Feasibility of 3D-Printed Locking Compression Plates with Polyether Ether Ketone (PEEK) in Tibial Comminuted Diaphyseal Fractures
Abstract
:1. Introduction
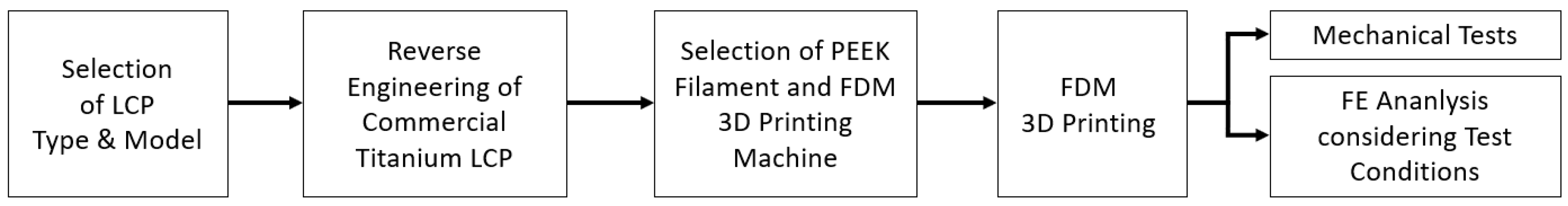
2. Material and Methods
2.1. Material Preparation
2.2. Specimen Preparation
2.3. Methods for Mechanical Tests
3. Data and Results
3.1. Experimental Results
3.2. Finite Element Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kubiak, E.N.; Fulkerson, E.; Strauss, E.; Egol, K.A. The evolution of locked plates. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. S4), 189–200. [Google Scholar] [CrossRef]
- Goodship, A.E.; Kenwright, J. The influence of induced micromovement upon the healing of experimental tibial fractures. J. Bone Jt. Surg. Br. 1985, 67, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.M. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J. Bone Jt. Surg. Br. 2002, 84, 1093–1110. [Google Scholar] [CrossRef]
- Augat, P.; Penzkofer, R.; Nolte, A.; Maier, M.; Panzer, S.; Oldenburg, G.v.; Pueschl, K.; Simon, U.; Bühren, V. Interfragmentary movement in diaphyseal tibia fractures fixed with locked intramedullary nails. J. Orthop. Trauma 2008, 22, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, M.C.; Kurtz, S.M.; Rimnac, C.M. Notch sensitivity of PEEK in monotonic tension. Biomaterials 2009, 30, 6485–6494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scolozzi, P.; Martinez, A.; Jaques, B. Complex orbito-fronto-temporal reconstruction using computer-designed PEEK implant. J. Craniofac. Surg. 2007, 18, 224–228. [Google Scholar] [CrossRef]
- Kurtz, S.M. An overview of PEEK biomaterials. In PEEK Biomaterials Handbook; William Andrew Publishing: Norwich, NY, USA, 2012; pp. 1–7. [Google Scholar]
- Wang, N.; Xie, H.; Xi, C.; Zhang, H.; Yan, J. A study to compare the efficacy of polyether ether ketone rod device with titanium devices in posterior spinal fusion in a canine model. J. Orthop. Surg. Res. 2017, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Sagomonyants, K.B.; Jarman-Smith, M.L.; Devine, J.N.; Aronow, M.S.; Gronowicz, G.A. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials 2008, 29, 1563–1572. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Bose, S. Additive Manufacturing; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Gibson, I.; Rosen, D.W.; Stucker, B. Additive Manufacturing Technologies: Rapid Prototyping to Direct Digital Manufacturing; Springer: New York, NY, USA, 2009. [Google Scholar]
- Nugraha, A.D.; Syahril, M.; Muflikhun, M.A. Excellent Performance of Hybrid Model Manufactured via Additive Manufacturing Process Reinforced with GFRP for Sport Climbing Equipment. Heliyon 2023, 9, e14706. [Google Scholar] [CrossRef]
- Hussain, T.; Gomez-Cia, T.; Valverde, I. Potential of 3D-printed models in planning structural interventional procedures. Interv. Cardiol. 2015, 7, 343–350. [Google Scholar]
- Haleem, A.; Javaid, M. Polyether ether ketone (PEEK) and its 3D printed implants applications in medical field: An overview. Clin. Epidemiol. Glob. Health 2019, 7, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Elhattab, K.; Sikder, P.; Walker, J.M.; Bottino, M.C.; Bhaduri, S.B. Fabrication and evaluation of 3-D printed PEEK scaffolds containing macropores by design. Mater. Lett. 2020, 263, 127227. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zheng, J.; Kang, J.; Dong, E.; Liu, S.; Sun, C.; Li, D.; Wang, L. 3rd-Printed Porous Peek Bone Implant with Enhanced Osseointegration- from Mechanical Design to Clinical Applications. Available online: https://ssrn.com/abstract=4348517 (accessed on 5 February 2023).
- Chavez, L.A.; Ibave, P.; Hassan, M.S.; Hall-Sanchez, S.E.; Billah, K.M.M.; Leyva, A.; Marquez, C.; Espalin, D.; Torres, S.; Robison, T.; et al. Low-temperature selective laser sintering 3D printingof PEEK-Nylon blends: Impact of thermal post-processingon mechanical properties and thermal stability. J. Appl. Polym. Sci. 2022, 139, e52290. [Google Scholar] [CrossRef]
- Von Wilmonsky, C.; Lutz, R.; Meisel, U.; Srour, S.; Rupprecht, S.; Toyoshima, T.; Nkenke, E.; Schlegel, K.A.; Pohle, D.; Münstedt, H.; et al. In Vivo Evaluation of ß-TCP Containing 3D Laser Sintered poly(ether ether ketone) Composites in Pigs. J. Bioact. Compat. Polym. 2009, 24, 169–184. [Google Scholar] [CrossRef] [Green Version]
- Nugraha, A.D.; Ruli; Supriyanto, E.; Rasgianti; Prawara, B.; Martides, E.; Junianto, E.; Wibowo, A.; Sentanuhady, J.; Muflikhun, M.A. First-Rate Manufacturing Process of Primary Air Fan (PAF) Coal Power Plant in Indonesia Using Laser Powder Bed Fusion (LPBF) Technology. J. Mater. Res. Technol. 2022, 18, 4075–4088. [Google Scholar] [CrossRef]
- Berretta, S.; Evans, K.E.; Ghita, O. Processability of PEEK, a new polymer for High Temperature Laser Sintering (HT-LS). Eur. Polym. J. 2015, 68, 243–266. [Google Scholar] [CrossRef] [Green Version]
- Honigmann, P.; Sharma, N.; Okolo, B.; Popp, U.; Msallem, B.; Thieringer, F.M. Patient-specific surgical implants made of 3D printed PEEK: Material, technology, and scope of surgical application. Biomed. Res. Int. 2018, 2018, 4520636. [Google Scholar] [CrossRef] [Green Version]
- Senra, M.R.; Marques, M.F.V.; Monteiro, S.N. Poly (ether-ether-ketone) for biomedical applications: From enhancing bioactivity to reinforced-bioactive composites-an overview. Polymers 2023, 15, 373. [Google Scholar] [CrossRef]
- Limaye, N.; Veschini, L.; Coward, T. Assessing biocompatibility & mechanical testing of 3D-printed PEEK versus milled PEEK. Heliyon 2022, 8, e12314. [Google Scholar] [CrossRef]
- Dua, R.; Rashad, Z.; Spears, J.; Dunn, G.; Maxwell, M. Applications of 3D-printed PEEK via fused filament fabrication: A systematic review. Polymers 2021, 13, 4046. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zou, B.; Ding, S.; Li, L.; Huang, C. Effects of FDM-3D printing parameters on mechanical properties and microstructure of CF/PEEK and GF/PEEK. Chin. J. Aeronaut. 2021, 34, 236–246. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, S.; Yin, B.; Liu, G.; Cheng, X.; Zhang, Y. Plating system design determines mechanical environment in long bone mid-shaft fractures: A finite element analysis. J. Investig. Surg. 2020, 33, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; He, X.; Tao, X.; Pan, F.; Yang, H. A biomechanical matched-pair comparison of two different locking plates for tibial diaphyseal comminuted fracture: Carbon fiber-reinforced poly-ether-ether-ketone (CF-PEEK) versus titanium plates. J. Orthop. Surg. Res. 2020, 15, 558. [Google Scholar] [CrossRef]
- Mehboob, H.; Son, D.S.; Chang, S.H. Finite element analysis of tissue differentiation process of a tibia with various fracture configurations when a composite intramedullary rod was applied. Compos. Sci. Technol. 2013, 80, 55–65. [Google Scholar] [CrossRef]
- Schliemann, B.; Seifert, R.; Theisen, C.; Gehweiler, D.; Wähnert, D.; Schulze, M.; Raschke, M.J.; Weimann, A. PEEK versus titanium locking plates for proximal humerus fracture fixation: A comparative biomechanical study in two- and three-part fractures. Arch. Orthop. Trauma Surg. 2017, 137, 63–71. [Google Scholar] [CrossRef]
- Schliemann, B.; Hartensuer, R.; Koch, T.; Theisen, C.; Raschke, M.J.; Kösters, C.; Weimann, A. Treatment of proximal humerus fractures with a CFR-PEEK plate: 2-year results of a prospective study and comparison to fixation with a conventional locking plate. J. Shoulder Elb. Surg. 2015, 24, 1282–1288. [Google Scholar] [CrossRef]
- Tarallo, L.; Mugnai, R.; Adani, R.; Zambianchi, F.; Catani, F. A new volar plate made of carbon-fiber-reinforced polyetheretherketon for distal radius fracture: Analysis of 40 cases. J. Orthop. Traumatol. 2014, 15, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Tarallo, L.; Giorgini, A.; Novi, M.; Zambianchi, F.; Porcellini, G.; Catani, F. Volar PEEK plate for distal radius fracture: Analysis of adverse events. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 1293–1298. [Google Scholar] [CrossRef]







| Item | Condition | Methods | Value |
|---|---|---|---|
| Tensile strength | Yield, 23 °C | ISO 527 | 100 MPa |
| Flexural strength | Yield, 23 °C | ISO 178 | 170 MPa |
| Flexural modulus | 23 °C | ISO 178 | 4.2 GPa |
| Compression strength | 23 °C | ISO 604 | 125 MPa |
| Melting point | ISO 11357 | 343 °C | |
| Glass transition | ISO 11357 | 143 °C |
| Item | Condition |
|---|---|
| Print speed | 35 mm/sec |
| Nozzle temperature | 410 °C |
| Chamber temperature | 90 °C |
| Bed temperature | 130 °C |
| Infill | 100% |
| Printing bottom direction | X-Y |
| Characteristics | 3D-Printed PEEK LCP | Commercial Titanium Alloy LCP | ||||
|---|---|---|---|---|---|---|
| #1 | #2 | Average | #1 | #2 | Average | |
| Stiffness (N/mm) | 11.01 | 9.15 | 10.08 | 104.78 | 127.61 | 116.20 |
| Yield load (N) | 74.69 | 78.87 | 76.78 | 761.85 | 718.68 | 740.27 |
| Bending structural stiffness (MN/mm2) | 0.89 | 0.74 | 0.82 | 8.45 | 10.29 | 9.37 |
| Bending strength (N/mm) | 2054 | 2169 | 2111 | 20,951 | 19,764 | 20,357 |
| Characteristics | 3D-Printed PEEK LCP | Commercial Titanium Alloy LCP | ||||
|---|---|---|---|---|---|---|
| #1 | #2 | Average | #1 | #2 | Average | |
| Stiffness (N/mm) | 533 | 586 | 560 | 1191 | 1314 | 1252 |
| Maximum displacement (mm) | 1.63 | 1.68 | 1.65 | 4.45 | 5.63 | 5.04 |
| Maximum load (kN) | 0.79 | 0.74 | 0.77 | 3.88 | 4.07 | 3.98 |
| Characteristics | 3D-Printed PEEK LCP | Commercial Titanium Alloy LCP | ||||
|---|---|---|---|---|---|---|
| #1 | #2 | Average | #1 | #2 | Average | |
| Stiffness (N-m/deg) | 0.15 | 0.11 | 0.13 | 1.02 | 1.25 | 1.13 |
| Maximum angle (degree) | 99.7 | 96.7 | 98.2 | 26.0 | 26.2 | 26.1 |
| Maximum torque (N-m) | 7.8 | 6.7 | 7.2 | 22.7 | 27.4 | 25.1 |
| Material | Density (g/cm3) | Young’s Modulus (GPa) | Poisson’s Ratio | Tensile Strength (MPa) | Compression Strength (MPa) |
|---|---|---|---|---|---|
| IEMAI PEEK | 1.3 | 3.76 | 0.39 | 100 | 125 |
| Cortical bone | 1.8 | 15 | 0.62 | 114 | 205 |
| Bending | Compression | Torsion | ||
|---|---|---|---|---|
| Yield Load [N] | Yield Load [N] | Yield Torque [N-m] | Maximum Torque Applied [N-m] | |
| FEA (ANSYS) | 80 | 900 | 1.7 | 10 |
| Experiment | 77 | 770 | 1.6 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.-J.; Lee, H.-B.; Park, K.-M.; Jung, T.-G.; Kim, S.-B.; Lee, B.-G.; Kim, W.-C.; Lee, J.-K. Feasibility of 3D-Printed Locking Compression Plates with Polyether Ether Ketone (PEEK) in Tibial Comminuted Diaphyseal Fractures. Polymers 2023, 15, 3057. https://doi.org/10.3390/polym15143057
Chung H-J, Lee H-B, Park K-M, Jung T-G, Kim S-B, Lee B-G, Kim W-C, Lee J-K. Feasibility of 3D-Printed Locking Compression Plates with Polyether Ether Ketone (PEEK) in Tibial Comminuted Diaphyseal Fractures. Polymers. 2023; 15(14):3057. https://doi.org/10.3390/polym15143057
Chicago/Turabian StyleChung, Hyung-Jin, Ho-Beom Lee, Kwang-Min Park, Tae-Gon Jung, Sang-Bum Kim, Byoung-Gu Lee, Wan-Chin Kim, and Jeong-Kil Lee. 2023. "Feasibility of 3D-Printed Locking Compression Plates with Polyether Ether Ketone (PEEK) in Tibial Comminuted Diaphyseal Fractures" Polymers 15, no. 14: 3057. https://doi.org/10.3390/polym15143057





