Bulk Polymerization of Acrylic Acid Using Dielectric-Barrier Discharge Plasma in a Mesoporous Material
Abstract
1. Introduction
2. Experiments and Materials
2.1. Materials
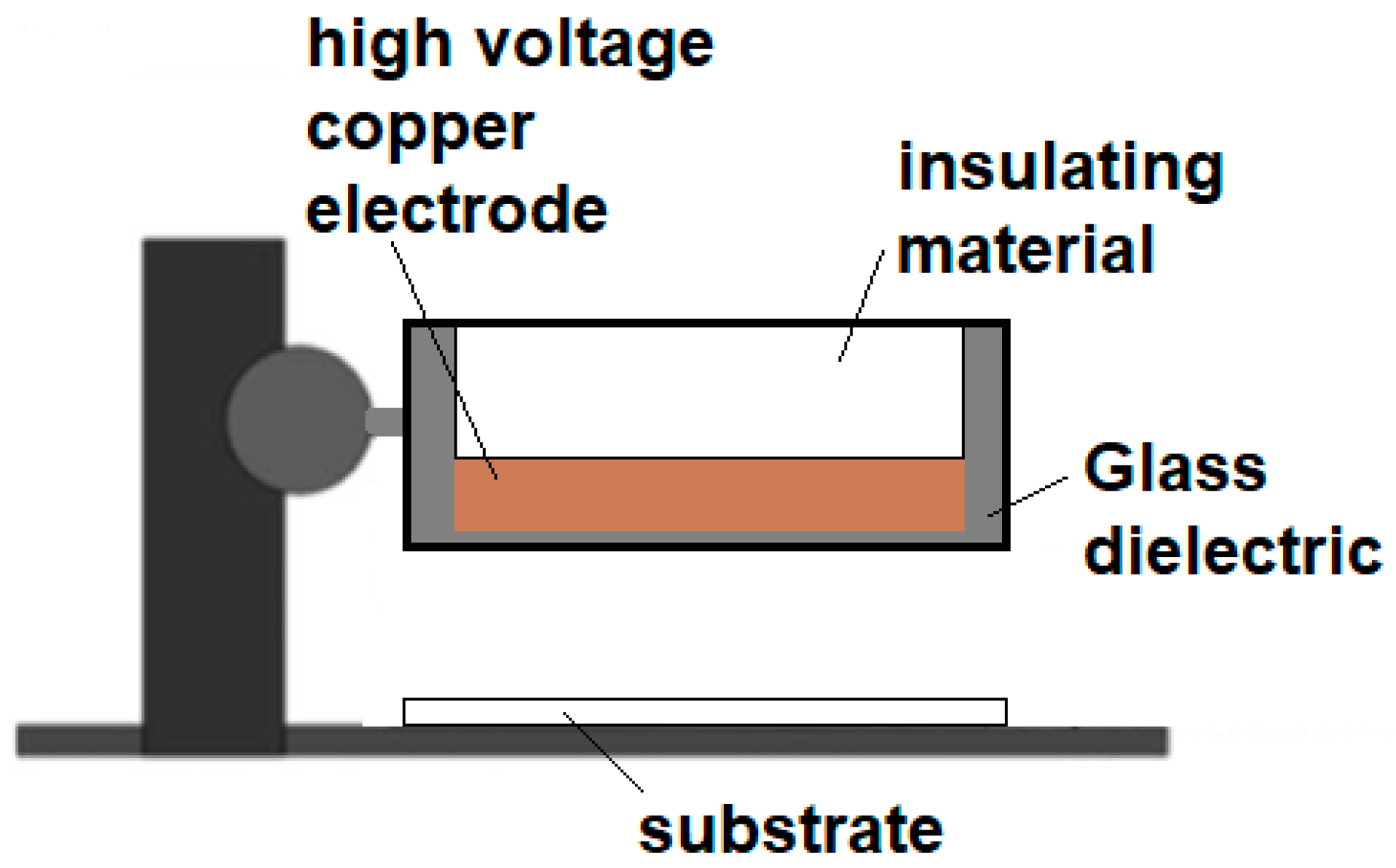
2.2. DBD Plasma Generation
2.3. DBD Plasma Polymerization of AA
2.4. FTIR Characterization and Analysis
2.5. Wet Wipe Application
3. Results and Discussion
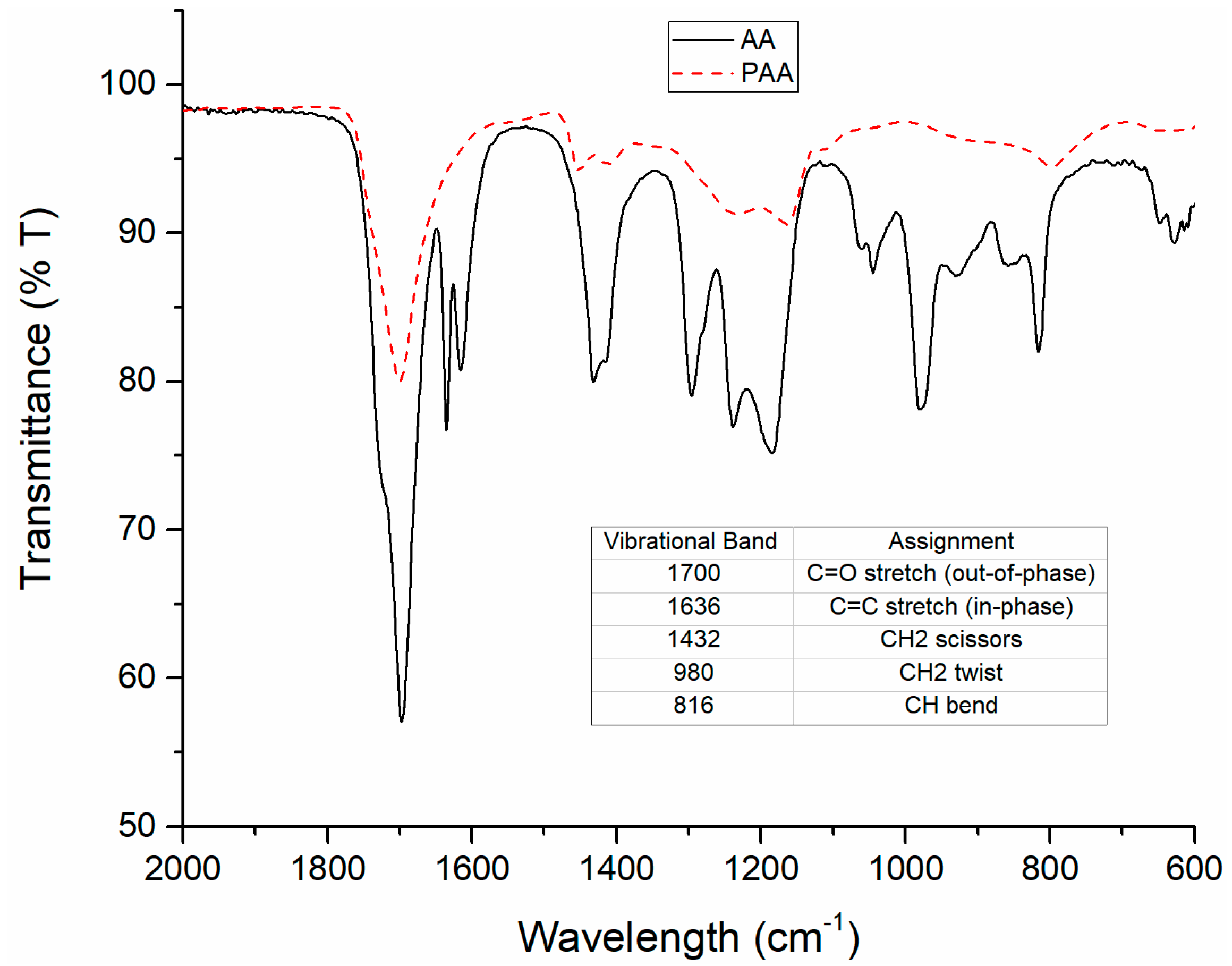
3.1. Characterization of AA and PAA Using Infrared Spectroscopy
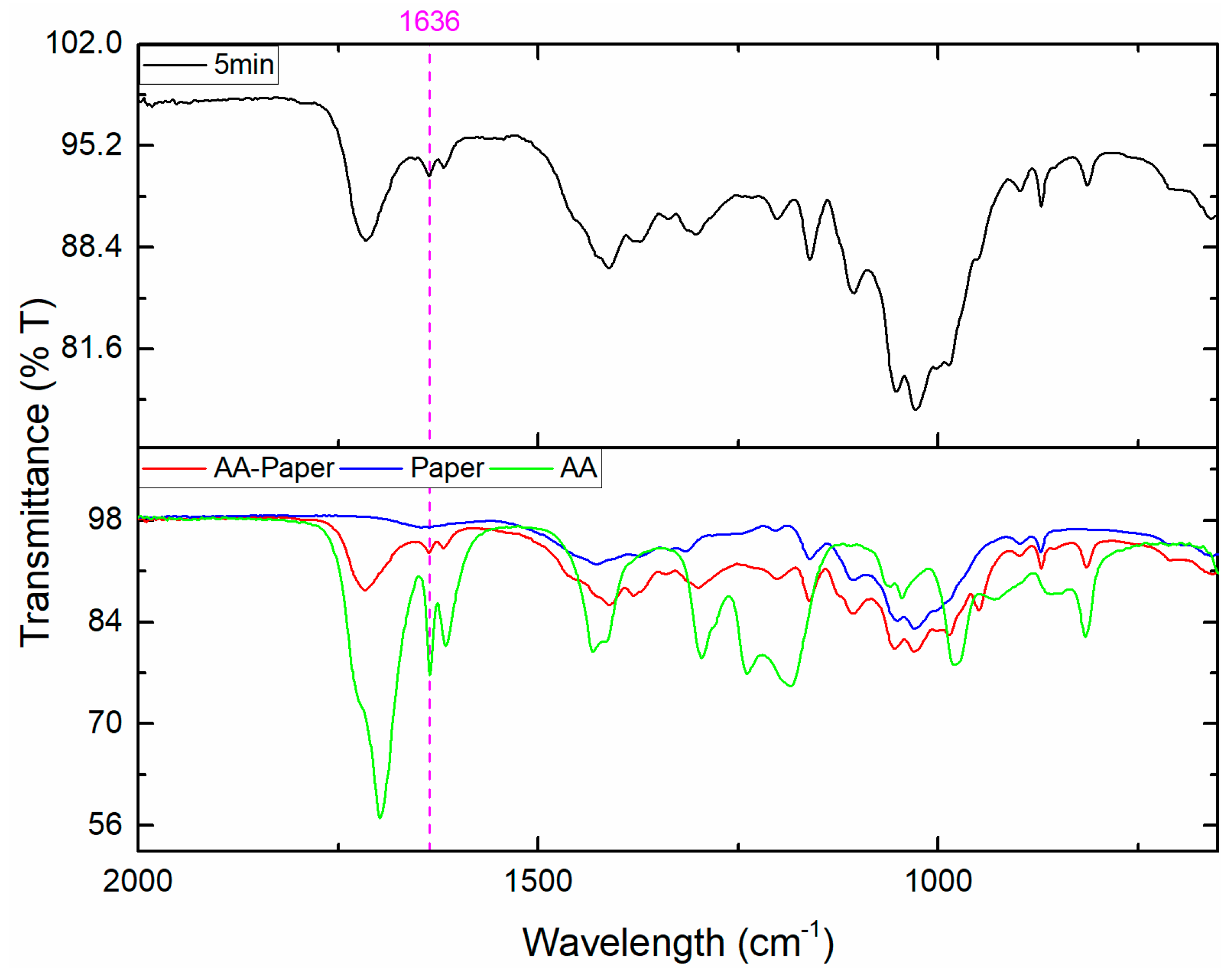
3.2. Kinetic Study
3.3. Wet Wipe Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nho, Y.-C.; Park, J.-S.; Lim, Y.-M. Preparation of Poly(acrylic acid) Hydrogel by Radiation Crosslinking and Its Application for Mucoadhesives. Polymers 2014, 6, 890–898. [Google Scholar] [CrossRef]
- Chazovachii, P.T.; Somers, M.J.; Robo, M.T.; Collias, D.I.; James, M.I.; Marsh, E.N.G.; Zimmerman, P.M.; Alfaro, J.F.; McNeil, A.J. Giving superabsorbent polymers a second life as pressure-sensitive adhesives. Nat. Commun. 2021, 12, 4524. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Lee, E.H.; Kwon, Y.-J.; Ye, S.-K.; Kim, K.O. Targeted drug release system based on pH-responsive PAA-POSS nanoparticles. RSC Adv. 2022, 12, 18209–18214. [Google Scholar] [CrossRef]
- Soni, V.; Pandey, V.; Tiwari, R.; Asati, S.; Tekade, R.K. Chapter 13—Design and Evaluation of Ophthalmic Delivery Formulations. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 473–538. [Google Scholar]
- Bitar, R.; Cools, P.; De Geyter, N.; Morent, R. Acrylic acid plasma polymerization for biomedical use. Appl. Surf. Sci. 2018, 448, 168–185. [Google Scholar] [CrossRef]
- Miar, S.; Perez, C.A.; Ong, J.L.; Guda, T. Polyvinyl alcohol-poly acrylic acid bilayer oral drug delivery systems: A comparison between thin films and inverse double network bilayers. J. Biomater. Appl. 2019, 34, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Raghunathan, K.; Solhaug, H.; Antony, J.; Stenvik, J.; Nilsen, A.M.; Einarsrud, M.-A.; Bandyopadhyay, S. Modulating acrylic acid content of nanogels for drug delivery & biocompatibility studies. J. Colloid Interface Sci. 2022, 607, 76–88. [Google Scholar] [PubMed]
- De Giglio, E.; Cafagna, D.; Ricci, M.A.; Sabbatini, L.; Cometa, S.; Ferretti, C.; Mattioli-Belmonte, M. Biocompatibility of Poly(Acrylic Acid) Thin Coatings Electro-synthesized onto TiAlV-based Implants. J. Bioact. Compat. Polym. 2010, 25, 374–391. [Google Scholar] [CrossRef]
- Odell, P.G.; Veregin, R.P.N.; Michalak, L.M.; Brousmiche, D.; Georges, M.K. Rate Enhancement of Living Free-Radical Polymerizations by an Organic Acid Salt. Macromolecules 1995, 28, 8453–8455. [Google Scholar] [CrossRef]
- Liu, T.; Jia, S.; Kowalewski, T.; Matyjaszewski, K.; Casado-Portilla, R.; Belmont, J. Grafting Poly(n-butyl acrylate) from a Functionalized Carbon Black Surface by Atom Transfer Radical Polymerization. Langmuir 2003, 19, 6342–6345. [Google Scholar] [CrossRef]
- Loiseau, J.; Doërr, N.; Suau, J.M.; Egraz, J.B.; Llauro, M.F.; Ladavière, C.; Claverie, J. Synthesis and Characterization of Poly(acrylic acid) Produced by RAFT Polymerization. Application as a Very Efficient Dispersant of CaCO3, Kaolin, and TiO2. Macromolecules 2003, 36, 3066–3077. [Google Scholar] [CrossRef]
- Clochard, M.C.; Bègue, J.; Lafon, A.; Caldemaison, D.; Bittencourt, C.; Pireaux, J.J.; Betz, N. Tailoring bulk and surface grafting of poly(acrylic acid) in electron-irradiated PVDF. Polymer 2004, 45, 8683–8694. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of Direct and Indirect Effects of Non-Thermal Atmospheric-Pressure Plasma on Bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Smith, J.B.; Adams, I.; Ji, H.-F. Biomolecule Response to Nonthermal Plasma. J. Plasma Med. 2017, 7, 427–443. [Google Scholar] [CrossRef]
- Hegemann, D.; Nisol, B.; Watson, S.; Wertheimer, M.R. Energy Conversion Efficiency in Plasma Polymerization—A Comparison of Low- And Atmospheric-Pressure Processes. Plasma Process. Polym. 2016, 13, 834–842. [Google Scholar] [CrossRef]
- Rittersdorf, I.M.; Ottinger, P.F.; Allen, R.J.; Schumer, J.W. Current density scaling expressions for a bipolar space-charge-limited cylindrical diode. IEEE Trans. Plasma Sci. 2015, 43, 3626–3636. [Google Scholar] [CrossRef]
- Ercan, U.K.; Joshi, S.S.; Yost, A.; Gogotsi, N.; O’Toole, S.; Paff, M.; Melchior, E.; Joshi, S.G. Inhibition of biofilms by non-thermal plasma treated novel solutions. Adv. Microbiol. 2014, 4, 1188–1196. [Google Scholar] [CrossRef]
- Eliasson, B.; Egli, W.; Kogelschatz, U. Modelling of dielectric barrier discharge chemistry. Pure Appl. Chem. 1994, 66, 1275–1286. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Balasubramanian, M.; Ayan, H.; Fridman, A.; Gutsol, A.; Brooks, A. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Serezhenkov, V.A.; Rudenko, T.G.; Pekshev, A.V.; Vanin, A.F. Beneficial effect of gaseous nitric oxide on the healing of skin wounds. Nitric Oxide Biol. Chem. 2005, 12, 210–219. [Google Scholar] [CrossRef]
- Lu, X.; Cao, Y.; Yang, P.; Xiong, Q.; Xiong, Z.; Xian, Y.; Pan, Y. An RC plasma device for sterilization of root canal of teeth. IEEE Trans. Plasma Sci. 2009, 37, 668–673. [Google Scholar]
- Coulombe, S.; Léveillé, V.; Yonson, S.; Leask, R.L. Miniature atmospheric pressure glow discharge torch (APGD-t) for local biomedical applications. Pure Appl. Chem. 2006, 78, 1147–1156. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef]
- Chen, K.; Cao, M.; Qiao, Z.; He, L.; Wei, Y.; Ji, H.-F. Polymerization of Solid-State 2,2′-Bithiophene Thin Film or Doped in Cellulose Paper Using DBD Plasma and Its Applications in Paper-Based Electronics. ACS Appl. Polym. Mater. 2020, 2, 1518–1527. [Google Scholar] [CrossRef]
- Chen, K.; Cao, M.; Feng, E.; Sohlberg, K.; Ji, H.-F. Polymerization of Solid-State Aminophenol to Polyaniline Derivative Using a Dielectric Barrier Discharge Plasma. Plasma 2020, 3, 187–195. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Van Vlierberghe, S.; Beaurain, A.; Dubruel, P.; Payen, E. Influence of operating parameters on plasma polymerization of acrylic acid in a mesh-to-plate dielectric barrier discharge. Prog. Org. Coat. 2011, 70, 336–341. [Google Scholar] [CrossRef]
- Topala, I.; Dumitrascu, N.; Popa, G. Properties of the acrylic acid polymers obtained by atmospheric pressure plasma polymerization. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 442–445. [Google Scholar] [CrossRef]
- Hegemann, D.; Körner, E.; Guimond, S. Plasma Polymerization of Acrylic Acid Revisited. Plasma Process. Polym. 2009, 6, 246–254. [Google Scholar] [CrossRef]
- Bashir, M.; Bashir, S. Polymerization of acrylic acid using atmospheric pressure DBD plasma jet. IOP Conf. Ser. Mater. Sci. Eng. 2016, 146, 012036. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Van Vlierberghe, S.; Vanderleyden, E.; Dubruel, P.; Leys, C.; Schacht, E. Deposition of Polyacrylic Acid Films by Means of an Atmospheric Pressure Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2009, 29, 103–117. [Google Scholar] [CrossRef]
- Mieles, M.; Stitt, C.; Ji, H.-F. Bulk Polymerization of PEGDA in Spruce Wood Using a DBD Plasma-Initiated Process to Improve the Flexural Strength of the Wood–Polymer Composite. Plasma 2022, 5, 146–153. [Google Scholar] [CrossRef]
- Michlíček, M.; Blahová, L.; Dvořáková, E.; Nečas, D.; Zajíčková, L. Deposition penetration depth and sticking probability in plasma polymerization of cyclopropylamine. Appl. Surf. Sci. 2021, 540, 147979. [Google Scholar] [CrossRef]
- Feairheller, W.R.; Katon, J.E. The vibrational spectra of acrylic acid and sodium acrylate. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 2225–2232. [Google Scholar] [CrossRef]
- Pintar, A.; Batista, J.; Levec, J. In situ Fourier transform infrared spectroscopy as an efficient tool for determination of reaction kinetics. Analyst 2002, 127, 1535–1540. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieles, M.; Harper, S.; Ji, H.-F. Bulk Polymerization of Acrylic Acid Using Dielectric-Barrier Discharge Plasma in a Mesoporous Material. Polymers 2023, 15, 2965. https://doi.org/10.3390/polym15132965
Mieles M, Harper S, Ji H-F. Bulk Polymerization of Acrylic Acid Using Dielectric-Barrier Discharge Plasma in a Mesoporous Material. Polymers. 2023; 15(13):2965. https://doi.org/10.3390/polym15132965
Chicago/Turabian StyleMieles, Matthew, Sky Harper, and Hai-Feng Ji. 2023. "Bulk Polymerization of Acrylic Acid Using Dielectric-Barrier Discharge Plasma in a Mesoporous Material" Polymers 15, no. 13: 2965. https://doi.org/10.3390/polym15132965
APA StyleMieles, M., Harper, S., & Ji, H.-F. (2023). Bulk Polymerization of Acrylic Acid Using Dielectric-Barrier Discharge Plasma in a Mesoporous Material. Polymers, 15(13), 2965. https://doi.org/10.3390/polym15132965








