S.; Sabbah, I.; Dosoretz, C.G. Co-metabolic oxidation of pharmaceutical compounds by a nitrifying bacterial enrichment. Bioresour. Technol. 2014, 167, 336–342. [Google Scholar] [CrossRef]
- Langenhoff, A.; Inderfurth, N.; Veuskens, T.; Schraa, G.; Blokland, M.; Kujawa-Roeleveld, K.; Rijnaarts, H. Microbial Removal of the Pharmaceutical Compounds Ibuprofen and Diclofenac from Wastewater. BioMed Res. Int. 2013, 2013, 325806. [Google Scholar] [CrossRef]
- Bessa, V.; Moreira, I.; Tiritan, M.; Castro, P. Enrichment of bacterial strains for the biodegradation of diclofenac and carbamazepine from activated sludge. Int. Biodeterior. Biodegrad. 2017, 120, 135–142. [Google Scholar] [CrossRef]
- Moreira, I.S.; Ribeiro, A.R.; Afonso, C.M.; Tiritan, M.E.; Castro, P.M. Enantioselective biodegradation of fluoxetine by the bacterial strain Labrys portucalensis F11. Chemosphere 2014, 111, 103–111. [Google Scholar] [CrossRef]
- Jathanna, N.N.; Krishnamurthy, G.K.; Paithankar, J.G.; Hegde, S.; Goveas, L.C.; Ravindranath, B.S.; Gowdru, M. Phyto-bacterial biosorption of basic fuchsine: A self-sustainable approach towards biomitigation of contaminant of emerging concern. J. Environ. Chem. Eng. 2023, 11, 109330. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Yang, S.; Kang, J.; Leusch, F.D.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of pharmaceuticals, steroid hormones, phytoestrogens, UV-filters, industrial chemicals and pesticides by Trametes versicolor: Role of biosorption and biodegradation. Int. Biodeterior. Biodegrad. 2014, 88, 169–175. [Google Scholar] [CrossRef]
- Vasiliadou, I.; Sánchez-Vázquez, R.; Molina, R.; Martínez, F.; Melero, J.; Bautista, L.; Iglesias, J.; Morales, G. Biological removal of pharmaceutical compounds using white-rot fungi with concomitant FAME production of the residual biomass. J. Environ. Manag. 2016, 180, 228–237. [Google Scholar] [CrossRef]
- Dalecka, B.; Juhna, T.; Rajarao, G.K. Constructive use of filamentous fungi to remove pharmaceutical substances from wastewater. J. Water Process. Eng. 2020, 33, 100992. [Google Scholar] [CrossRef]
- Li, X.; Li, G. A review: Pharmaceutical wastewater treatment technology and research in China. In Proceedings of the Asia-Pacific Energy Equipment Engineering Research Conference, Zhuhai, China, 13–14 June 2015; Atlantis Press: Amsterdam, The Netherlands, 2015; pp. 345–348. [Google Scholar]
- Zhang, Y.; Geißen, S.-U. Elimination of carbamazepine in a non-sterile fungal bioreactor. Bioresour. Technol. 2012, 112, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Della Giustina, S.V.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase—Degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef]
- Becker, D.; Rodriguez-Mozaz, S.; Insa, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; Misovic, A.; Oehlmann, J.; et al. Removal of Endocrine Disrupting Chemicals in Wastewater by Enzymatic Treatment with Fungal Laccases. Org. Process. Res. Dev. 2017, 21, 480–491. [Google Scholar] [CrossRef]
- Simelane, N.P.; Asante, J.K.; Ndibewu, P.P.; Mramba, A.S.; Sibali, L.L. Biopolymer composites for removal of toxic organic compounds in pharmaceutical effluents—A review. Carbohydr. Polym. Technol. Appl. 2022, 4, 100239. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Krumm, B.; Kofen, M. Synthesis and characterization of new insensitive and high-energy dense cellulosic biopolymers. Fuel 2021, 292, 120347. [Google Scholar] [CrossRef]
- Gowthaman, N.; Lim, H.; Sreeraj, T.; Amalraj, A.; Gopi, S. Advantages of biopolymers over synthetic polymers: Social, economic, and environmental aspects. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. [Google Scholar]
- Zia, Z.; Hartland, A.; Mucalo, M.R. Use of low-cost biopolymers and biopolymeric composite systems for heavy metal removal from water. Int. J. Environ. Sci. Technol. 2020, 17, 4389–4406. [Google Scholar] [CrossRef]
- Kong, Y.; Zhuang, Y.; Han, K.; Shi, B. Enhanced tetracycline adsorption using alginate-graphene-ZIF67 aerogel. Colloids Surf. A Physicochem. Eng. Asp. 2019, 588, 124360. [Google Scholar] [CrossRef]
- Gu, J.; Liu, Z.; Jia, A.; Wang, Y.; Li, N.; Liu, Z.; Li, Y.; Zhang, H. New insight into adsorption and co-adsorption of chlortetracycline hydrochloride and ciprofloxacin hydrochloride by Ga-based metal-organic gel/sodium alginate composite beads. Sep. Purif. Technol. 2023, 312, 123408. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Strong adsorption properties and mechanism of action with regard to tetracycline adsorption of double-network polyvinyl alcohol-copper alginate gel beads. J. Hazard. Mater. 2021, 422, 126863. [Google Scholar] [CrossRef]
- Shamsudin, M.S.; Azha, S.F.; Sellaoui, L.; Badawi, M.; Bonilla-Petriciolet, A.; Ismail, S. Performance and interactions of diclofenac adsorption using Alginate/Carbon-based Films: Experimental investigation and statistical physics modelling. Chem. Eng. J. 2021, 428, 131929. [Google Scholar] [CrossRef]
- Silva, E.C.; Soares, V.R.; Fajardo, A.R. Removal of pharmaceuticals from aqueous medium by alginate/polypyrrole/ZnFe2O4 beads via magnetic field enhanced adsorption. Chemosphere 2023, 316, 137734. [Google Scholar] [CrossRef]
- de Araújo, T.P.; Quesada, H.B.; Dos Santos, D.F.; da Silva Fonseca, B.C.; Barbieri, J.Z.; Bergamasco, R.; de Barros, M.A.S.D. Acetaminophen removal by calcium alginate/activated hydrochar composite beads: Batch and fixed-bed studies. International J. Biol. Macromol. 2022, 203, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Tunioli, F.; Khaliha, S.; Mantovani, S.; Bianchi, A.; Kovtun, A.; Xia, Z.; Bafqi, M.S.S.; Okan, B.S.; Marforio, T.D.; Calvaresi, M.; et al. Adsorption of emerging contaminants by graphene related materials and their alginate composite hydrogels. J. Environ. Chem. Eng. 2023, 11, 109566. [Google Scholar] [CrossRef]
- Ajebli, S.; Kaichouh, G.; Khachani, M.; Babas, H.; EL Karbane, M.; Safi, Z.S.; Berisha, A.; Mehmeti, V.; Warad, I.; Zarrouk, A.; et al. Modeling of tenofovir disoproxil fumarate decontamination using sodium alginate-encapsulated activated carbon: Molecular dynamics, monte carlo and density functional theory. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131057. [Google Scholar] [CrossRef]
- Filho, F.G.N.; Filho, E.C.S.; Osajima, J.A.; de Melo Alves, A.P.; Fonseca, M.G. Adsorption of tetracycline using chitosan–alginate–bentonite composites. Appl. Clay Sci. 2023, 239, 106952. [Google Scholar] [CrossRef]
- Shahrin, E.W.E.; Narudin, N.A.H.; Shahri, N.N.M.; Nur, M.; Lim, J.-W.; Bilad, M.R.; Mahadi, A.H.; Hobley, J.; Usman, A. A comparative study of adsorption behavior of rifampicin, streptomycin, and ibuprofen contaminants from aqueous solutions onto chitosan: Dynamic interactions, kinetics, diffusions, and mechanisms. Emerg. Contam. 2023, 9, 100199. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Y.; Yang, Z.; Yang, W.; Wu, J.; Dong, C. Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem. Eng. J. 2016, 304, 325–334. [Google Scholar] [CrossRef]
- Turan, B.; Sarigol, G.; Demircivi, P. Adsorption of tetracycline antibiotics using metal and clay embedded cross-linked chitosan. Mater. Chem. Phys. 2022, 279, 125781. [Google Scholar] [CrossRef]
- Nasiri, A.; Rajabi, S.; Amiri, A.; Fattahizade, M.; Hasani, O.; Lalehzari, A.; Hashemi, M. Adsorption of tetracycline using CuCoFe2O4@Chitosan as a new and green magnetic nanohybrid adsorbent from aqueous solutions: Isotherm, kinetic and thermodynamic study. Arab. J. Chem. 2022, 15, 104014. [Google Scholar] [CrossRef]
- Valizadeh, K.; Bateni, A.; Sojoodi, N.; Rafiei, R.; Behroozi, A.H.; Maleki, A. Preparation and characterization of chitosan-curdlan composite magnetized by zinc ferrite for efficient adsorption of tetracycline antibiotics in water. Int. J. Biol. Macromol. 2023, 235, 123826. [Google Scholar] [CrossRef]
- Yaqubi, O.; Tai, M.H.; Mitra, D.; Gerente, C.; Neoh, K.G.; Wang, C.-H.; Andres, Y. Adsorptive removal of tetracycline and amoxicillin from aqueous solution by leached carbon black waste and chitosan-carbon composite beads. J. Environ. Chem. Eng. 2021, 9, 104988. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, J.; Chen, W.; Zheng, Z.; Yang, Z.; Liu, Z.; Zhou, L. Fabrication and adsorption mechanism of chitosan/Zr-MOF (UiO-66) composite foams for efficient removal of ketoprofen from aqueous solution. Chem. Eng. J. 2022, 431, 134045. [Google Scholar] [CrossRef]
- Şahin, M.; Arslan, Y.; Tomul, F. Removal of naproxen and diclofenac using magnetic nanoparticles/nanocomposites. Res. Chem. Intermed. 2022, 48, 5209–5226. [Google Scholar] [CrossRef]
- Quesada, H.B.; de Araújo, T.P.; Cusioli, L.F.; de Barros, M.A.S.D.; Gomes, R.G.; Bergamasco, R. Caffeine removal by chitosan/activated carbon composite beads: Adsorption in tap water and synthetic hospital wastewater. Chem. Eng. Res. Des. 2022, 184, 1–12. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Effect of humic acid on pharmaceuticals adsorption using sulfonic acid grafted chitosan. J. Mol. Liq. 2017, 230, 1–5. [Google Scholar] [CrossRef]
- Davarnejad, R.; Sarvmeili, K.; Safari, Z.; Kennedy, J.F. Estrogen adsorption from an aqueous solution on the chitosan nanoparticles. Int. J. Biol. Macromol. 2023, 237, 124224. [Google Scholar] [CrossRef] [PubMed]
- Kgomo, H.; Dube, S.; Nindi, M.M. Evaluating the Performance of Ball-Milled Silk Fibroin Films for Simultaneous Adsorption of Eight Pharmaceuticals from Water. Int. J. Environ. Res. Public Health 2022, 19, 14922. [Google Scholar] [CrossRef]
- Shokri, M.; Mojtabavi, S.; Jafari-Nodoushan, H.; Vojdanitalab, K.; Golshani, S.; Jahandar, H.; Faramarzi, M.A. Laccase-loaded magnetic dialdehyde inulin nanoparticles as an efficient heterogeneous natural polymer-based biocatalyst for removal and detoxification of ofloxacin. Biodegradation 2022, 33, 489–508. [Google Scholar] [CrossRef]
- Quesada, H.B.; de Araújo, T.P.; Vareschini, D.T.; de Barros, M.A.S.D.; Gomes, R.G.; Bergamasco, R. Chitosan, alginate and other macromolecules as activated carbon immobilizing agents: A review on composite adsorbents for the removal of water contaminants. Int. J. Biol. Macromol. 2020, 164, 2535–2549. [Google Scholar] [CrossRef]
- Hayeeye, F.; Sattar, M.; Chinpa, W.; Sirichote, O. Kinetics and thermodynamics of Rhodamine B adsorption by gelatin/activated carbon composite beads. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 259–266. [Google Scholar] [CrossRef]
- Sattar, M.; Hayeeye, F.; Chinpa, W.; Sirichote, O. Preparation and characterization of poly (lactic acid)/activated carbon composite bead via phase inversion method and its use as adsorbent for Rhodamine B in aqueous solution. J. Environ. Chem. Eng. 2017, 5, 3780–3791. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Omer, A.M.; Hassan, M.A.; Hassan, M.E.; Sabet, M.M.; Eldin, M.S.M. Development of thermo-sensitive poly N-isopropyl acrylamide grafted chitosan derivatives. J. Appl. Pharm. Sci. 2015, 5, 001–006. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, Z.; Ren, R.; Sui, X.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B. Chitosan adsorbent reinforced with citric acid modified β-cyclodextrin for highly efficient removal of dyes from reactive dyeing effluents. Eur. Polym. J. 2018, 108, 212–218. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.; Hanafiah, M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium. Pharmaceutics 2021, 13, 338. [Google Scholar] [CrossRef]
- Solano, R.A.; De León, L.D.; De Ávila, G.; Herrera, A.P. Polycyclic aromatic hydrocarbons (PAHs) adsorption from aqueous solution using chitosan beads modified with thiourea, TiO2 and Fe3O4 nanoparticles. Environ. Technol. Innov. 2021, 21, 101378. [Google Scholar] [CrossRef]
- Liakos, E.V.; Lazaridou, M.; Michailidou, G.; Koumentakou, I.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Chitosan Adsorbent Derivatives for Pharmaceuticals Removal from Effluents: A Review. Macromol 2021, 1, 130–154. [Google Scholar] [CrossRef]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Adsorption of Cu (II)and Co (II) from aqueous solution using lignosulfonate/chitosan adsorbent. Int. J. Biol. Macromol. 2020, 163, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Tanhaei, B.; Ayati, A.; Iakovleva, E.; Sillanpää, M. Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: Synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 2020, 164, 3621–3631. [Google Scholar] [CrossRef] [PubMed]
- Asgari, E.; Sheikhmohammadi, A.; Yeganeh, J. Application of the Fe3O4-chitosan nano-adsorbent for the adsorption of metronidazole from wastewater: Optimization, kinetic, thermodynamic and equilibrium studies. Int. J. Biol. Macromol. 2020, 164, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Ranjbari, S.; Tanhaei, B.; Ayati, A.; Orooji, Y.; Alizadeh, M.; Karimi, F.; Salmanpour, S.; Rouhi, J.; Sillanpää, M.; et al. Novel 1-butyl-3-methylimidazolium bromide impregnated chitosan hydrogel beads nanostructure as an efficient nanobio-adsorbent for cationic dye removal: Kinetic study. Environ. Res. 2021, 195, 110809. [Google Scholar] [CrossRef] [PubMed]
- Shebl, A.; Omer, A.; Tamer, T. Adsorption of cationic dye using novel O-amine functionalized chitosan Schiff base derivatives: Isotherm and kinetic studies. Desalination Water Treat. 2018, 130, 132–141. [Google Scholar] [CrossRef]
- Shankar, P.; Gomathi, T.; Vijayalakshmi, K.; Sudha, P. Comparative studies on the removal of heavy metals ions onto cross linked chitosan-g-acrylonitrile copolymer. Int. J. Biol. Macromol. 2014, 67, 180–188. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Chen, D.-Y.; Pathak, J.L.; Long, J.; Subbaiah, M.V.; Wen, J.-C.; Pan, C.-L. Preparation of novel aminated chitosan schiff’s base derivative for the removal of methyl orange dye from aqueous environment and its biological applications. Int. J. Biol. Macromol. 2020, 146, 1100–1110. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2021, 33, 100565. [Google Scholar] [CrossRef]
- Tofan, L.; Bojoaga, C.-N.; Paduraru, C. Biosorption for the recovery and analysis of rare earth elements and platinum group metals from real samples. A review. Environ. Chem. Lett. 2022, 20, 1225–1248. [Google Scholar] [CrossRef]
- Velkova, Z.; Kirova, G.; Stoytcheva, M.; Kostadinova, S.; Todorova, K.; Gochev, V. Immobilized microbial biosorbents for heavy metals removal. Eng. Life Sci. 2018, 18, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2018, 17, 241–257. [Google Scholar] [CrossRef]
- Brahma, B.; Das, M.; Sarkar, P.; Sarkar, U. Biosorption of p-chloro meta xylenol (PCMX) by bacterium-encapsulated calcium alginate beads in a novel plug flow process. J. Environ. Manag. 2023, 337, 117764. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).