Sol–Gel Approach for Fabricating Silica/Epoxy Nanocomposites
Abstract
:1. Introduction
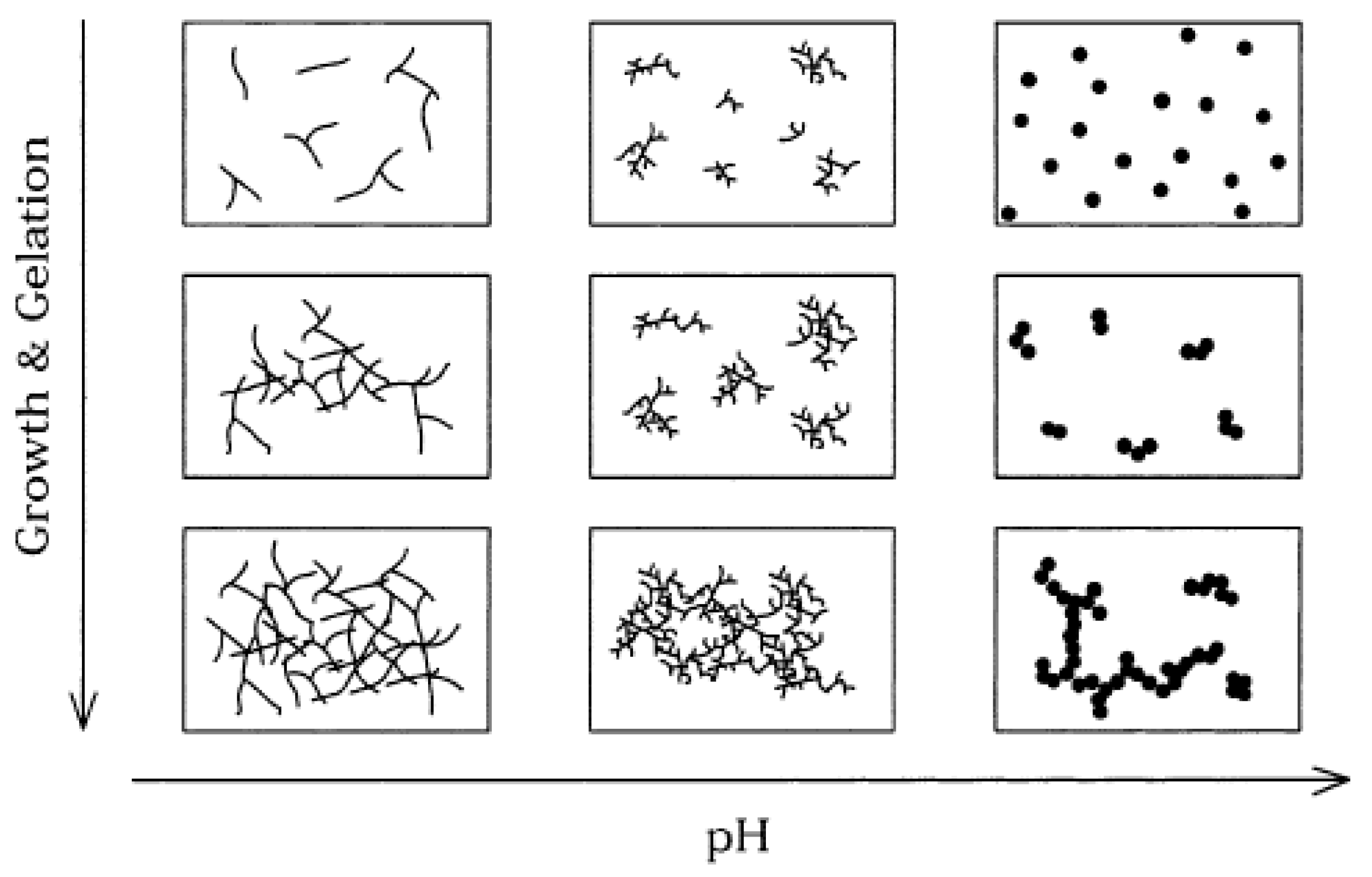
2. The Sol–Gel Route—A Few Basic Concepts
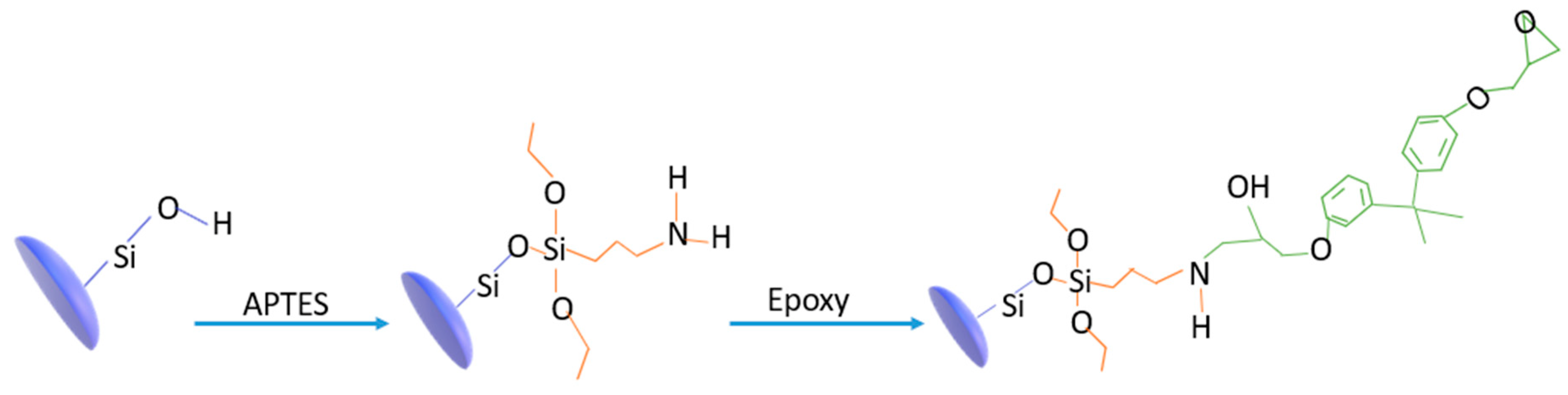
Functionalized Nanoparticles through Sol–Gel Chemistry
3. Nanocomposite Synthesis
- Extra situ: It consists in making the nanoparticles and disperse them in the matrix.
- In situ: It exploits the “mildness” of the reaction conditions that allows us to prepare nanoparticles in the presence of organic moieties like polymers or monomers.
3.1. Synthesis by Dispersion of Preformed Nanoparticles (Ex Situ Process)
3.2. In Situ Sol–Gel Chemistry
- A “one-step” procedure: All the reagents are mixed simultaneously.
- A “simultaneous two-step” procedure: The silica precursor is first pre-hydrolyzed in a first step, then the monomer and the curing agent are added in order to build up both organic and inorganic networks.
- A “sequential two-step” procedure: The polymer is cured; successive swelling allows the reagent mixture of inorganic precursor to diffuse into its pores where the inorganic network is built up.
- A “chronological two-step” procedure: A mixture of epoxy resin and a coupling agent is left to react and produce a hybrid molecule; the successive addition of the remaining reagents of the inorganic precursor mixture and the curing agent finally leads to the nanocomposite.
4. Syntheses That Progress beyond the Classical In Situ Batch Composition
- (a)
- A DOPO derivative phosphorus (P) flame-retardant, i.e., 6H-dibenz [c, e] [1,2] oxaphosphorin,6-[(1-oxido-2,6,7-trioxa-1-phosphabicyclo [2.2.2] oct-4-yl) methoxy]-, 6-oxide (DOPO-DP) and melamine (Mel) (Figure 8):
- (b)
- A DOPO derivative phosphorus (P) flame-retardant, i.e., 3-(6-oxidodibenzo [c, e] [1,2] oxaphosphinin-6-yl) propenamide (DOPO-DA) and melamine (Figure 9):
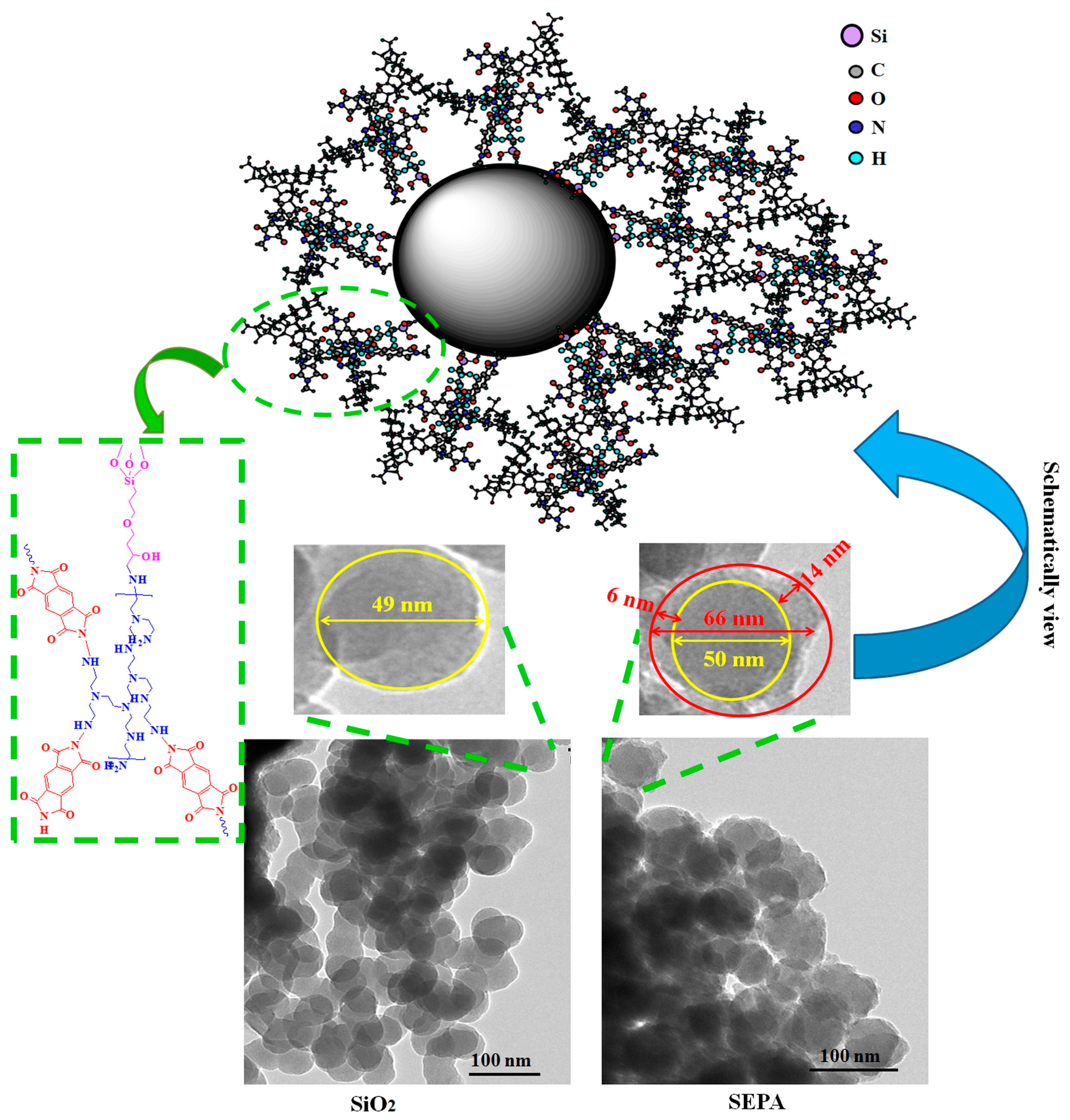
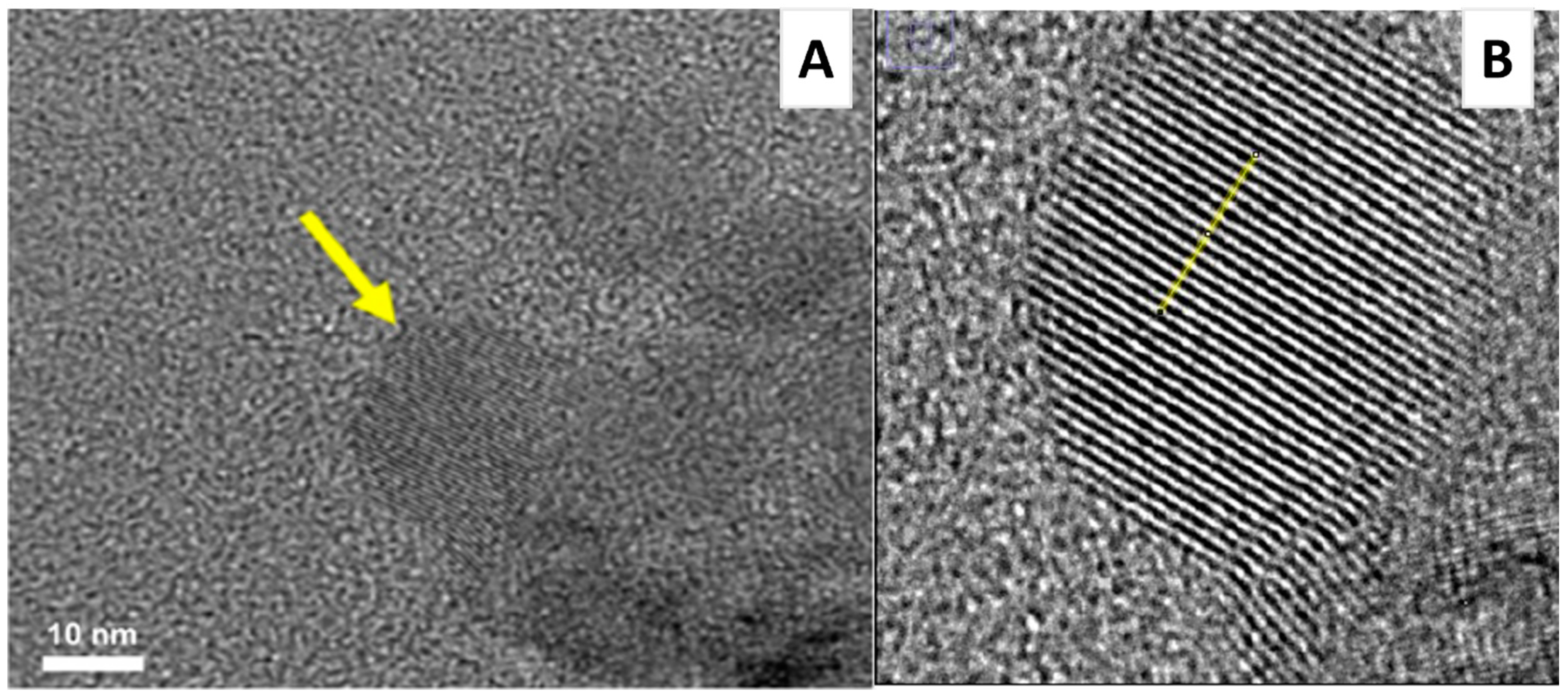
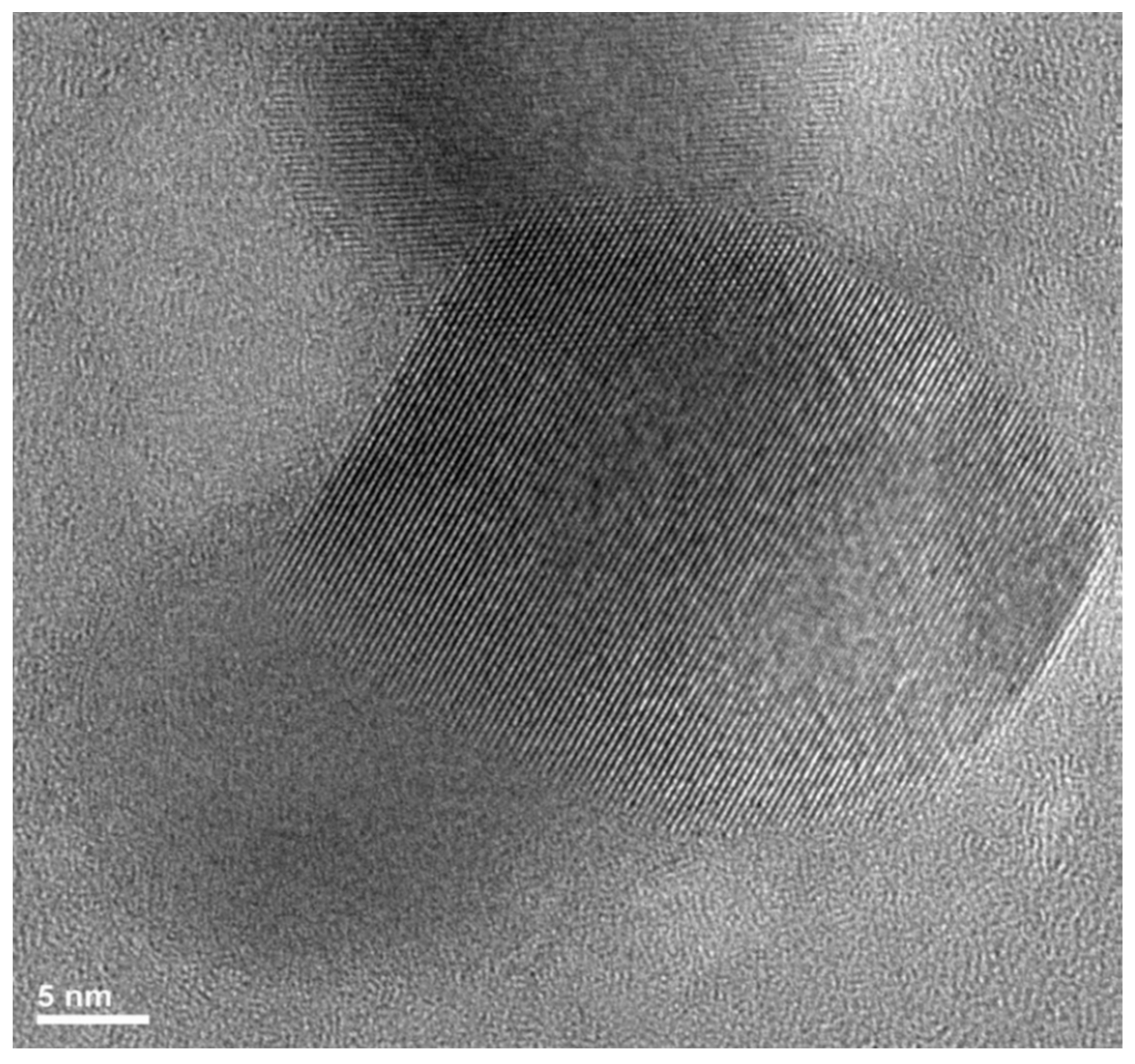
5. Insights into the In Situ Nanocomposite Structure and Formation through HRTEM and SAXS
- Solubility in the hydrophobic resin;
- The ability to result in the new phase through hydrolysis and, possibly, polycondensation.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Serra, A.; Ramis, X.; Fernández-Francos, X. Epoxy Sol–Gel Hybrid Thermosets. Coatings 2016, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Chu, P.; Zhang, H.; Zhao, J.; Gao, F.; Guo, Y.; Dang, B.; Zhang, Z. On the volume resistivity of silica nanoparticle filled epoxy with different surface modifications. Compos. Part A Appl. Sci. Manuf. 2017, 99, 139–148. [Google Scholar] [CrossRef]
- Bell, M.; Krentz, T.; Nelson, J.K.; Schadler, L.; Wu, K.; Breneman, C.; Zhao, S.; Hillborg, H.; Benicewicz, B. Investigation of dielectric breakdown in silica-epoxy nanocomposites using designed interfaces. J. Colloid Interface Sci. 2017, 495, 130–139. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Guo, Z.; Wang, H.; Zhang, Y.; Hong, R.; Peng, Z. Effects of silane coupling agents on the electrical properties of silica/epoxy nanocomposites. In Proceedings of the IEEE International Conference on Dielectrics, Montpellier, France, 3–7 July 2016. [Google Scholar] [CrossRef]
- Marouf, B.T.; Mai, Y.-W.; Bagheri, R.; Pearson, R.A. Toughening of Epoxy Nanocomposites: Nano and Hybrid Effects. Polym. Rev. 2016, 56, 70–112. [Google Scholar] [CrossRef]
- Prasad, T.; Halder, S.; Dhar, S.S.; Goyat, M.S. Epoxy/imidazole functionalized silica epoxy nanocomposites: Mechanical and fracture behaviour. Express Polym. Lett. 2021, 15, 203–223. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Wu, Y. Computational Thermomechanical Properties of Silica–Epoxy Nanocomposites by Molecular Dynamic Simulation. Polymers 2017, 9, 430. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, P.; Li, G.; Wen, J.; Wang, T.; Lu, D.; Sun, R.; Wong, C. Study on the effects of interfacial interaction on the rheological and thermal performance of silica nanoparticles reinforced epoxy nanocomposites. Compos. Part B Eng. 2017, 116, 388–397. [Google Scholar] [CrossRef]
- Karnati, S.R.; Agbo, P.; Zhang, L. Applications of silica nanoparticles in glass/carbon fiber-reinforced epoxy nanocomposite. Compos. Commun. 2019, 17, 32–41. [Google Scholar] [CrossRef]
- Kongparakul, S.; Kornprasert, S.; Suriya, P.; Le, D.; Samart, C.; Chantarasiri, N.; Prasassarakich, P.; Guan, G. Self-healing hybrid nanocomposite anticorrosive coating from epoxy/modified nanosilica/perfluorooctyl triethoxysilane. Prog. Org. Coat. 2017, 104, 173–179. [Google Scholar] [CrossRef]
- Abenojar, J.; Tutor, J.; Ballesteros, Y.; del Real, J.; Martínez, M. Erosion-wear, mechanical and thermal properties of silica filled epoxy nanocomposites. Compos. Part B Eng. 2017, 120, 42–53. [Google Scholar] [CrossRef]
- Caseri, W. Nanocomposites of Polymers and Inorganic Particles. In Hybrid Materials: Synthesis, Characterization, and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2007; pp. 49–86. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Sanchez, C.; Ribot, F.; Lebeau, B. Materials Molecular design of hybrid organic-inorganic nanocomposites synthesized via sol-gel chemistry. J. Mater. Chem. 1999, 9, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.; Ribot, F.; Rozes, L.; Alonso, B. Design of hybrid organic-inorganic nanocomposites synthesized via sol-gel chemistry. In Molecular Crystals and Liquid Crystals Science and Technology, Section A: Molecular Crystals and Liquid Crystals; Gordon and Breach Science Publishers Inc.: Philadelphia, PA, USA, 2000; pp. 143–158. [Google Scholar] [CrossRef]
- Sanchez, C.; Soler-Illia, G.J.A.; Ribot, F.; Grosso, D. Design of functional nano-structured materials through the use of controlled hybrid organic–inorganic interfaces. Comptes Rendus Chim. 2003, 6, 1131–1151. [Google Scholar] [CrossRef]
- Gómez-Romero, P.; Sanchez, C. (Eds.) Functional Hybrid Materials; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Pierre, A. Introduction to Sol-Gel Processing, 2nd ed.; Springer Nature: Berlin, Germany, 2020. [Google Scholar]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent Advances in the Liquid-Phase Syntheses of Inorganic Nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef]
- Branda, F. The Sol-Gel Route to Nanocomposites. Adv. Nanocomposites-Synth. Charact. Ind. Appl. 2011, 14, 323–340. [Google Scholar]
- Bourgeat-Lami, E. Hybrid Organic/Inorganic Particles. Hybrid Mater. Synth. Charact. Appl. 2007, 1, 87–149. [Google Scholar] [CrossRef]
- Adnan, M.M.; Dalod, A.R.M.; Balci, M.H.; Glaum, J.; Einarsrud, M.-A. In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites. Polymers 2018, 10, 1129. [Google Scholar] [CrossRef] [Green Version]
- Bifulco, A.; Tescione, F.; Capasso, A.; Mazzei, P.; Piccolo, A.; Durante, M.; Lavorgna, M.; Malucelli, G.; Branda, F. Effects of post cure treatment in the glass transformation range on the structure and fire behavior of in situ generated silica/epoxy hybrids. J. Sol-Gel Sci. Technol. 2018, 87, 156–169. [Google Scholar] [CrossRef]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Nazir, R.; Lehner, S.; Stämpfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Fire and mechanical properties of DGEBA-based epoxy resin cured with a cycloaliphatic hardener: Combined action of silica, melamine and DOPO-derivative. Mater. Des. 2020, 193, 108862. [Google Scholar] [CrossRef]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Lehner, S.; Stämpfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Improving flame retardancy of in-situ silica-epoxy nanocomposites cured with aliphatic hardener: Combined effect of DOPO-based flame-retardant and melamine. Compos. Part C Open Access 2020, 2, 100022. [Google Scholar] [CrossRef]
- Bifulco, A.; Marotta, A.; Passaro, J.; Costantini, A.; Cerruti, P.; Gentile, G.; Ambrogi, V.; Malucelli, G.; Branda, F. Thermal and Fire Behavior of a Bio-Based Epoxy/Silica Hybrid Cured with Methyl Nadic Anhydride. Polymers 2020, 12, 1661. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, A.; Avolio, R.; Lehner, S.; Errico, M.E.; Clayden, N.J.; Pauer, R.; Gaan, S.; Malucelli, G.; Aronne, A.; Imparato, C. In Situ P-Modified Hybrid Silica–Epoxy Nanocomposites via a Green Hydrolytic Sol–Gel Route for Flame-Retardant Applications. ACS Appl. Nano Mater. 2023, 6, 7422–7435. [Google Scholar] [CrossRef]
- Venezia, V.; Matta, S.; Lehner, S.; Vitiello, G.; Costantini, A.; Gaan, S.; Malucelli, G.; Branda, F.; Luciani, G.; Bifulco, A. Detailed Thermal, Fire, and Mechanical Study of Silicon-Modified Epoxy Resin Containing Humic Acid and Other Additives. ACS Appl. Polym. Mater. 2021, 3, 5969–5981. [Google Scholar] [CrossRef]
- Gao, T.-Y.; Wang, F.-D.; Xu, Y.; Wei, C.-X.; Zhu, S.-E.; Yang, W.; Lu, H.-D. Luteolin-based epoxy resin with exceptional heat resistance, mechanical and flame retardant properties. Chem. Eng. J. 2021, 428, 131173. [Google Scholar] [CrossRef]
- Branda, F.; Bifulco, A.; Jehnichen, D.; Parida, D.; Pauer, R.; Passaro, J.; Gaan, S.; Pospiech, D.; Durante, M. Structure and Bottom-up Formation Mechanism of Multisheet Silica-Based Nanoparticles Formed in an Epoxy Matrix through an In Situ Process. Langmuir 2021, 37, 8886–8893. [Google Scholar] [CrossRef] [PubMed]
- Branda, F.; Parida, D.; Pauer, R.; Durante, M.; Gaan, S.; Malucelli, G.; Bifulco, A. Effect of the Coupling Agent (3-Aminopropyl) Triethoxysilane on the Structure and Fire Behavior of Solvent-Free One-Pot Synthesized Silica-Epoxy Nanocomposites. Polymers 2022, 14, 3853. [Google Scholar] [CrossRef]
- Branda, F.; Passaro, J.; Pauer, R.; Gaan, S.; Bifulco, A. Solvent-Free One-Pot Synthesis of Epoxy Nanocomposites Containing Mg(OH)2 Nanocrystal–Nanoparticle Formation Mechanism. Langmuir 2022, 38, 5795–5802. [Google Scholar] [CrossRef]
- Freundlich, S. Colloid and capillary Chemistry; Methuen: London, UK, 1926. [Google Scholar]
- Dislich, H. New Routes to Multicomponent Oxide Glasses. Angew. Chem. Int. Ed. 1971, 10, 363–370. [Google Scholar] [CrossRef]
- Sakka, S. Birth of the sol–gel method: Early history. J. Sol-Gel Sci. Technol. 2021, 102, 478–481. [Google Scholar] [CrossRef]
- Nogami, J.M.; Moriya, Y. Glass formation through hydrolysis of Si (OC2H5)4 with NH4OH and HCl solution. J. Non-Cryst. Solids 1980, 37, 191–201. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Gallagher, D.; Ring, T.A. Sol-Gel processing of ceramic films. Chimia 1989, 43, 298. [Google Scholar]
- Bogush, G.; Tracy, M.; Zukoski, C. Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non-Cryst. Solids 1988, 104, 95–106. [Google Scholar] [CrossRef]
- Di Renzo, F.; Cambon, H.; Dutartre, R. A 28-year-old synthesis of micelle-templated mesoporous silica. Microporous Mater. 1997, 10, 283–286. [Google Scholar] [CrossRef]
- Katiyar, A.; Yadav, S.; Smirniotis, P.G.; Pinto, N.G. Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules. J. Chromatogr. A 2006, 1122, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Branda, F. Synthesis and Functionalization of Mesoporous Bioactive Glasses for Drug Delivery. In Clinical Applications of Biomaterials—State of the art Progress Trends and Novel Approaches; Kaur, G., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; ISBN 978-3-319-56058-8. ISBN 978-3-319-56059-5 (eBook). [Google Scholar] [CrossRef]
- Vartuli, J.; Schmitt, K.; Kresge, C.; Roth, W.; Leonowicz, M.; McCullen, S.; Hellring, S.; Beck, J.; Schlenker, J.; Olson, D.; et al. Development of a formation mechanism for M41S materials. Stud. Surf. Sci. Catal. 1994, 84, 53–60. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beckt, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Firouzi, A.; Kumar, D.; Bull, L.M.; Besier, T.; Sieger, P.; Huo, Q.; Walker, S.A.; Zasadzinski, J.A.; Glinka, C. REPORTS Cooperative Organization of Inorganic-Surfactant and Biomimetic Assemblies. [Online]. Available online: https://www.science.org (accessed on 6 July 2023).
- Huo, Q.; Margolese, D.I.; Ciesla, U.; Demuth, D.G.; Feng, P.; Gier, T.E.; Sieger, P.; Firouzi, A.; Chmelka, B.F. Organization of Organic Molecules with Inorganic Molecular Species into Nanocomposite Biphase Arrays. Chem. Mater. 1994, 6, 1176–1191. [Google Scholar] [CrossRef]
- Stucky, G.D.; Huo, Q.; Firouzi, A.; Chmelka, B.F.; Schacht, S.; Voigt-Martin, I.G.; Schüth, F. Directed synthesis of organic/inorganic composite structures. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1997; pp. 3–28. [Google Scholar]
- Nakamura, T.; Mizutani, M.; Nozaki, H.; Suzuki, N.; Yano, K. Formation Mechanism for Monodispersed Mesoporous Silica Spheres and Its Application to the Synthesis of Core/Shell Particles. J. Phys. Chem. C 2007, 111, 1093–1100. [Google Scholar] [CrossRef]
- Moon, D.-S.; Lee, J.-K. Tunable Synthesis of Hierarchical Mesoporous Silica Nanoparticles with Radial Wrinkle Structure. Langmuir 2012, 28, 12341–12347. [Google Scholar] [CrossRef]
- Kickelbick, G. Introduction to Hybrid Materials. In Hybrid Materials: Synthesis, Characterization, and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2007; pp. 1–48. [Google Scholar] [CrossRef] [Green Version]
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent Progress in Silane Coupling Agent with Its Emerging Applications. J. Polym. Environ. 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
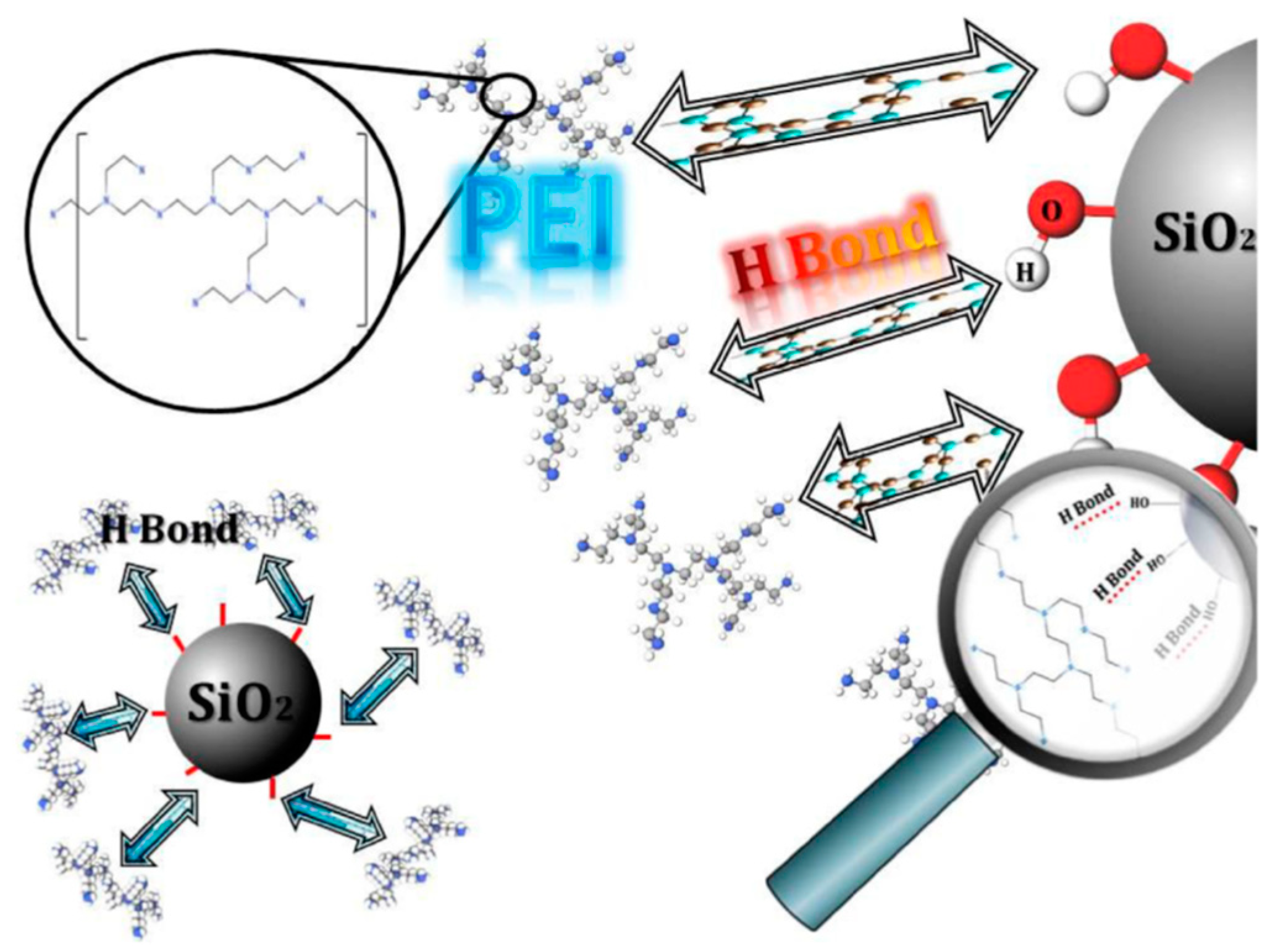
- Ghiyasi, S.; Sari, M.G.; Shabanian, M.; Hajibeygi, M.; Zarrintaj, P.; Rallini, M.; Torre, L.; Puglia, D.; Vahabi, H.; Jouyandeh, M.; et al. Hyperbranched poly(ethyleneimine) physically attached to silica nanoparticles to facilitate curing of epoxy nanocomposite coatings. Prog. Org. Coat. 2018, 120, 100–109. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Surface engineering of nanoparticles with macromolecules for epoxy curing: Development of super-reactive nitrogen-rich nanosilica through surface chemistry manipulation. Appl. Surf. Sci. 2018, 447, 152–164. [Google Scholar] [CrossRef]
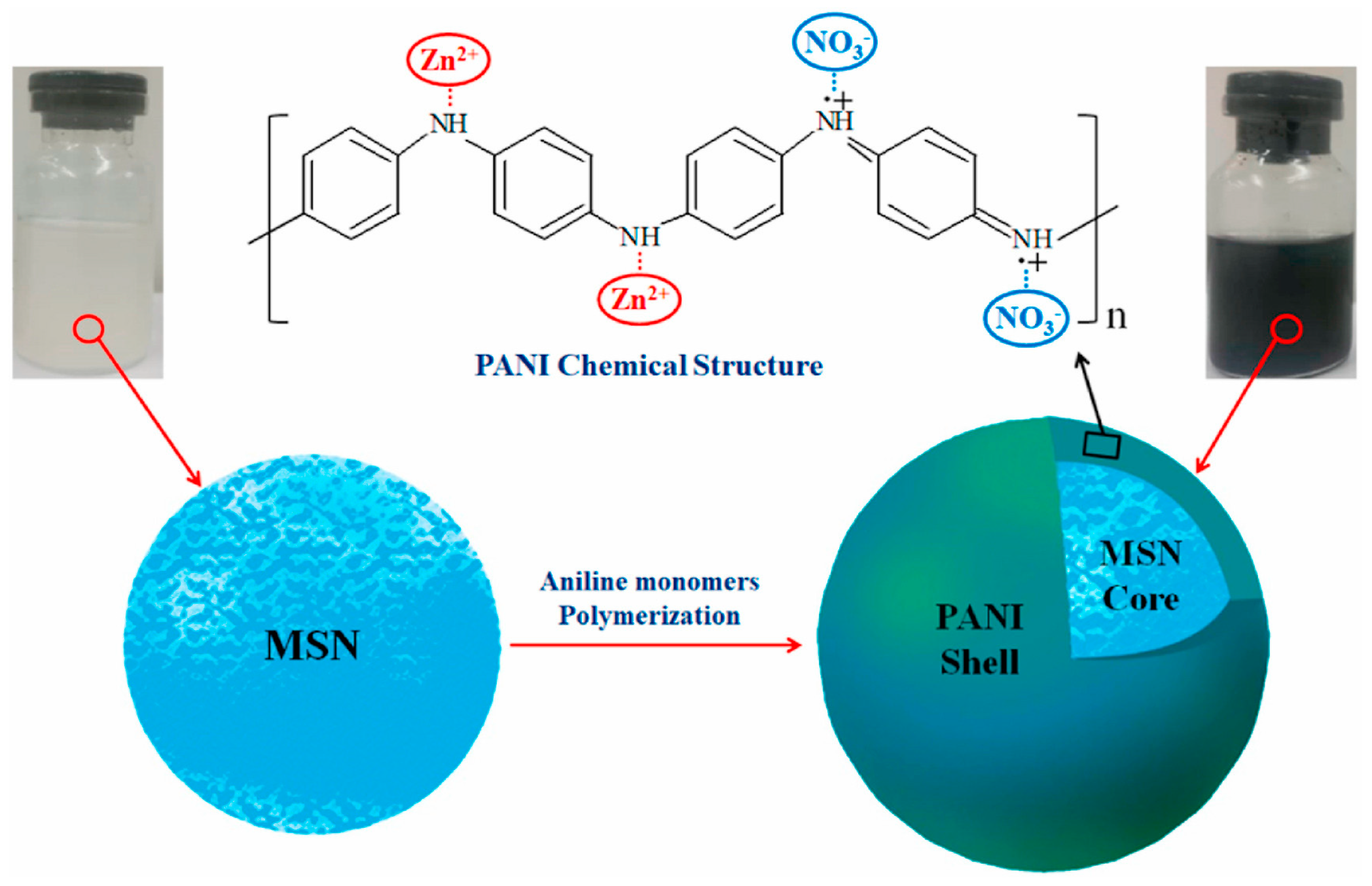
- Haddadi, S.A.; Mehmandar, E.; Jabari, H.; SA, A.R.; Mohammadkhani, R.; Yan, N.; Arjmand, M. Zinc-doped silica/polyaniline core/shell nanoparticles towards corrosion protection epoxy nanocomposite coatings. Compos. Part B Eng. 2021, 212, 108713. [Google Scholar] [CrossRef]
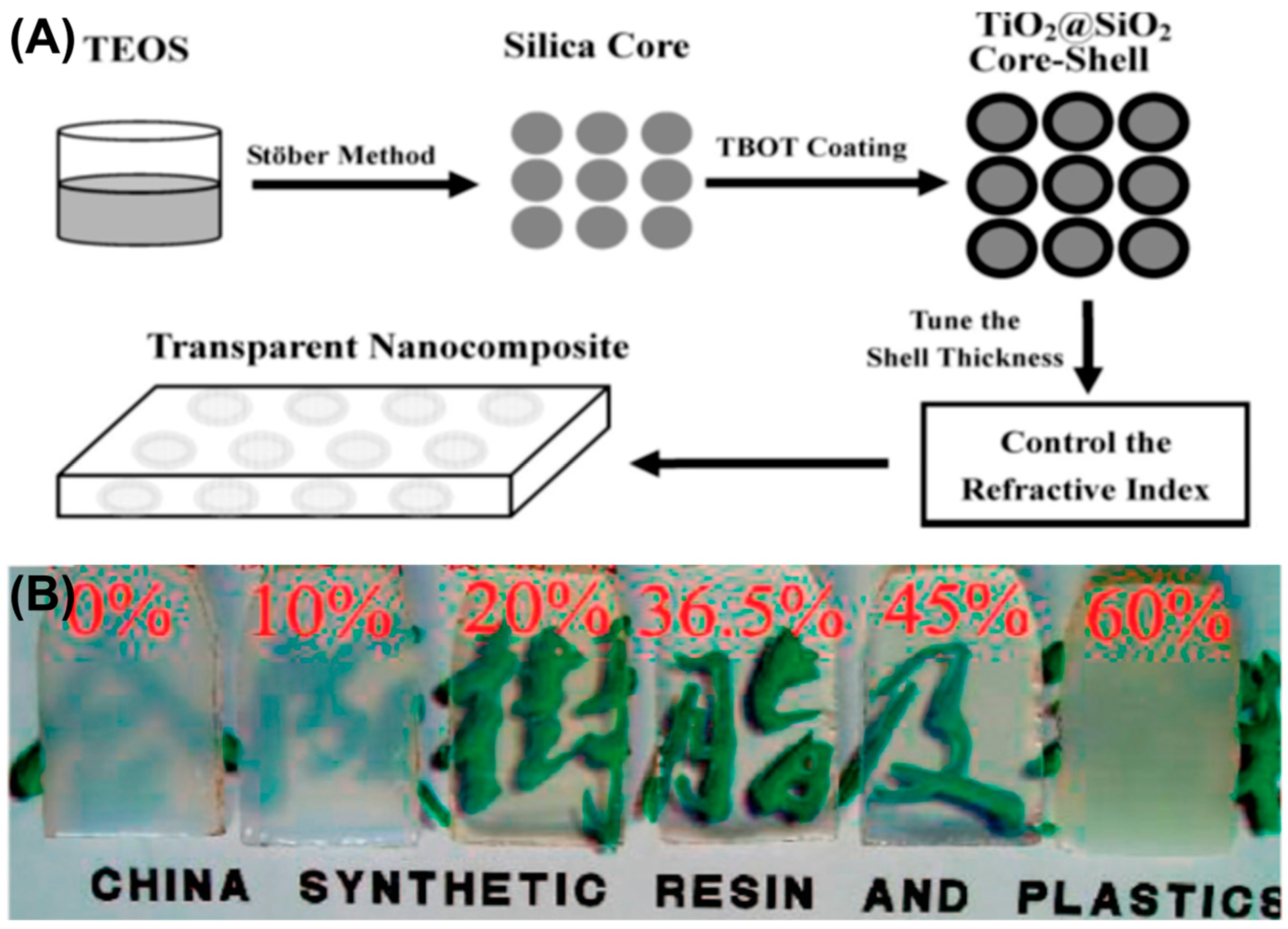
- Li, Y.-Q.; Fu, S.-Y.; Yang, Y.; Mai, Y.-W. Facile Synthesis of Highly Transparent Polymer Nanocomposites by Introduction of Core–Shell Structured Nanoparticles. Chem. Mater. 2008, 20, 2637–2643. [Google Scholar] [CrossRef]
- Picu, C.R.; Krawczyk, K.K.; Wang, Z.; Pishvazadeh-Moghaddam, H.; Sieberer, M.; Lassnig, A.; Kern, W.; Hadar, A.; Constantinescu, D.M. Toughening in nanosilica-reinforced epoxy with tunable filler-matrix interface properties. Compos. Sci. Technol. 2019, 183, 107799. [Google Scholar] [CrossRef]
- Mark, J.E.; Pan, S.-J. Reinforcement of Polydimethylsiloxane Networks by in-situ Precipitation of Silica: A New Method for Preparation of Filled Elastomers. Makromol. Chem. Rapid Commun. 1982, 3, 681–685. [Google Scholar] [CrossRef]
- Ning, Y.-P.; Tang, M.-Y.; Jiang, C.-Y.; Mark, J.E.; Roth, W.C. Particle sizes of reinforcing silica precipitated into elastomeric networks. J. Appl. Polym. Sci. 1984, 29, 3209–3212. [Google Scholar] [CrossRef]
- Matějka, L.; Pleštil, J.; Dušek, K. Structure evolution in epoxy-silica hybrids: Sol-gel process. J. Non-Cryst. Solids 1998, 226, 114–121. [Google Scholar] [CrossRef]
- Matejka, L.; Dusek, K.; Plegtil, J.; Kg, J.; Lednicklj, F. Formation and structure of the epoxy-silica aybrids. Polymer 1999, 40, 171–181. [Google Scholar] [CrossRef]
- Matĕjka, L.; Dukh, O.; Kolařıík, J. Reinforcement of crosslinked rubbery epoxies by in-situ formed silica. Polymer 2000, 41, 1449–1459. [Google Scholar] [CrossRef]
- Bauer, B.J.; Liu, D.W.; Jackson, C.L.; Barnes, J.D. Epoxy/SiO2 interpenetrating polymer networks. Polym. Adv. Technol. 1996, 7, 333–339. [Google Scholar] [CrossRef]
- Piscitelli, F.; Lavorgna, M.; Buonocore, G.G.; Verdolotti, L.; Galy, J.; Mascia, L. Plasticizing and Reinforcing Features of Siloxane Domains in Amine-Cured Epoxy/Silica Hybrids. Macromol. Mater. Eng. 2013, 298, 896–909. [Google Scholar] [CrossRef]
- Piscitelli, F.; Buonocore, G.; Lavorgna, M.; Verdolotti, L.; Pricl, S.; Gentile, G.; Mascia, L. Peculiarities in the structure–Properties relationship of epoxy-silica hybrids with highly organic siloxane domains. Polymer 2015, 63, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Afzal, A.; Siddiqi, H.M.; Iqbal, N.; Ahmad, Z. The effect of SiO2 filler content and its organic compatibility on thermal stability of epoxy resin. J. Therm. Anal. Calorim. 2012, 111, 247–252. [Google Scholar] [CrossRef]
- Wu, C.-C.; Hsu, S.L.-C. Preparation of Epoxy/Silica and Epoxy/Titania Hybrid Resists via a Sol-Gel Process for Nanoimprint Lithography. J. Phys. Chem. C 2010, 114, 2179–2183. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Ma, C.-C.M. Synthesis, Characterization and Thermal Properties of Novel Epoxy Containing Silicon and Phosphorus Nanocomposites by Sol–Gel Method. Eur. Polym. J. 2002, 38, 2219–2224. [Google Scholar] [CrossRef]
- Bi, Y.-T.; Li, Z.-J.; Liang, W. Preparation and characterization of epoxy/SiO2 nanocomposites by cationic photopolymerization and sol-gel process. Polym. Adv. Technol. 2014, 25, 173–178. [Google Scholar] [CrossRef]
- Yang, C.-F.; Wang, L.-F.; Wu, S.-M.; Su, C.-C. Characterization and Curing Kinetics of Epoxy/Silica Nano-Hybrids. Materials 2015, 8, 7032–7040. [Google Scholar] [CrossRef] [Green Version]
- Phonthamachai, N.; Chia, H.; Li, X.; Wang, F.; Tjiu, W.W.; He, C. Solvent-Free One-Pot Synthesis of high performance silica/epoxy nanocomposites. Polymer 2010, 51, 5377–5384. [Google Scholar] [CrossRef]
- Nazir, T.; Afzal, A.; Siddiqi, H.M.; Ahmad, Z.; Dumon, M. Thermally and mechanically superior hybrid epoxy–silica polymer films via sol–gel method. Prog. Org. Coat. 2010, 69, 100–106. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, P.; Wang, L.; Cai, Y. One-step synthesis of improved silica/epoxy nanocomposites with inorganic-organic hybrid network. J. Polym. Res. 2013, 20, 1–8. [Google Scholar] [CrossRef]
- Adnan, M.M.; Tveten, E.G.; Miranti, R.; Hvidsten, S.; Ese, M.-H.G.; Glaum, J.; Einarsrud, M.-A. In situ synthesis of epoxy nanocomposites with hierarchical surface-modified SiO2 clusters. J. Sol-Gel Sci. Technol. 2020, 95, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.M.; Ve, T.A.; Hvidsten, S.; Ese, M.-H.G.; Glaum, J.; Einarsrud, M.-A. Electrical Treeing and Partial Discharge Behaviour in Epoxy Nanocomposites with in situ Synthesized SiO2. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 936–945. [Google Scholar] [CrossRef]
- Adnan, M.M.; Kroyan, A.; Srivastava, C.; Grammatikos, S.; Glaum, J.; Glomm Ese, M.H.; Schnell, S.K.; Einarsrud, M.A. Theoretical and Experimental Determination of Thermomechanical Properties of Epoxy-SiO2 Nanocomposites. ChemPhysChem 2023, 24, e202200443. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Siddiqi, H.M. A comprehensive study of the bicontinuous epoxy–silica hybrid polymers: I. Synthesis, characterization and glass transition. Polymer 2011, 52, 1345–1355. [Google Scholar] [CrossRef]
- Mascia, L.; Prezzi, L.; Lavorgna, M. Peculiarities in the solvent absorption characteristics of epoxy-siloxane hybrids. Polym. Eng. Sci. 2005, 45, 1039–1048. [Google Scholar] [CrossRef]
- Mascia, L.; Prezzi, L.; Haworth, B. Substantiating the role of phase bicontinuity and interfacial bonding in epoxy-silica nanocomposites. J. Mater. Sci. 2006, 41, 1145–1155. [Google Scholar] [CrossRef]
- Prezzi, L.; Mascia, L. Network density control in epoxy-silica hybrids by selective silane functionalization of precursors. Adv. Polym. Technol. 2005, 24, 91–102. [Google Scholar] [CrossRef]
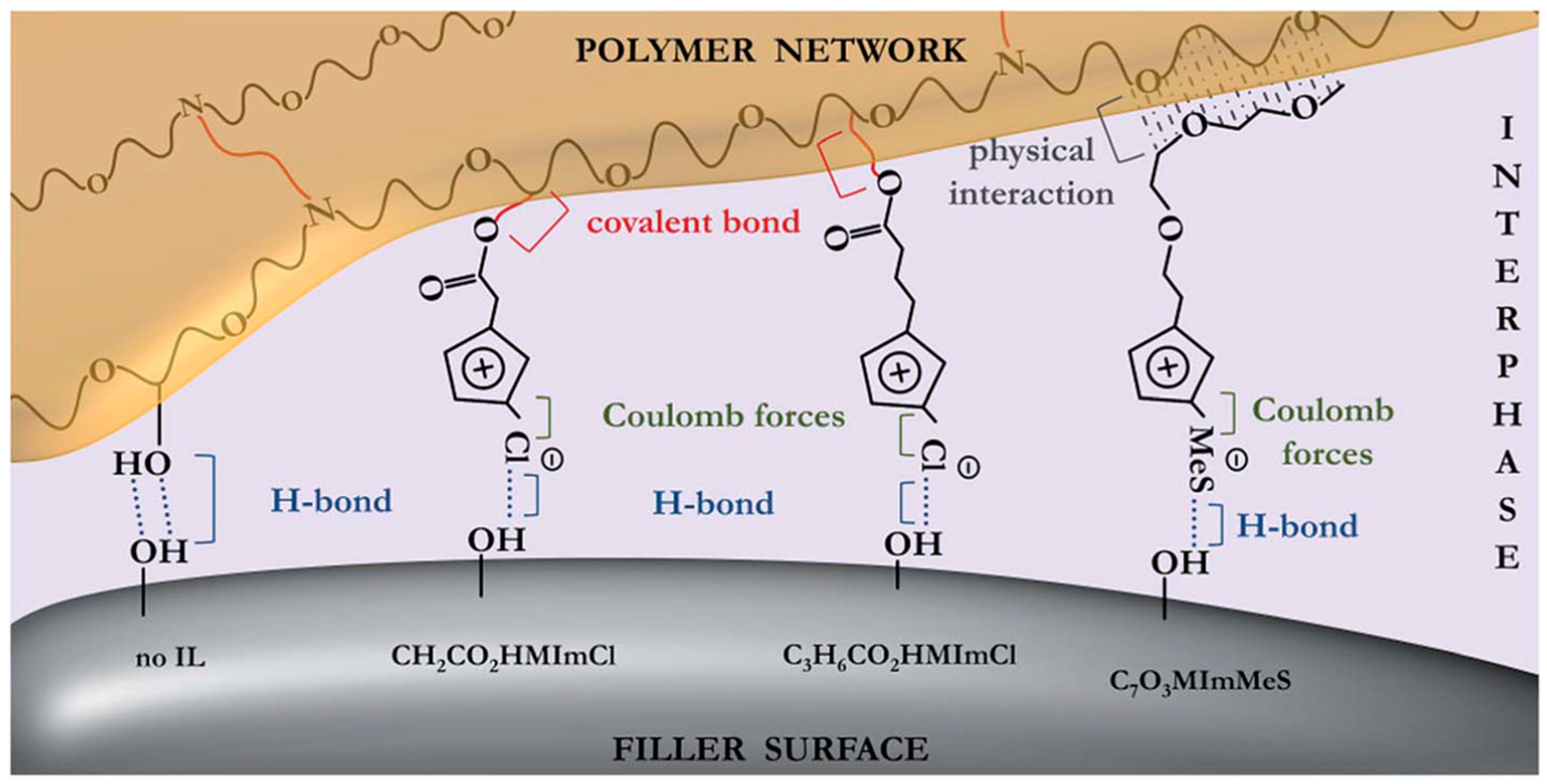
- Donato, R.K.; Matějka, L.; Schrekker, H.S.; Pleštil, J.; Jigounov, A.; Brus, J.; Šlouf, M. The multifunctional role of ionic liquids in the formation of epoxy-silica nanocomposites. J. Mater. Chem. 2011, 21, 13801–13810. [Google Scholar] [CrossRef]
- Donato, R.K.; Donato, K.Z.; Schrekker, H.S.; Matějka, L. Tunable reinforcement of epoxy-silica nanocomposites with ionic liquids. J. Mater. Chem. 2012, 22, 9939–9948. [Google Scholar] [CrossRef] [Green Version]
- Donato, R.K.; Perchacz, M.; Ponyrko, S.; Donato, K.Z.; Schrekker, H.S.; Beneš, H.; Matějka, L. Epoxy–silica nanocomposite interphase control using task-specific ionic liquids via hydrolytic and non-hydrolytic sol–gel processes. RSC Adv. 2015, 5, 91330–91339. [Google Scholar] [CrossRef]
- McMillan, P.W.; Hodgson, B.P.; Booth, R.E. Mechanical strength and surface microstructure of partially crystallised glasses. J. Mater. Sci. 1969, 4, 1029–1038. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branda, F.; Grappa, R.; Costantini, A.; Luciani, G. Sol–Gel Approach for Fabricating Silica/Epoxy Nanocomposites. Polymers 2023, 15, 2987. https://doi.org/10.3390/polym15142987
Branda F, Grappa R, Costantini A, Luciani G. Sol–Gel Approach for Fabricating Silica/Epoxy Nanocomposites. Polymers. 2023; 15(14):2987. https://doi.org/10.3390/polym15142987
Chicago/Turabian StyleBranda, Francesco, Rossella Grappa, Aniello Costantini, and Giuseppina Luciani. 2023. "Sol–Gel Approach for Fabricating Silica/Epoxy Nanocomposites" Polymers 15, no. 14: 2987. https://doi.org/10.3390/polym15142987







