Laminar Biomaterial Composite of PVA Cryogel with Amnion as Potential Wound Dressing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods for Preparation of the Dressing
2.2.1. PVA Cryogel Preparation
2.2.2. Dressing Preparation
2.2.3. E-Beam Irradiation
2.3. Characterization of Dressing Properties
2.3.1. Measurements of Gel Fraction Yield and Equilibrium Degree of Swelling
2.3.2. Mechanical Properties
2.3.3. Scanning Electron Microscopy
2.3.4. X-ray Studies
2.4. Biological Studies
2.4.1. Cytotoxicity Assay of Extract of PVA/Amnion Material
2.4.2. Cell Proliferation and Death Studies
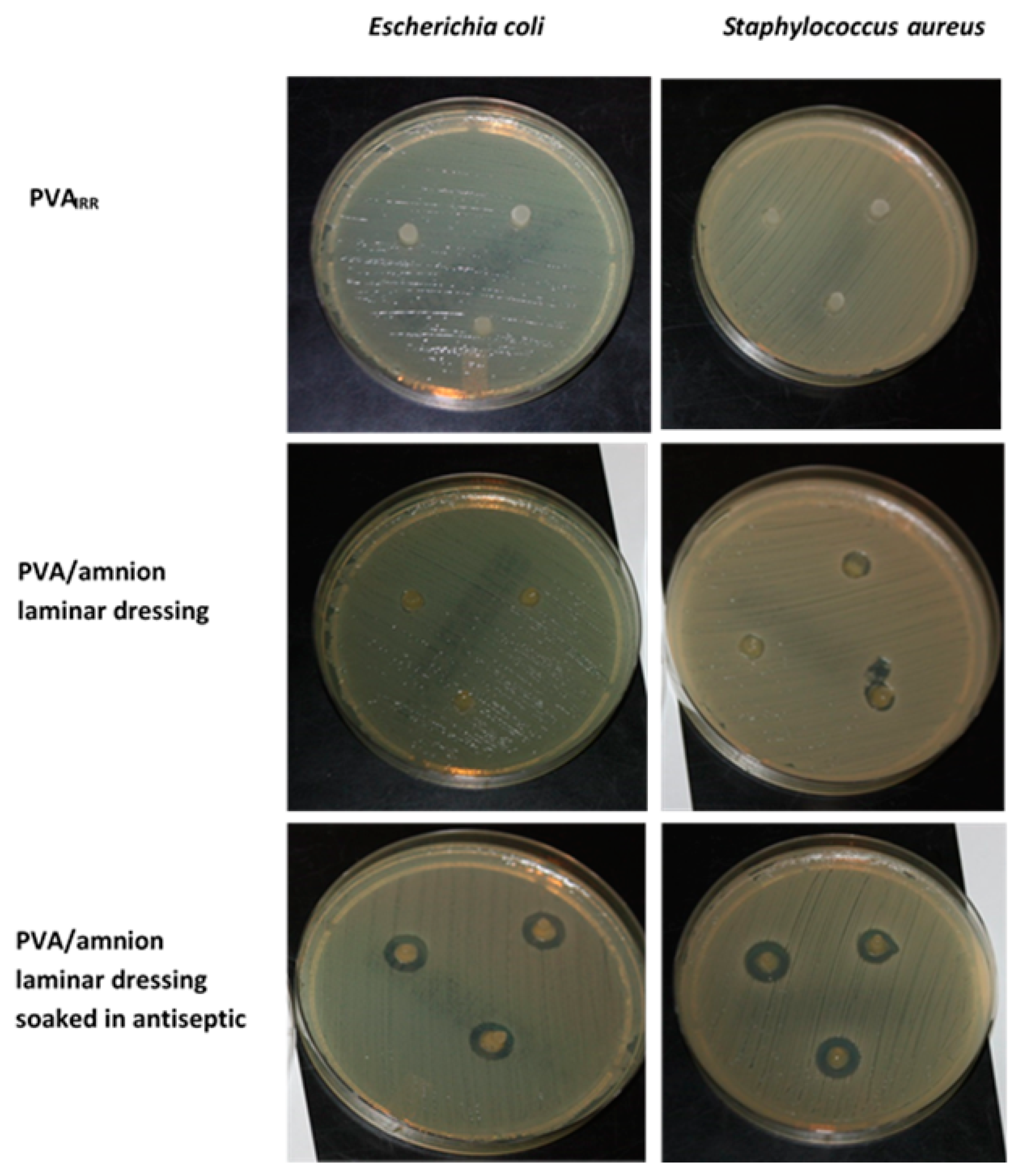
2.4.3. Antibacterial Studies
2.4.4. Histology of the Amnion and Its Laminar Dressing with Cryogel
3. Results and Discussion
3.1. Cryogel Formation and Characterization
3.2. Preparation of the Dressing
3.3. Biological Studies
3.3.1. Cytotoxicity Studies of the Extract from Amnion/PVA Material via MTT Test
3.3.2. Cell Proliferation and Death Studies
3.3.3. Antibacterial Studies
3.3.4. Histological Examinations of the Dressing with the Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dissemond, J.; Augustin, M.; Eming, S.A.; Goerge, T.; Horn, T.; Karrer, S.; Schumann, H.; Stücker, M. Modern wound care—Practical aspects of non-interventional topical treatment of patients with chronic wounds. JDDG J. Dtsch. Dermatol. Ges. 2014, 12, 541–554. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S. Synthetic Hydrogels for Biomedical Applications. In Hydrogels for Medical and Related Applications; ACS Symposium Series; Andrade, J.D., Ed.; American Chemical Society: Washington, DC, USA, 1976; Volume 31, pp. 1–36. ISBN 9780841203389. [Google Scholar]
- Wan, W.; Bannerman, A.D.; Yang, L.; Mak, H. Poly(vinyl alcohol) Cryogels for Biomedical Applications. In Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Okay, O., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 283–321. ISBN 978-3-319-05846-7. [Google Scholar]
- Hassan, C.M.; Peppas, N.A. Structure and Applications of Poly(vinyl alcohol) Hydrogels Produced by Conventional Crosslinking or by Freezing/Thawing Methods. In Biopolymers · PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2000; pp. 37–65. ISBN 978-3-540-46414-3. [Google Scholar]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A Comprehensive Review of Advanced Biopolymeric Wound Healing Systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Gajra, B.; Pandya, S.; Vidyasagar, G.; Rabari, H.; Dedania, R.; Rao, S. Poly vinyl alcohol Hydrogel and its Pharmaceutical and Biomedical Applications: A Review. Int. J. Pharm. Res. 2011, 4, 20–26. [Google Scholar]
- He, W.; Benson, R. 8—Polymeric Biomaterials. In Plastics Design Library, Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 145–164. ISBN 978-0-323-39040-8. [Google Scholar]
- Okay, O.; Lozinsky, V.I. Synthesis and Structure–Property Relationships of Cryogels. In Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Okay, O., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 103–157. ISBN 978-3-319-05846-7. [Google Scholar]
- Afshari, M.J.; Sheikh, N.; Afarideh, H. PVA/CM-chitosan/honey hydrogels prepared by using the combined technique of irradiation followed by freeze-thawing. Radiat. Phys. Chem. 2015, 113, 28–35. [Google Scholar] [CrossRef]
- Puspitasari, T.; Raja, K.M.L.; Pangerteni, D.S.; Patriati, A.; Putra, E.G.R. Structural Organization of Poly(vinyl alcohol) Hydrogels Obtained by Freezing/Thawing and γ-Irradiation Processes: A Small-Angle Neutron Scattering (SANS) Study. Procedia Chem. 2012, 4, 186–193. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, X.; Hu, Q.; Liu, S.; Guan, S. PVA/PEG hybrid hydrogels prepared by freeze-thawing and high energy electron beam irradiation. Chem. Res. Chin. Univ. 2017, 33, 995–999. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Gray, H. Anatomy of the Human Body, 20th ed.; Lewis, W.H., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1918. [Google Scholar]
- Hilmy, N.; Yusof, N.; Nather, A. Anatomy and Histology of Amnion. In Human Amniotic Membrane: Basic Science and Clinical Application; World Scientific: Singapore, 2018; pp. 87–101. [Google Scholar] [CrossRef]
- Niknejad, H.; Paeini-Vayghan, G.; Tehrani, F.A.; Khayat-Khoei, M.; Peirovi, H. Side dependent effects of the human amnion on angiogenesis. Placenta 2013, 34, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Farhadihosseinabadi, B.; Farahani, M.; Tayebi, T.; Jafari, A.; Biniazan, F.; Modaresifar, K.; Moravvej, H.; Bahrami, S.; Redl, H.; Tayebi, L.; et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Kogan, S.; Sood, A.; Granick, M.S. Amniotic Membrane Adjuncts and Clinical Applications in Wound Healing: A Review of the Literature. Wounds Compend. Clin. Res. Pract. 2018, 30, 168–173. [Google Scholar]
- Sabol, T.J.; Tran, G.S.; Matuszewski, J.; Weston, W.W. Standardized reporting of amnion and amnion/chorion allograft data for wound care. Health Sci. Rep. 2022, 5, e794. [Google Scholar] [CrossRef] [PubMed]
- Mencucci, R.; Menchini, U.; Dei, R. Antimicrobial activity of antibiotic-treated amniotic membrane: An in vitro study. Cornea 2006, 25, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Ramuta, T.Ž.; Tratnjek, L.; Janev, A.; Seme, K.; Starčič Erjavec, M.; Kreft, M.E. The antibacterial activity of human amniotic membrane against multidrug-resistant bacteria associated with urinary tract infections: New insights from normal and cancerous urothelial models. Biomedicines 2021, 9, 218. [Google Scholar] [CrossRef]
- Mao, Y.; Hoffman, T.; Singh-Varma, A.; Duan-Arnold, Y.; Moorman, M.; Danilkovitch, A.; Kohn, J. Antimicrobial Peptides Secreted From Human Cryopreserved Viable Amniotic Membrane Contribute to its Antibacterial Activity. Sci. Rep. 2017, 7, 13722. [Google Scholar] [CrossRef]
- Favaron, P.O.; Carvalho, R.C.; Borghesi, J.; Anunciação, A.R.A.; Miglino, M.A. The Amniotic Membrane: Development and Potential Applications—A Review. Reprod. Domest. Anim. 2015, 50, 881–892. [Google Scholar] [CrossRef]
- John, T.; Foulks, G.N.; John, M.E.; Cheng, K.; Hu, D. Amniotic membrane in the surgical management of acute toxic epidermal necrolysis. Ophthalmology 2002, 109, 351–360. [Google Scholar] [CrossRef]
- Forbes, J.; Fetterolf, D.E. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: A case series. J. Wound Care 2012, 21, 290–296. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Harrison, J. An Evaluation of Wound Healing Efficacy of a Film Dressing Made from Polymer-integrated Amnion Membrane. Organogenesis 2020, 16, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cañada, C.; Bernabé-García, Á.; Liarte, S.; Rodríguez-Valiente, M.; Nicolás, F.J. Chronic wound healing by amniotic membrane: TGF-β and EGF signaling modulation in re-epithelialization. Front. Bioeng. Biotechnol. 2021, 9, 689328. [Google Scholar] [CrossRef]
- Davis, J.S. Skin transplantation. Johns Hopkins Hosp. Rep. 1910, 15, 307–396. [Google Scholar]
- Bose, B. Burn wound dressing with human amniotic membrane. Ann. R. Coll. Surg. Engl. 1979, 61, 444–447. [Google Scholar] [PubMed]
- Fu, Y.; Liu, J.; Tseng, S.C.G. Ocular surface deficits contributing to persistent epithelial defect after penetrating keratoplasty. Cornea 2012, 31, 723–729. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Nelson, R.A., Jr.; Sunnon, K.; Reid, T.; Clouse, C.; Kock, N.D.; Jackson, J.; Soker, S.; Atala, A. Amnion membrane hydrogel and amnion membrane powder accelerate wound healing in a full thickness porcine skin wound model. Stem Cells Transl. Med. 2020, 9, 80–92. [Google Scholar] [CrossRef]
- Pazos, V.; Mongrain, R.; Tardif, J.C. Polyvinyl alcohol cryogel: Optimizing the parameters of cryogenic treatment using hyperelastic models. J. Mech. Behav. Biomed. Mater. 2009, 2, 542–549. [Google Scholar] [CrossRef]
- Gent, A.N. On the Relation between Indentation Hardness and Young’s Modulus. Rubber Chem. Technol. 1958, 31, 896–906. [Google Scholar] [CrossRef]
- Ricciardi, R.; Auriemma, F.; Gaillet, C.; De Rosa, C.; Lauprêtre, F. Investigation of the Crystallinity of Freeze/Thaw Poly(vinyl alcohol) Hydrogels by Different Techniques. Macromolecules 2004, 37, 9510–9516. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- Huang, Y.; Dan, N.; Dan, W.; Zhao, W. Reinforcement of Polycaprolactone/Chitosan with Nanoclay and Controlled Release of Curcumin for Wound Dressing. ACS Omega 2019, 4, 22292–22301. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, J.; Lin, H.; Ren, X.; Tian, H.; Liang, Y.; Wang, W.; Wang, Y.; Yin, M.; Huang, Y.; et al. In situ Fabrication of Nano ZnO/BCM Biocomposite Based on MA Modified Bacterial Cellulose Membrane for Antibacterial and Wound Healing. Int. J. Nanomed. 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Caló, E.; Barros, J.; Ballamy, L.; Khutoryanskiy, V.V. Poly(vinyl alcohol)–Gantrez® AN cryogels for wound care applications. RSC Adv. 2016, 6, 105487–105494. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, R.; Reinwald, Y.; Bhat, S. Cryogels: Freezing unveiled by thawing. Mater. Today 2010, 13, 42–44. [Google Scholar] [CrossRef]
- Kanaya, T.; Ohkura, M.; Takeshita, H.; Kaji, K.; Furusaka, M.; Yamaoka, H.; Wignall, G.D. Gelation Process of Poly(vinyl alcohol) As Studied by Small-Angle Neutron and Light Scattering. Macromolecules 1995, 28, 3168–3174. [Google Scholar] [CrossRef]
- Wan, W.K.; Campbell, G.; Zhang, Z.F.; Hui, A.J.; Boughner, D.R. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J. Biomed. Mater. Res. 2002, 63, 854–861. [Google Scholar] [CrossRef]
- Hunt, J.W. Early events in radiation chemistry. In Advances in Radiation Chemistry; Burton, M.M.J., Ed.; John Wiley & Sons: New York, NY, USA, 1976; pp. 185–315. ISBN 978-0471016694. [Google Scholar]
- Charlesby, A. Atomic Radiation and Polymers, 1st ed.; Charlesby, A., Ed.; Pergamon: Oxford, UK, 1960; ISBN 9781483181301. [Google Scholar]
- Sievers, J.; Sperlich, K.; Stahnke, T.; Kreiner, C.; Eickner, T.; Martin, H.; Guthoff, R.F.; Schünemann, M.; Bohn, S.; Stachs, O. Determination of hydrogel swelling factors by two established and a novel non-contact continuous method. J. Appl. Polym. Sci. 2021, 138, 50326. [Google Scholar] [CrossRef]
- Millon, L.E.; Oates, C.J.; Wan, W. Compression properties of polyvinyl alcohol—Bacterial cellulose nanocomposite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 922–929. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Ben Halima, N. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Cournane, S.; Cannon, L.; Browne, J.E.; Fagan, A.J. Assessment of the accuracy of an ultrasound elastography liver scanning system using a PVA-cryogel phantom with optimal acoustic and mechanical properties. Phys. Med. Biol. 2010, 55, 5965–5983. [Google Scholar] [CrossRef] [PubMed]
- Fromageau, J.; Gennisson, J.; Schmitt, C.; Maurice, R.L.; Mongrain, R.; Cloutier, G. Estimation of polyvinyl alcohol cryogel mechanical properties with four ultrasound elastography methods and comparison with gold standard testings. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Duboeuf, F.; Basarab, A.; Liebgott, H.; Brusseau, E.; Delachartre, P.; Vray, D. Investigation of PVA cryogel Young’s modulus stability with time, controlled by a simple reliable technique. Med. Phys. 2009, 36, 656–661. [Google Scholar] [CrossRef]
- Łabuś, W.; Kitala, D.; Klama-Baryła, A.; Szapski, M.; Kraut, M.; Smętek, W.; Glik, J.; Kucharzewski, M.; Rojczyk, E.; Utrata-Wesołek, A.; et al. Influence of electron beam irradiation on extracellular matrix of the human allogeneic skin grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Ramuta, T.Ž.; Starčič Erjavec, M.; Kreft, M.E. Amniotic Membrane Preparation Crucially Affects Its Broad Spectrum Activity Against Uropathogenic Bacteria. Front. Microbiol. 2020, 11, 469. [Google Scholar] [CrossRef]









| Sample | Temperature a | GF [%] | Equilibrium SD [%] | |
|---|---|---|---|---|
| PVANOT-IRR | PVA 5% | 40 °C | 81 | 505 |
| 90 °C | disintegration | |||
| PVA 10% | 40 °C | 96 | 325 | |
| 90 °C | disintegration | |||
| PVAIRR | PVA 5% 25 kGy | 40 °C | 96 ± 1 | 510 ± 2 |
| PVA 5% 35 kGy | 97 ± 1 | 506 ± 3 | ||
| PVA 10% 25 kGy | 96 ± 1 | 348 ± 4 | ||
| PVA 10% 35 kGy | 96 ± 1 | 357 ± 7 | ||
| PVA 5% 25 kGy | 90 °C | 90 ± 1 | 310 ± 3 | |
| PVA 5% 35 kGy | 91 ± 1 | 334 ± 3 | ||
| PVA 10% 25 kGy | 89 ± 1 | 247 ± 5 | ||
| PVA 10% 35 kGy | 91 ± 1 | 242 ± 8 | ||
| Relative Crystallinity, % | 5% PVA | 10% PVA |
|---|---|---|
| PVANOT-IRR | 0.69 ± 0.53 | 2.16 ± 1.43 |
| PVAIRR | 0.61 ± 0.55 | 2.12 ± 1.37 |
| Sample | Shore Hardness | Young Modulus [KPa] |
|---|---|---|
| 5% PVA 25 kGy | 0.15 ± 0.02 | 161 |
| 5% PVA 35 kGy | 0.75 ± 0.05 | 171 |
| 10% PVA 25 kGy | 2.95 ± 0.11 | 227 |
| 10% PVA 35 kGy | 4.50 ± 0.10 | 266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otulakowski, Ł.; Klama-Baryła, A.; Celny, A.; Kasprów, M.; Hercog, A.; Godzierz, M.; Sitkowska, A.; Kadłubowski, S.; Jaworska, M.; Chmielik, E.; et al. Laminar Biomaterial Composite of PVA Cryogel with Amnion as Potential Wound Dressing. Polymers 2023, 15, 2955. https://doi.org/10.3390/polym15132955
Otulakowski Ł, Klama-Baryła A, Celny A, Kasprów M, Hercog A, Godzierz M, Sitkowska A, Kadłubowski S, Jaworska M, Chmielik E, et al. Laminar Biomaterial Composite of PVA Cryogel with Amnion as Potential Wound Dressing. Polymers. 2023; 15(13):2955. https://doi.org/10.3390/polym15132955
Chicago/Turabian StyleOtulakowski, Łukasz, Agnieszka Klama-Baryła, Anna Celny, Maciej Kasprów, Anna Hercog, Marcin Godzierz, Anna Sitkowska, Sławomir Kadłubowski, Magdalena Jaworska, Ewa Chmielik, and et al. 2023. "Laminar Biomaterial Composite of PVA Cryogel with Amnion as Potential Wound Dressing" Polymers 15, no. 13: 2955. https://doi.org/10.3390/polym15132955
APA StyleOtulakowski, Ł., Klama-Baryła, A., Celny, A., Kasprów, M., Hercog, A., Godzierz, M., Sitkowska, A., Kadłubowski, S., Jaworska, M., Chmielik, E., Trzebicka, B., & Utrata-Wesołek, A. (2023). Laminar Biomaterial Composite of PVA Cryogel with Amnion as Potential Wound Dressing. Polymers, 15(13), 2955. https://doi.org/10.3390/polym15132955











