Unraveling Exclusive In-Plasma Initiated Oxidation Processes Occurring at Polymeric Surfaces upon O2 Admixtures to Medium Pressure Ar and N2 DBD Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
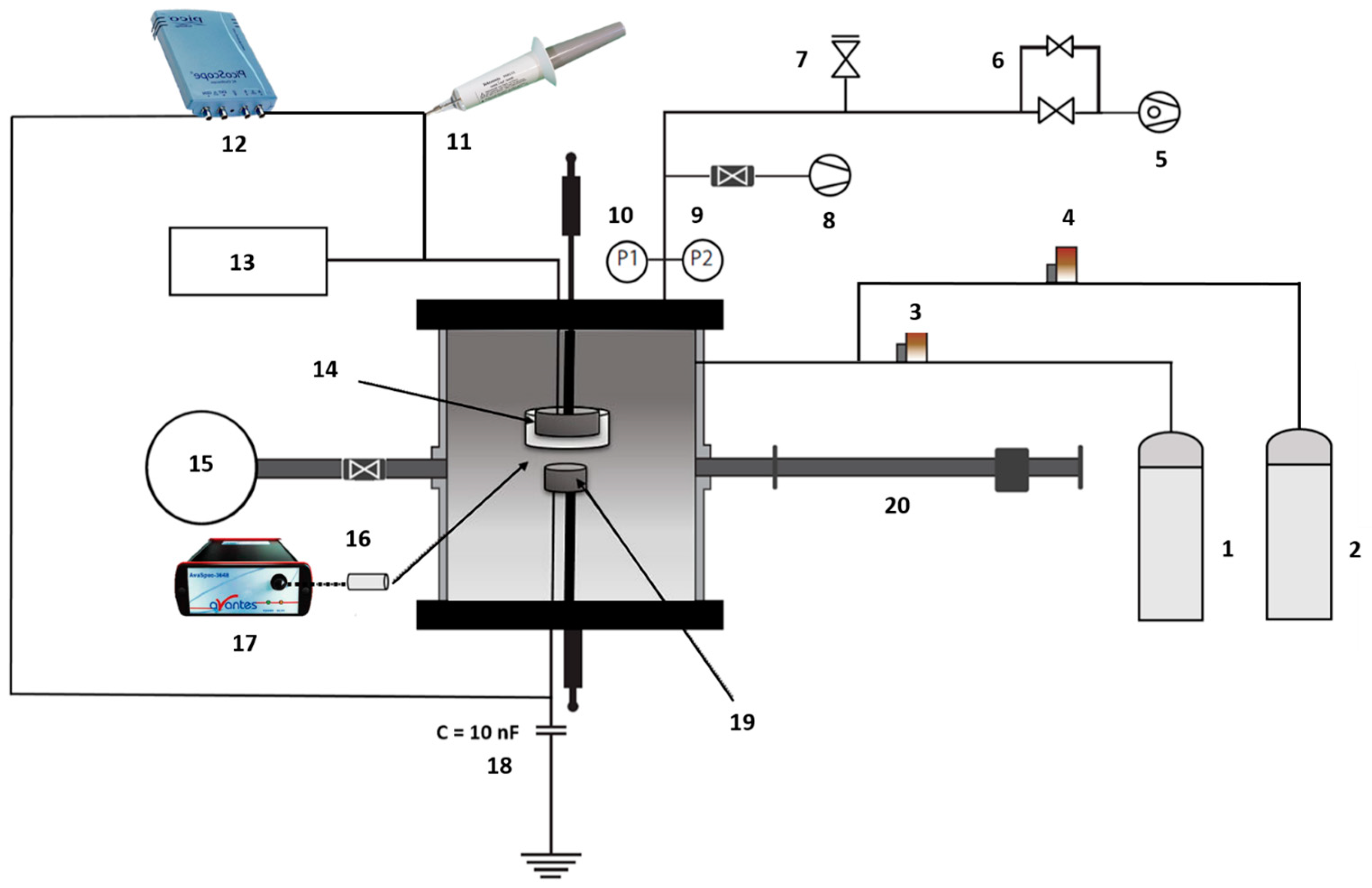
2.2. Plasma Treatment
2.3. Electrical and Optical Characterization
2.4. Surface Analysis Techniques
2.4.1. Water Contact Angle Measurements
2.4.2. XPS Measurements
3. Results and Discussion
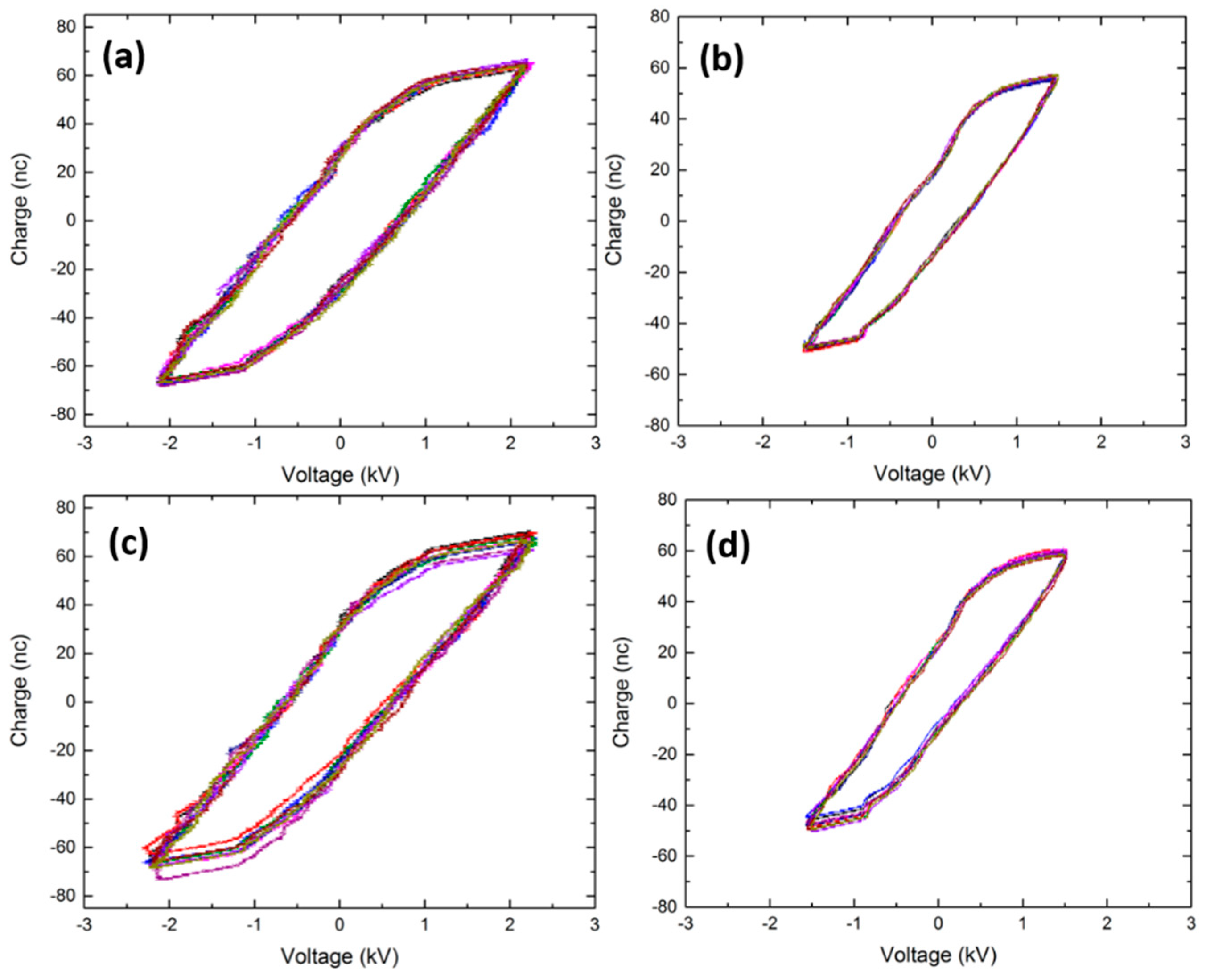
3.1. Electrical Measurements
3.2. OES Analyses of the Different Discharges
3.2.1. N2 and N2/O2 Plasma Spectral Analyses
3.2.2. Ar and Ar/O2 Plasma Spectral Analyses
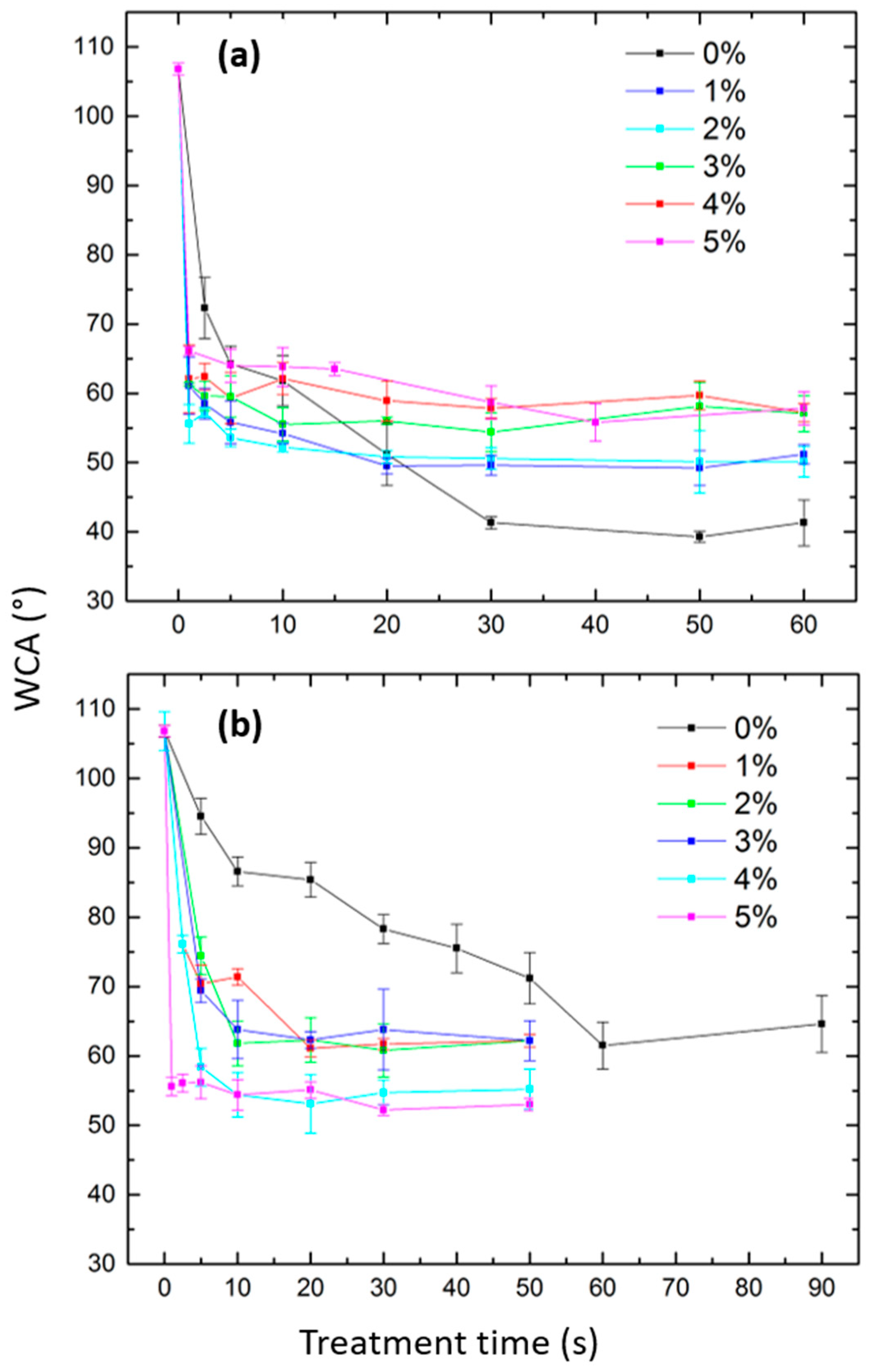
3.3. WCA Results
3.3.1. N2 and N2/O2 Plasma Treatments
3.3.2. Ar and Ar/O2 Plasma Treatments
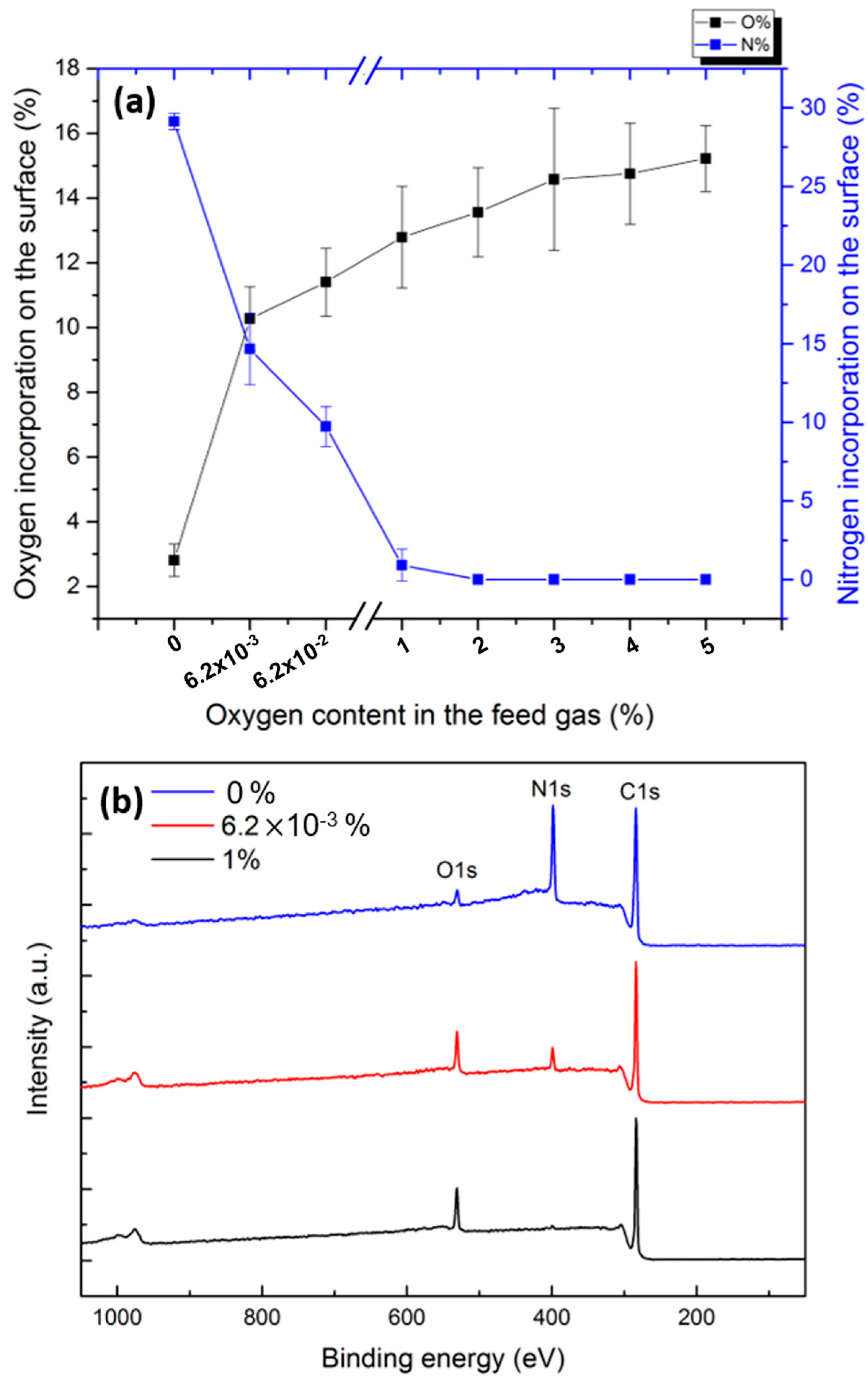
3.4. In Situ XPS Results
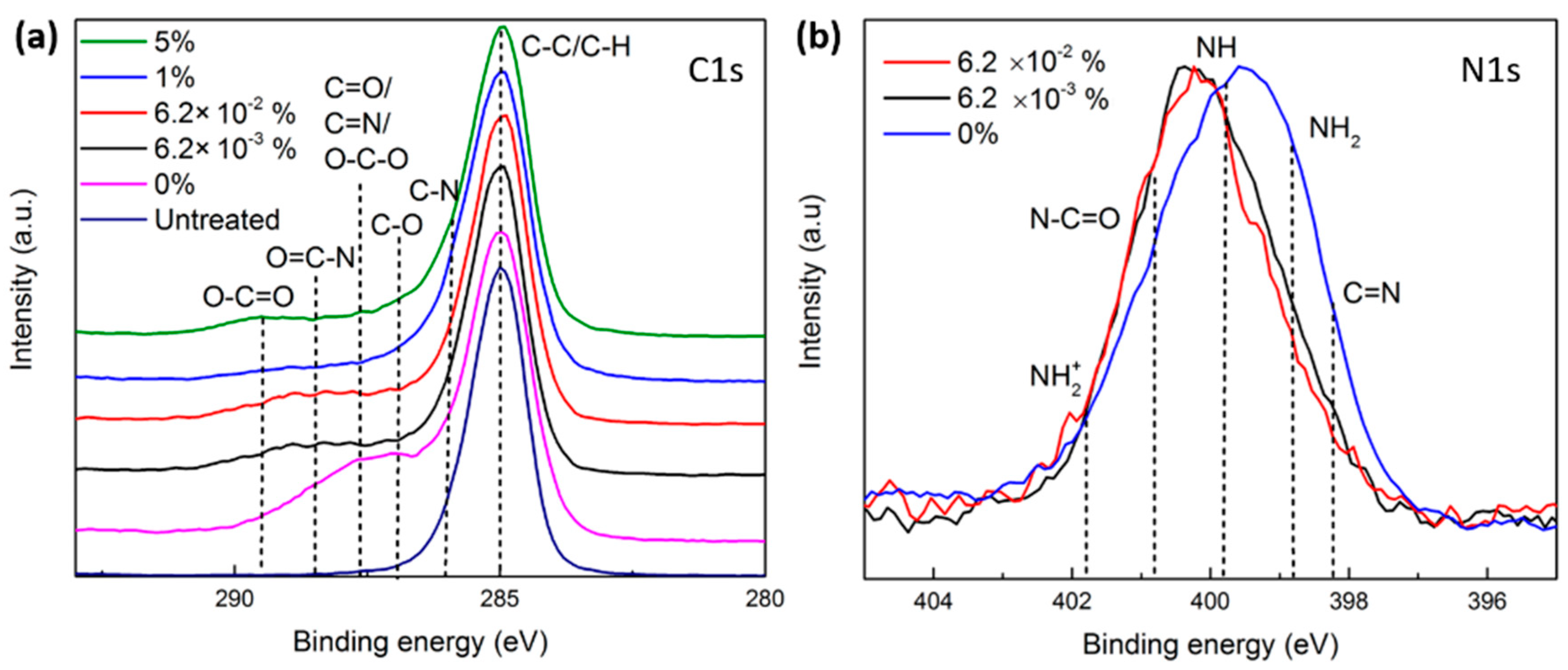
3.4.1. N2 and N2/O2 Plasma Treatments
- In pure N2 plasma, reactions of carbon-centered surface radicals (C•) with active species (N atoms and N2+ ions) resulted in the formation of N-containing functional groups, namely C-N and C=N, on the surface.
- In N2/O2 plasmas containing very low amounts of O2 (6.2 × 10−3% and 6.2 × 10−2%), the formed polymer radicals reacted with reactive nitrogen species and O2 in the feed gas, inserting N- and O-containing functional groups such as C-O, C=N, and O=C-N onto the surface.
- In N2/O2 plasmas sustained in gas mixtures containing O2 concentrations exceeding 1%, the plasma-induced surface radicals solely reacted with O2 molecules that completely depleted the incorporation of N onto the surface. In fact, O2 molecules are highly reactive and electronegative, thus rapidly reacting with the surface radicals and overpowering the reactions with nitrogen species.
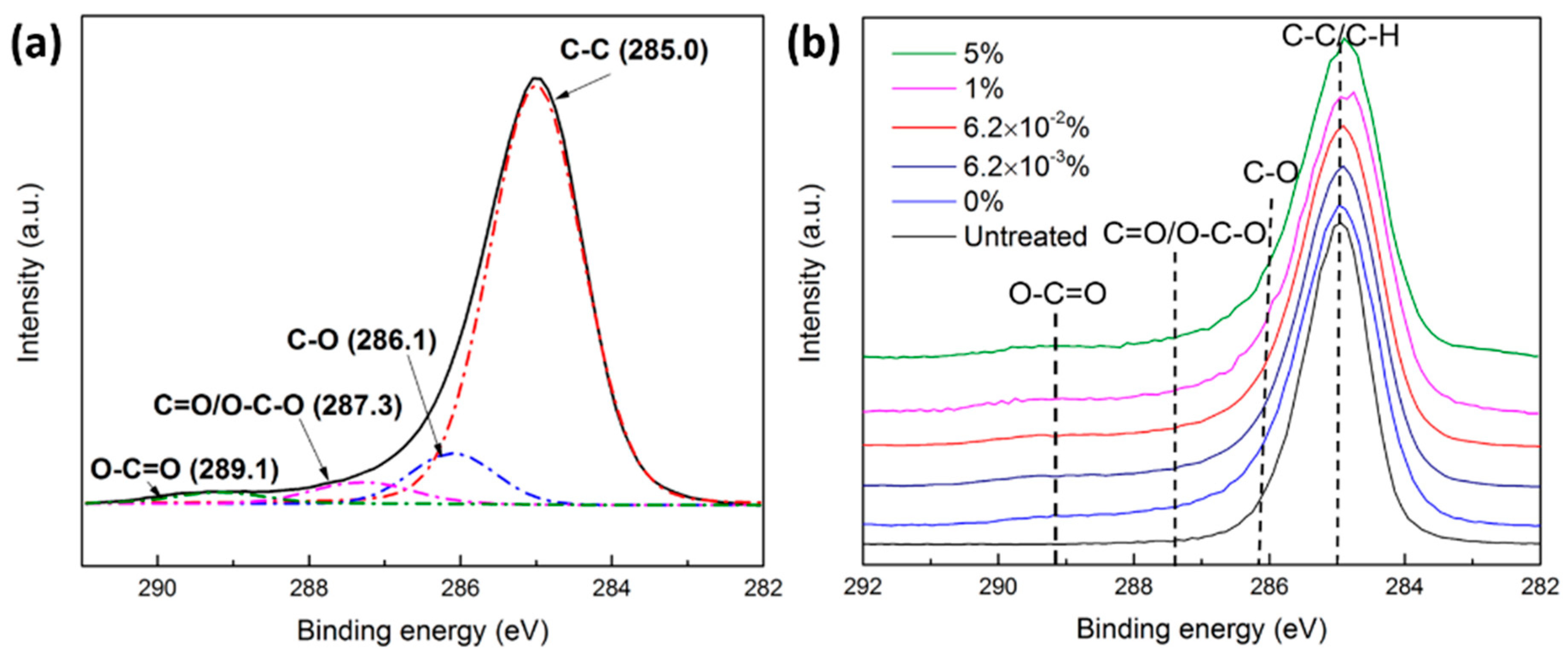
3.4.2. Ar and Ar/O2 Plasma Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hoque, M.; McDonagh, C.; Tiwari, B.K.; Kerry, J.P.; Pathania, S. Effect of Cold Plasma Treatment on the Packaging Properties of Biopolymer-Based Films: A Review. Appl. Sci. 2022, 12, 1346. [Google Scholar]
- Ghobeira, R.; De Geyter, N.; Morent, R. Plasma surface functionalization of biodegradable electrospun scaffolds for tissue engineering applications. In Biodegradable Polymers: Recent Developments and New Perspectives; IAPC Publishing: Zagreb, Croatia, 2017; pp. 191–236. [Google Scholar]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and plasma modification of nanofibrous tissue engineering scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [PubMed] [Green Version]
- Sundriyal, P.; Pandey, M.; Bhattacharya, S. Plasma-assisted surface alteration of industrial polymers for improved adhesive bonding. Int. J. Adhes. Adhes. 2020, 101, 102626. [Google Scholar]
- Ghobeira, R.; Wieringa, P.; Van Vrekhem, S.; Aliakbarshirazi, S.; Narimisa, M.; Onyshchenko, Y.; De Geyter, N.; Moroni, L.; Morent, R. Multifaceted polymeric nerve guidance conduits with distinctive double-layered architecture and plasma-induced inner chemistry gradient for the repair of critical-sized defects. Biomater. Adv. 2022, 143, 213183. [Google Scholar]
- Ghobeira, R.; Philips, C.; Liefooghe, L.; Verdonck, M.; Asadian, M.; Cools, P.; Declercq, H.; De Vos, W.H.; De Geyter, N.; Morent, R. Synergetic effect of electrospun PCL fiber size, orientation and plasma-modified surface chemistry on stem cell behavior. Appl. Surf. Sci. 2019, 485, 204–221. [Google Scholar]
- Esbah Tabaei, P.S.; Asadian, M.; Ghobeira, R.; Cools, P.; Thukkaram, M.; Derakhshandeh, P.G.; Abednatanzi, S.; Van Der Voort, P.; Verbeken, K.; Vercruysse, C.; et al. Combinatorial effects of coral addition and plasma treatment on the properties of chitosan/polyethylene oxide nanofibers intended for bone tissue engineering. Carbohydr. Polym. 2021, 253, 117211. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Huang, X.; Zhao, Y.; Li, X.; Wei, W.; Li, H.; Wu, G. Interfacial reinforcement of composites by the electrostatic self-assembly of graphene oxide and NH3 plasma-treated carbon fiber. Appl. Surf. Sci. 2022, 585, 152717. [Google Scholar]
- Shao, T.; Zhang, C.; Long, K.; Zhang, D.; Wang, J.; Yan, P.; Zhou, Y. Surface modification of polyimide films using unipolar nanosecond-pulse DBD in atmospheric air. Appl. Surf. Sci. 2010, 256, 3888–3894. [Google Scholar]
- Ghobeira, R.; Tabaei, P.S.E.; Morent, R.; De Geyter, N. Chemical characterization of plasma-activated polymeric surfaces via XPS analyses: A review. Surf. Interfaces 2022, 31, 102087. [Google Scholar]
- Cools, P.; Astoreca, L.; Esbah Tabaei, P.S.; Thukkaram, M.; De Smet, H.; Morent, R.; De Geyter, N. Surface treatment of polymers by plasma. In Surface Modification of Polymers: Methods and Applications; Wiley VCH: Weinheim, Germany, 2019; pp. 31–65. [Google Scholar]
- De Geyter, N.; Morent, R.; Desmet, T.; Trentesaux, M.; Gengembre, L.; Dubruel, P.; Leys, C.; Payen, E. Plasma modification of polylactic acid in a medium pressure DBD. Surf. Coat. Technol. 2010, 204, 3272–3279. [Google Scholar]
- Tabaei, P.S.E.; Ghobeira, R.; Cools, P.; Rezaei, F.; Nikiforov, A.; Morent, R.; De Geyter, N. Comparative study between in-plasma and post-plasma chemical processes occurring at the surface of UHMWPE subjected to medium pressure Ar and N2 plasma activation. Polymer 2020, 193, 122383. [Google Scholar]
- Massines, F.; Gouda, G.; Gherardi, N.; Duran, M.; Croquesel, E. The role of dielectric barrier discharge atmosphere and physics on polypropylene surface treatment. Plasmas Polym. 2001, 6, 35–49. [Google Scholar]
- Morent, R.; De Geyter, N.; Gengembre, L.; Leys, C.; Payen, E.; Van Vlierberghe, S.; Schacht, E. Surface treatment of a polypropylene film with a nitrogen DBD at medium pressure. Eur. Phys. J. Appl. Phys. 2008, 43, 289–294. [Google Scholar]
- Cheng, L.; Ghobeira, R.; Cools, P.; Liu, Z.; Yan, K.; De Geyter, N.; Morent, R. Comparative study of different nitrogen-containing plasma modifications applied on 3D porous PCL scaffolds and 2D PCL films. Appl. Surf. Sci. 2020, 516, 146067. [Google Scholar]
- Aziz, G.; Cools, P.; De Geyter, N.; Declercq, H.; Cornelissen, R.; Morent, R. Dielectric barrier discharge plasma treatment of ultrahigh molecular weight polyethylene in different discharge atmospheres at medium pressure: A cell-biomaterial interface study. Biointerphases 2015, 10, 029502. [Google Scholar]
- Borcia, G.; Anderson, C.; Brown, N. Using a nitrogen dielectric barrier discharge for surface treatment. Plasma Sources Sci. Technol. 2005, 14, 259. [Google Scholar]
- Gerenser, L. XPS studies of in situ plasma-modified polymer surfaces. J. Adhes. Sci. Technol. 1993, 7, 1019–1040. [Google Scholar]
- Ghobeira, R.; Philips, C.; Declercq, H.; Cools, P.; De Geyter, N.; Cornelissen, R.; Morent, R. Effects of different sterilization methods on the physico-chemical and bioresponsive properties of plasma-treated polycaprolactone films. Biomed. Mater. 2017, 12, 015017. [Google Scholar]
- Cools, P.; Van Vrekhem, S.; De Geyter, N.; Morent, R. The use of DBD plasma treatment and polymerization for the enhancement of biomedical UHMWPE. Thin Solid Film. 2014, 572, 251–259. [Google Scholar]
- Deng, X.; Nikiforov, A.Y.; Vanraes, P.; Leys, C. Direct current plasma jet at atmospheric pressure operating in nitrogen and air. J. Appl. Phys. 2013, 113, 023305. [Google Scholar]
- Schulz-Von Der Gathen, V.; Schaper, L.; Knake, N.; Reuter, S.; Niemi, K.; Gans, T.; Winter, J. Spatially resolved diagnostics on a microscale atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2008, 41, 194004. [Google Scholar]
- Fang, Z.; Wang, X.; Shao, T.; Zhang, C. Influence of oxygen content on argon/oxygen dielectric barrier discharge plasma treatment of polyethylene terephthalate film. IEEE Trans. Plasma Sci. 2016, 45, 310–317. [Google Scholar]
- Hao, Y.; Chen, J.; Yang, L.; Wang, X. Lissajous figures of glow and filamentary dielectric barrier discharges under high frequency voltage at atmospheric pressure in helium. In Proceedings of the 2009 IEEE 9th International Conference on the Properties and Applications of Dielectric Materials, Harbin, China, 19–23 July 2009; pp. 626–629. [Google Scholar]
- Radu, I.; Bartnikas, R.; Wertheimer, M.R. Frequency and voltage dependence of glow and pseudoglow discharges in helium under atmospheric pressure. IEEE Trans. Plasma Sci. 2003, 31, 1363–1378. [Google Scholar]
- Bretagnol, F.; Tatoulian, M.; Arefi-Khonsari, F.; Lorang, G.; Amouroux, J. Surface modification of polyethylene powder by nitrogen and ammonia low pressure plasma in a fluidized bed reactor. React. Funct. Polym. 2004, 61, 221–232. [Google Scholar]
- Akishev, Y.; Grushin, M.; Karalnik, V.; Petryakov, A.; Trushkin, N. Non-equilibrium constricted dc glow discharge in N2 flow at atmospheric pressure: Stable and unstable regimes. J. Phys. D Appl. Phys. 2010, 43, 075202. [Google Scholar]
- Liu, D.; Niu, J.; Yu, N. Optical emission characteristics of medium-to high-pressure N2 dielectric barrier discharge plasmas during surface modification of polymers. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 061506. [Google Scholar]
- Lommatzsch, U.; Pasedag, D.; Baalmann, A.; Ellinghorst, G.; Wagner, H.E. Atmospheric pressure plasma jet treatment of polyethylene surfaces for adhesion improvement. Plasma Process. Polym. 2007, 4, S1041–S1045. [Google Scholar]
- Xiao, D.; Cheng, C.; Shen, J.; Lan, Y.; Xie, H.; Shu, X.; Meng, Y.; Li, J.; Chu, P.K. Characteristics of atmospheric-pressure non-thermal N2 and N2/O2 gas mixture plasma jet. J. Appl. Phys. 2014, 115, 033303. [Google Scholar]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of plasma discharges and materials processing. MRS Bull. 1994, 30, 899–901. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Wagner, A.; Fairbrother, D.; Reniers, F. A comparison of PE surfaces modified by plasma generated neutral nitrogen species and nitrogen ions. Plasmas Polym. 2003, 8, 119–134. [Google Scholar]
- Ono, R.; Oda, T. NO formation in a pulsed spark discharge in N2/O2/Ar mixture at atmospheric pressure. J. Phys. D Appl. Phys. 2002, 35, 309. [Google Scholar]
- Thomas, J.M.; Kaufman, F. An Upper Limit on the Formation of NO (X2Pr) in the Reactions N2 (A3S+/u)+ O (3P) and N2 (A3S+/u)+ O2 (X3S-/g) at 298 K. J. Phys. Chem. 1996, 100, 8901–8906. [Google Scholar]
- De Benedictis, S.; Dilecce, G. Rate constants for deactivation of N2 (A) v = 2–7 by O, O2, and NO. J. Chem. Phys. 1997, 107, 6219–6229. [Google Scholar]
- Gatilova, L.; Allegraud, K.; Guillon, J.; Ionikh, Y.; Cartry, G.; Röpcke, J.; Rousseau, A. NO formation mechanisms studied by infrared laser absorption in a single low-pressure plasma pulse. Plasma Sources Sci. Technol. 2007, 16, S107. [Google Scholar]
- Gherardi, N.; Gouda, G.; Gat, E.; Ricard, A.; Massines, F. Transition from glow silent discharge to micro-discharges in nitrogen gas. Plasma Sources Sci. Technol. 2000, 9, 340. [Google Scholar]
- Wang, W.; Patil, B.; Heijkers, S.; Hessel, V.; Bogaerts, A. Nitrogen fixation by gliding arc plasma: Better insight by chemical kinetics modelling. ChemSusChem 2017, 10, 2145–2157. [Google Scholar]
- Chung, T.; Ra Kang, H.; Keun Bae, M. Optical emission diagnostics with electric probe measurements of inductively coupled Ar/O2/Ar-O2 plasmas. Phys. Plasmas 2012, 19, 113502. [Google Scholar]
- Xiong, Q.; Nikiforov, A.; Lu, X.; Leys, C. High-speed dispersed photographing of an open-air argon plasma plume by a grating–ICCD camera system. J. Phys. D Appl. Phys. 2010, 43, 415201. [Google Scholar]
- Jõgi, I.; Raud, J.; Hein, K.; Laan, M. Spectral characterization of medium-pressure RF discharge in argon–oxygen mixture. J. Phys. D Appl. Phys. 2014, 47, 335206. [Google Scholar]
- Taghizadeh, L.; Brackman, G.; Nikiforov, A.; van der Mullen, J.; Leys, C.; Coenye, T. Inactivation of biofilms using a low power atmospheric pressure argon plasma jet; the role of entrained nitrogen. Plasma Process. Polym. 2015, 12, 75–81. [Google Scholar]
- Matra, K. Atmospheric non-thermal argon–oxygen plasma for sunflower seedling growth improvement. Jpn. J. Appl. Phys. 2017, 57, 01AG03. [Google Scholar]
- Hippler, R.; Cada, M.; Stranak, V.; Hubicka, Z. Time-resolved optical emission spectroscopy of a unipolar and a bipolar pulsed magnetron sputtering discharge in an argon/oxygen gas mixture with a cobalt target. Plasma Sources Sci. Technol. 2019, 28, 115020. [Google Scholar]
- Park, G.; Lee, H.; Kim, G.; Lee, J.K. Global model of He/O2 and Ar/O2 atmospheric pressure glow discharges. Plasma Process. Polym. 2008, 5, 569–576. [Google Scholar]
- Fricke, K.; Koban, I.; Tresp, H.; Jablonowski, L.; Schröder, K.; Kramer, A.; Weltmann, K.-D.; von Woedtke, T.; Kocher, T. Atmospheric pressure plasma: A high-performance tool for the efficient removal of biofilms. PLoS ONE 2012, 7, e42539. [Google Scholar]
- Cullen, P.; Milosavljević, V. Spectroscopic characterization of a radio-frequency argon plasma jet discharge in ambient air. Prog. Theor. Exp. Phys. 2015, 2015, 063J01. [Google Scholar]
- Cools, P.; Asadian, M.; Nicolaus, W.; Declercq, H.; Morent, R.; De Geyter, N. Surface treatment of PEOT/PBT (55/45) with a dielectric barrier discharge in air, helium, argon and nitrogen at medium pressure. Materials 2018, 11, 391. [Google Scholar]
- Chen, Y.; Gao, Q.; Wan, H.; Yi, J.; Wei, Y.; Liu, P. Surface modification and biocompatible improvement of polystyrene film by Ar, O2 and Ar + O2 plasma. Appl. Surf. Sci. 2013, 265, 452–457. [Google Scholar]
- Egghe, T.; Van Guyse, J.F.; Ghobeira, R.; Morent, R.; Hoogenboom, R.; De Geyter, N. Evaluation of cross-linking and degradation processes occurring at polymer surfaces upon plasma activation via size-exclusion chromatography. Polym. Degrad. Stab. 2021, 187, 109543. [Google Scholar]
- Aziz, G.; De Geyter, N.; Declercq, H.; Cornelissen, R.; Morent, R. Incorporation of amine moieties onto ultra-high molecular weight polyethylene (UHMWPE) surface via plasma and UV polymerization of allylamine. Surf. Coat. Technol. 2015, 271, 39–47. [Google Scholar]
- Van Vrekhem, S.; Cools, P.; Declercq, H.; Van Tongel, A.; Vercruysse, C.; Cornelissen, M.; De Geyter, N.; Morent, R. Application of atmospheric pressure plasma on polyethylene for increased prosthesis adhesion. Thin Solid Film. 2015, 596, 256–263. [Google Scholar]
- Grace, J.M.; Gerenser, L.J. Plasma Treatment of Polymers. J. Dispers. Sci. Technol. 2003, 24, 305–341. [Google Scholar] [CrossRef]
- Normand, F.; Granier, A.; Leprince, P.; Marec, J.; Shi, M.; Clouet, F. Polymer treatment in the flowing afterglow of an oxygen microwave discharge: Active species profile concentrations and kinetics of the functionalization. Plasma Chem. Plasma Process. 1995, 15, 173–198. [Google Scholar]
- Borcia, G.; Anderson, C.; Brown, N. Dielectric barrier discharge for surface treatment: Application to selected polymers in film and fibre form. Plasma Sources Sci. Technol. 2003, 12, 335. [Google Scholar]
- Manakhov, A.; Makhneva, E.; Skládal, P.; Nečas, D.; Čechal, J.; Kalina, L.; Eliáš, M.; Zajíčková, L. The robust bio-immobilization based on pulsed plasma polymerization of cyclopropylamine and glutaraldehyde coupling chemistry. Appl. Surf. Sci. 2016, 360, 28–36. [Google Scholar]
- Gengenbach, T.R.; Griesser, H.J. Aging of 1, 3-diaminopropane plasma-deposited polymer films: Mechanisms and reaction pathways. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 2191–2206. [Google Scholar]
- Sanchis, M.; Calvo, O.; Fenollar, O.; Garcia, D.; Balart, R. Characterization of the surface changes and the aging effects of low-pressure nitrogen plasma treatment in a polyurethane film. Polym. Test. 2008, 27, 75–83. [Google Scholar]
- Teodoru, S.; Kusano, Y.; Rozlosnik, N.; Michelsen, P.K. Continuous plasma treatment of ultra-high-molecular-weight polyethylene (UHMWPE) fibres for adhesion improvement. Plasma Process. Polym. 2009, 6, S375–S381. [Google Scholar]
- Belmonte, T.; Thiébaut, J.; Mézerette, D. Role of active species in surface cleaning by an Ar-N2 atmospheric pressure post-discharge. J. Phys. D Appl. Phys. 2002, 35, 1919. [Google Scholar]
- Van Deynse, A.; De Geyter, N.; Leys, C.; Morent, R. Influence of Water Vapor Addition on the Surface Modification of Polyethylene in an Argon Dielectric Barrier Discharge. Plasma Process. Polym. 2014, 11, 117–125. [Google Scholar] [CrossRef]


| Plasma Gas | Discharge Power (w) | Treatment Time (s) | Energy Density at Saturated Point (J/cm2) |
|---|---|---|---|
| N2 | 2.1 | 0–60 | 12.8 |
| N2/O2 (O2: 6.2 × 10−3%) | 2.4 | 30 | 14.6 |
| N2/O2 (O2: 6.2 × 10−2%) | 2.4 | 30 | 14.6 |
| N2/O2 (99/1) | 2.4 | 0–60 | 9.7 |
| N2/O2 (98/2) | 2.4 | 0–60 | 9.7 |
| N2/O2 (97/3) | 2.4 | 0–60 | 14.6 |
| N2/O2 (96/4) | 2.4 | 0–60 | 14.6 |
| N2/O2 (95/5) | 2.4 | 0–60 | 19.5 |
| Ar | 1.2 | 0–90 | 14.6 |
| Ar/O2 (O2: 6.2 × 10−3%) | 1.8 | 60 | 22.0 |
| Ar/O2 (O2: 6.2 × 10−2%) | 1.8 | 60 | 22.0 |
| Ar/O2 (99/1) | 1.8 | 0–50 | 7.3 |
| Ar/O2 (98/2) | 1.8 | 0–50 | 3.6 |
| Ar/O2 (97/3) | 1.8 | 0–50 | 7.3 |
| Ar/O2 (96/4) | 1.8 | 0–50 | 7.3 |
| Ar/O2 (95/5) | 1.8 | 0–50 | 14.6 |
| Untreated | Pure Ar | Ar + O2 (6.2 × 10−3%) | Ar + O2 (6.2 × 10−2%) | Ar + O2 (1%) | Ar + O2 (2%) | Ar + O2 (3%) | Ar + O2 (4%) | Ar + O2 (5%) | |
|---|---|---|---|---|---|---|---|---|---|
| C-C | 95.3 ± 0.5 | 83.8 ± 1.3 | 84.3 ± 0.5 | 84.4 ± 1.1 | 82.7 ± 1.8 | 85.1 ± 1.7 | 83.6 ± 0.8 | 83.3 ± 1.1 | 82.1 ± 1.7 |
| C-O | 4.6 ± 0.5 | 10.7 ± 0.9 | 10.1 ± 0.3 | 9.8± 0.6 | 10.9 ± 1.6 | 9.4 ± 0.9 | 10.9 ± 1.0 | 10.9 ± 1.3 | 10.8 ± 1.5 |
| C=O/O-C-O | - | 3.6 ±0.1 | 3.3 ± 0.3 | 3.3 ± 0.2 | 3.8 ± 0.4 | 3.2 ± 0.3 | 3.9 ± 0.4 | 3.5 ± 0.3 | 4.3 ± 0.5 |
| O-C=O | - | 1.6 ± 0.3 | 2.1 ± 0.2 | 2.2 ± 0.3 | 2.3 ± 0.5 | 2.1 ± 0.5 | 2.1 ± 0.3 | 2.1 ± 0.4 | 2.7 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghobeira, R.; Esbah Tabaei, P.S.; Nikiforov, A.; Morent, R.; De Geyter, N. Unraveling Exclusive In-Plasma Initiated Oxidation Processes Occurring at Polymeric Surfaces upon O2 Admixtures to Medium Pressure Ar and N2 DBD Treatments. Polymers 2023, 15, 2978. https://doi.org/10.3390/polym15142978
Ghobeira R, Esbah Tabaei PS, Nikiforov A, Morent R, De Geyter N. Unraveling Exclusive In-Plasma Initiated Oxidation Processes Occurring at Polymeric Surfaces upon O2 Admixtures to Medium Pressure Ar and N2 DBD Treatments. Polymers. 2023; 15(14):2978. https://doi.org/10.3390/polym15142978
Chicago/Turabian StyleGhobeira, Rouba, Parinaz Saadat Esbah Tabaei, Anton Nikiforov, Rino Morent, and Nathalie De Geyter. 2023. "Unraveling Exclusive In-Plasma Initiated Oxidation Processes Occurring at Polymeric Surfaces upon O2 Admixtures to Medium Pressure Ar and N2 DBD Treatments" Polymers 15, no. 14: 2978. https://doi.org/10.3390/polym15142978






