Using Pegylated Graphene Oxide to Achieve High Performance Solid Polymer Electrolyte Based on Poly(ethylene oxide)/Polyvinyl Alcohol Blend (PEO/PVA)
Abstract
1. Introduction
2. Experimental
2.1. Material
2.2. Preparation of Graphene Oxide (GO)
2.3. Preparation of Functionalized Graphene (FGO)
2.4. Preparation of Polymer Electrolytes
2.5. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Z.; Wang, J.; Wu, H.; Li, Q.; Fan, S.; Wang, J. Progress and Challenges of Flexible Lithium Ion Batteries. J. Power Sources 2020, 454, 227932. [Google Scholar] [CrossRef]
- Tao, T.; Lu, S.; Chen, Y. A Review of Advanced Flexible Lithium-Ion Batteries. Adv. Mater. Technol. 2018, 3, 1700375. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Q.; Ma, W.; Yang, L. Polyethylene Oxide-Based Composites as Solid-State Polymer Electrolytes for Lithium Metal Batteries: A Mini Review. Front. Chem. 2020, 8, 640. [Google Scholar] [CrossRef]
- Meabe, L.; Peña, S.R.; Martinez-Ibañez, M.; Zhang, Y.; Lobato, E.; Manzano, H.; Armand, M.; Carrasco, J.; Zhang, H. Insight into the Ionic Transport of Solid Polymer Electrolytes in Polyether and Polyester Blends. J. Phys. Chem. C 2020, 124, 17981–17991. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Pandey, G.P. Solid Polymer Electrolytes: Materials Designing and All-Solid-State Battery Applications: An Overview. J. Phys. D Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Bergfelt, A.; Hernández, G.; Mogensen, R.; Lacey, M.J.; Mindemark, J.; Brandell, D.; Bowden, T.M. Mechanically Robust Yet Highly Conductive Diblock Copolymer Solid Polymer Electrolyte for Ambient Temperature Battery Applications. ACS Appl. Polym. Mater. 2020, 2, 939–948. [Google Scholar] [CrossRef]
- Schroers, M.; Kokil, A.; Weder, C. Solid Polymer Electrolytes Based on Nanocomposites of Ethylene Oxide-Epichlorohydrin Copolymers and Cellulose Whiskers. J. Appl. Polym. Sci. 2004, 93, 2883–2888. [Google Scholar] [CrossRef]
- Higa, M.; Fujino, Y.; Koumoto, T.; Kitani, R.; Egashira, S. All Solid-State Polymer Electrolytes Prepared from a Hyper-Branched Graft Polymer Using Atom Transfer Radical Polymerization. Electrochim. Acta 2005, 50, 3832–3837. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Cao, X.; He, Y.; Wu, K.; Yang, J.; Zhou, H.; Liu, W.; Sun, X. Thiol-Branched Solid Polymer Electrolyte Featuring High Strength, Toughness, and Lithium Ionic Conductivity for Lithium-Metal Batteries. Adv. Mater. 2020, 32, 2001259. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R.J. Influence of Solid Polymer Electrolyte Preparation Methods on the Performance of (PEO–PMMA)–LiBF4 Films for Lithium-Ion Battery Applications. Polym. Bull. 2018, 75, 5645–5666. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Jia, Y.; Zhu, P.; Jing, M.; Yao, S.; Shen, X.; Li, S.; Tu, F. Toward High Performance Solid-State Lithium-Ion Battery with a Promising PEO/PPC Blend Solid Polymer Electrolyte. Int. J. Energy Res. 2020, 44, 10168–10178. [Google Scholar] [CrossRef]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.C.; Liu, K.; Cui, Y. High Ionic Conductivity of Composite Solid Polymer Electrolyte via in Situ Synthesis of Monodispersed SiO2 Nanospheres in Poly(Ethylene Oxide). Nano Lett. 2016, 16, 459–465. [Google Scholar] [CrossRef]
- Vignarooban, K.; Dissanayake, M.A.K.L.; Albinsson, I.; Mellander, B.E. Effect of TiO2 Nano-Filler and EC Plasticizer on Electrical and Thermal Properties of Poly(Ethylene Oxide) (PEO) Based Solid Polymer Electrolytes. Solid State Ion. 2014, 266, 25–28. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent Advances in Solid Polymer Electrolytes for Lithium Batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Kesavan, K.; Mathew, C.M.; Rajendran, S.; Ulaganathan, M. Preparation and Characterization of Novel Solid Polymer Blend Electrolytes Based on Poly (Vinyl Pyrrolidone) with Various Concentrations of Lithium Perchlorate. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2014, 184, 26–33. [Google Scholar] [CrossRef]
- Mallaiah, Y.; Jeedi, V.R.; Swarnalatha, R.; Raju, A.; Narender Reddy, S.; Sadananda Chary, A. Impact of Polymer Blending on Ionic Conduction Mechanism and Dielectric Properties of Sodium Based PEO-PVdF Solid Polymer Electrolyte Systems. J. Phys. Chem. Solids 2021, 155, 110096. [Google Scholar] [CrossRef]
- Farea, M.O.; Abdelghany, A.M.; Meikhail, M.S.; Oraby, A.H. Effect of Cesium Bromide on the Structural, Optical, Thermal and Electrical Properties of Polyvinyl Alcohol and Polyethylene Oxide. J. Mater. Res. Technol. 2020, 9, 1530–1538. [Google Scholar] [CrossRef]
- Sarada, B.A.; Balaji Bhargav, P.; Sharma, A.K.; Rao, V.V.R.N. Studies on (PEO+PVA+KIO3) Polymer Blend Electrolyte Films for Electrochemical Cell Applications. Energy Mater. Mater. Sci. Eng. Energy Syst. 2012, 6, 394–400. [Google Scholar] [CrossRef]
- Kammoun, M.; Berg, S.; Ardebili, H. Flexible Thin-Film Battery Based on Graphene-Oxide Embedded in Solid Polymer Electrolyte. Nanoscale 2015, 7, 17516–17522. [Google Scholar] [CrossRef]
- Yuan, M.; Erdman, J.; Tang, C.; Ardebili, H. High Performance Solid Polymer Electrolyte with Graphene Oxide Nanosheets. RSC Adv. 2014, 4, 59637–59642. [Google Scholar] [CrossRef]
- Jia, W.; Li, Z.; Wu, Z.; Wang, L.; Wu, B.; Wang, Y.; Cao, Y.; Li, J. Graphene Oxide as a Filler to Improve the Performance of PAN-LiClO4 Flexible Solid Polymer Electrolyte. Solid State Ion. 2018, 315, 7–13. [Google Scholar] [CrossRef]
- Wen, J.; Zhao, Q.; Jiang, X.; Ji, G.; Wang, R.; Lu, G.; Long, J.; Hu, N.; Xu, C. Graphene Oxide Enabled Flexible PEO-Based Solid Polymer Electrolyte for All-Solid-State Lithium Metal Battery. ACS Appl. Energy Mater. 2021, 4, 3660–3669. [Google Scholar] [CrossRef]
- Bao, W.; Hu, Z.; Wang, Y.; Jiang, J.; Huo, S.; Fan, W.; Chen, W.; Jing, X.; Long, X.; Zhang, Y. Poly(Ionic Liquid)-Functionalized Graphene Oxide towards Ambient Temperature Operation of All-Solid-State PEO-Based Polymer Electrolyte Lithium Metal Batteries. Chem. Eng. J. 2022, 437, 135420. [Google Scholar] [CrossRef]
- Gomari, S.; Esfandeh, M.; Ghasemi, I. All-Solid-State Flexible Nanocomposite Polymer Electrolytes Based on Poly(Ethylene Oxide): Lithium Perchlorate Using Functionalized Graphene. Solid State Ion. 2017, 303, 37–46. [Google Scholar] [CrossRef]
- Romero, A.; Lavin-Lopez, M.P.; Sanchez-Silva, L.; Valverde, J.L.; Paton-Carrero, A. Comparative Study of Different Scalable Routes to Synthesize Graphene Oxide and Reduced Graphene Oxide. Mater. Chem. Phys. 2018, 203, 284–292. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Farooqui, U.R.; Hamid, N.A. Effect of Graphene Oxide (GO) on Poly(Vinylidene Fluoride-Hexafluoropropylene) (PVDF- HFP) Polymer Electrolyte Membrane. Polymer 2018, 142, 330–336. [Google Scholar] [CrossRef]
- Banerjee, A.; Blasiak, B.; Pasquier, E.; Tomanek, B.; Trudel, S. Synthesis, Characterization, and Evaluation of PEGylated First-Row Transition Metal Ferrite Nanoparticles as: T 2 Contrast Agents for High-Field MRI. RSC Adv. 2017, 7, 38125–38134. [Google Scholar] [CrossRef]
- Karimi, S.; Ghasemi, I.; Abbassi-Sourki, F. A Study on the Crystallization Kinetics of PLLA in the Presence of Graphene Oxide and PEG-Grafted-Graphene Oxide: Effects on the Nucleation and Chain Mobility. Compos. Part B Eng. 2019, 158, 302–310. [Google Scholar] [CrossRef]
- Kumar, K.K.; Ravi, M.; Pavani, Y.; Bhavani, S.; Sharma, A.K.; Narasimha Rao, V.V.R. Investigations on PEO/PVP/NaBr Complexed Polymer Blend Electrolytes for Electrochemical Cell Applications. J. Memb. Sci. 2014, 454, 200–211. [Google Scholar] [CrossRef]
- Anilkumar, K.M.; Jinisha, B.; Manoj, M.; Jayalekshmi, S. Poly(Ethylene Oxide) (PEO)—Poly(Vinyl Pyrrolidone) (PVP) Blend Polymer Based Solid Electrolyte Membranes for Developing Solid State Magnesium Ion Cells. Eur. Polym. J. 2017, 89, 249–262. [Google Scholar] [CrossRef]
- Jinisha, B.; Femy, A.F.; Ashima, M.S.; Jayalekshmi, S. Polyethylene Oxide (PEO)/Polyvinyl Alcohol (PVA) Complexed with Lithium Perchlorate (LiClO4) as a Prospective Material for Making Solid Polymer Electrolyte Films. Mater. Today Proc. 2018, 5, 21189–21194. [Google Scholar] [CrossRef]
- Qi, X.; Yao, X.; Deng, S.; Zhou, T.; Fu, Q. Water-Induced Shape Memory Effect of Graphene Oxide Reinforced Polyvinyl Alcohol Nanocomposites. J. Mater. Chem. A 2014, 2, 2240–2249. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Qu, L.; Zhu, S.; Sun, Y.; Han, G. Enhanced Thermal, UV Blocking and Dye Absorptive Properties of Chitosan/Poly(Vinyl Alcohol)/Graphene Oxide Fibers. Fibers Polym. 2015, 16, 2011–2020. [Google Scholar] [CrossRef]
- Kartick, B.; Srivastava, S.K.; Srivastava, I. Green Synthesis of Graphene. J. Nanosci. Nanotechnol. 2013, 13, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Li, H.; Zhang, J.; Hu, D.; Cai, Z.; Yin, X.; Dong, L.; Huang, L.; Xiong, C.; Jiang, M. Latent Heat and Thermal Conductivity Enhancements in Polyethylene Glycol/Polyethylene Glycol-Grafted Graphene Oxide Composites. Adv. Compos. Hybrid Mater. 2019, 2, 471–480. [Google Scholar] [CrossRef]
- Najafi-Shoa, S.; Barikani, M.; Ehsani, M.; Ghaffari, M. Cobalt-Based Ionic Liquid Grafted on Graphene as a Heterogeneous Catalyst for Poly (Ethylene Terephthalate) Glycolysis. Polym. Degrad. Stab. 2021, 192, 109691. [Google Scholar] [CrossRef]
- Kesavan, K.; Mathew, C.M.; Rajendran, S.; Subbu, C.; Ulaganathan, M. Solid Polymer Blend Electrolyte Based on Poly(Ethylene Oxide) and Poly(Vinyl Pyrrolidone) for Lithium Secondary Batteries. Braz. J. Phys. 2015, 45, 19–27. [Google Scholar] [CrossRef]
- Elashmawi, I.S.; Abdelrazek, E.M.; Hezma, A.M.; Rajeh, A. Modification and Development of Electrical and Magnetic Properties of PVA/PEO Incorporated with MnCl2. Phys. B Condens. Matter 2014, 434, 57–63. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Amarasekara, Y.; Jayaweera, V.; Rajaphaksha, C.; Gunasekara, C.; Perera, I.C.; Amaratunga, G.A.J.; Weerasinghe, L. Graphene Oxide–Based Nanocomposite for Sustained Release of Cephalexin. J. Pharm. Sci. 2020, 109, 1130–1135. [Google Scholar] [CrossRef]
- Wu, W.; Tu, J.; Li, H.; Zhan, Z.; Huang, L.; Cai, Z.; Li, Q.; Jiang, M.; Huang, J. Suppressed Dielectric Loss and Enhanced Thermal Conductivity in Poly(Vinylidene Fluoride) Nanocomposites Using Polyethylene Glycol-Grafted Graphene Oxide. J. Mater. Sci. Mater. Electron. 2020, 31, 807–813. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, P.; Yang, X.; Wang, X. Novel PEG Functionalized Graphene Nanosheets: Enhancement of Dispersibility and Thermal Stability. Nanoscale 2011, 3, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Kim, D.G.; Kim, H.J.; Lee, J.H.; Baik, J.H.; Lee, J.C. Novel Composite Polymer Electrolytes Containing Poly(Ethylene Glycol)-Grafted Graphene Oxide for All-Solid-State Lithium-Ion Battery Applications. J. Mater. Chem. A 2014, 2, 13873–13883. [Google Scholar] [CrossRef]
- Wong, D.H.C.; Thelen, J.L.; Fu, Y.; Devaux, D.; Pandya, A.A.; Battaglia, V.S.; Balsara, N.P.; DeSimone, J.M. Nonflammable Perfluoropolyether-Based Electrolytes for Lithium Batteries. Proc. Natl. Acad. Sci. USA 2014, 111, 3327–3331. [Google Scholar] [CrossRef] [PubMed]
- El-Newehy, M.H.; Al-Deyab, S.S.; Kenawy, E.R.; Abdel-Megeed, A. Fabrication of Electrospun Antimicrobial Nanofibers Containing Metronidazole Using Nanospider Technology. Fibers Polym. 2012, 13, 709–717. [Google Scholar] [CrossRef]
- Putri, R.M.; Sundari, C.D.D.; Floweri, O.; Mayangsari, T.R.; Ivansyah, A.L.; Santosa, S.P.; Arcana, I.M.; Iskandar, F. PEO/PVA/LiOH Solid Polymer Electrolyte Prepared via Ultrasound-Assisted Solution Cast Method. J. Non-Cryst. Solids 2021, 556, 120549. [Google Scholar] [CrossRef]
- Yuan, B.; Bao, C.; Song, L.; Hong, N.; Liew, K.M.; Hu, Y. Preparation of Functionalized Graphene Oxide/Polypropylene Nanocomposite with Significantly Improved Thermal Stability and Studies on the Crystallization Behavior and Mechanical Properties. Chem. Eng. J. 2014, 237, 411–420. [Google Scholar] [CrossRef]
- Galano, A. Carbon Nanotubes: Promising Agents against Free Radicals. Nanoscale 2010, 2, 373–380. [Google Scholar] [CrossRef]
- Abd El-Kader, F.H.; Hakeem, N.A.; Elashmawi, I.S.; Ismail, A.M. Enhancement of Structural and Thermal Properties of PEO/PVA Blend Embedded with TiO2 Nanoparticles. Indian J. Phys. 2013, 87, 983–990. [Google Scholar] [CrossRef]
- Falqi, F.H.; Bin-Dahman, O.A.; Khair, A.; Al-Harthi, M.A. PVA/PEG/Graphene Shape Memory Composites Responsive to Multi-Stimuli. Appl. Phys. A Mater. Sci. Process. 2022, 128, 427. [Google Scholar] [CrossRef]
- Falqi, F.H.; Bin-Dahman, O.A.; Hussain, M.; Al-Harthi, M.A. Preparation of Miscible PVA/PEG Blends and Effect of Graphene Concentration on Thermal, Crystallization, Morphological, and Mechanical Properties of PVA/PEG (10 wt%) Blend. Int. J. Polym. Sci. 2018, 2018, 8527693. [Google Scholar] [CrossRef]
- Han, S.; Meng, Q.; Araby, S.; Liu, T.; Demiral, M. Mechanical and Electrical Properties of Graphene and Carbon Nanotube Reinforced Epoxy Adhesives: Experimental and Numerical Analysis. Compos. Part A Appl. Sci. Manuf. 2019, 120, 116–126. [Google Scholar] [CrossRef]
- Sreeprasad, T.S.; Berry, V. How Do the Electrical Properties of Graphene Change with Its Functionalization? Small 2013, 9, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Koduru, H.K.; Scarpelli, F.; Marinov, Y.G.; Hadjichristov, G.B.; Rafailov, P.M.; Miloushev, I.K.; Petrov, A.G.; Godbert, N.; Bruno, L.; Scaramuzza, N. Characterization of PEO/PVP/GO Nanocomposite Solid Polymer Electrolyte Membranes: Microstructural, Thermo-Mechanical, and Conductivity Properties. Ionics 2018, 24, 3459–3473. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, X.; Liu, J.; Zhu, Y. Ion Liquid Modified GO Filler to Improve the Performance of Polymer Electrolytes for Li Metal Batteries. Front. Chem. 2020, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Putri, R.M.; Floweri, O.; Mayangsari, T.R.; Aimon, A.H.; Iskandar, F. Preliminary Study of Electrochemical Properties of Polyethylene Oxide (PEO) and Polyvinyl Alcohol (PVA) Composites as Material for Solid Polymer Electrolyte. Mater. Today Proc. 2020, 44, 3375–3377. [Google Scholar] [CrossRef]


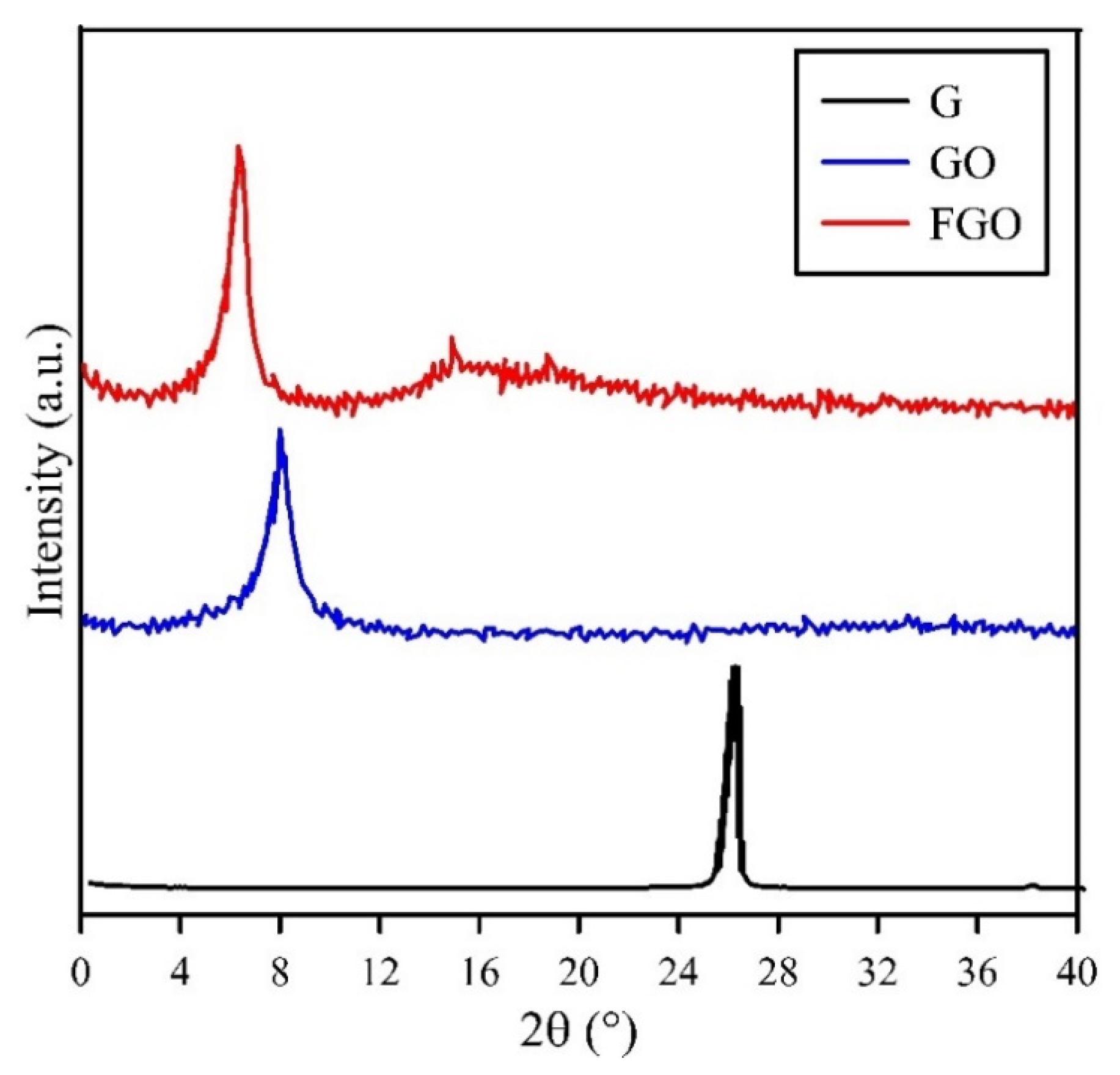
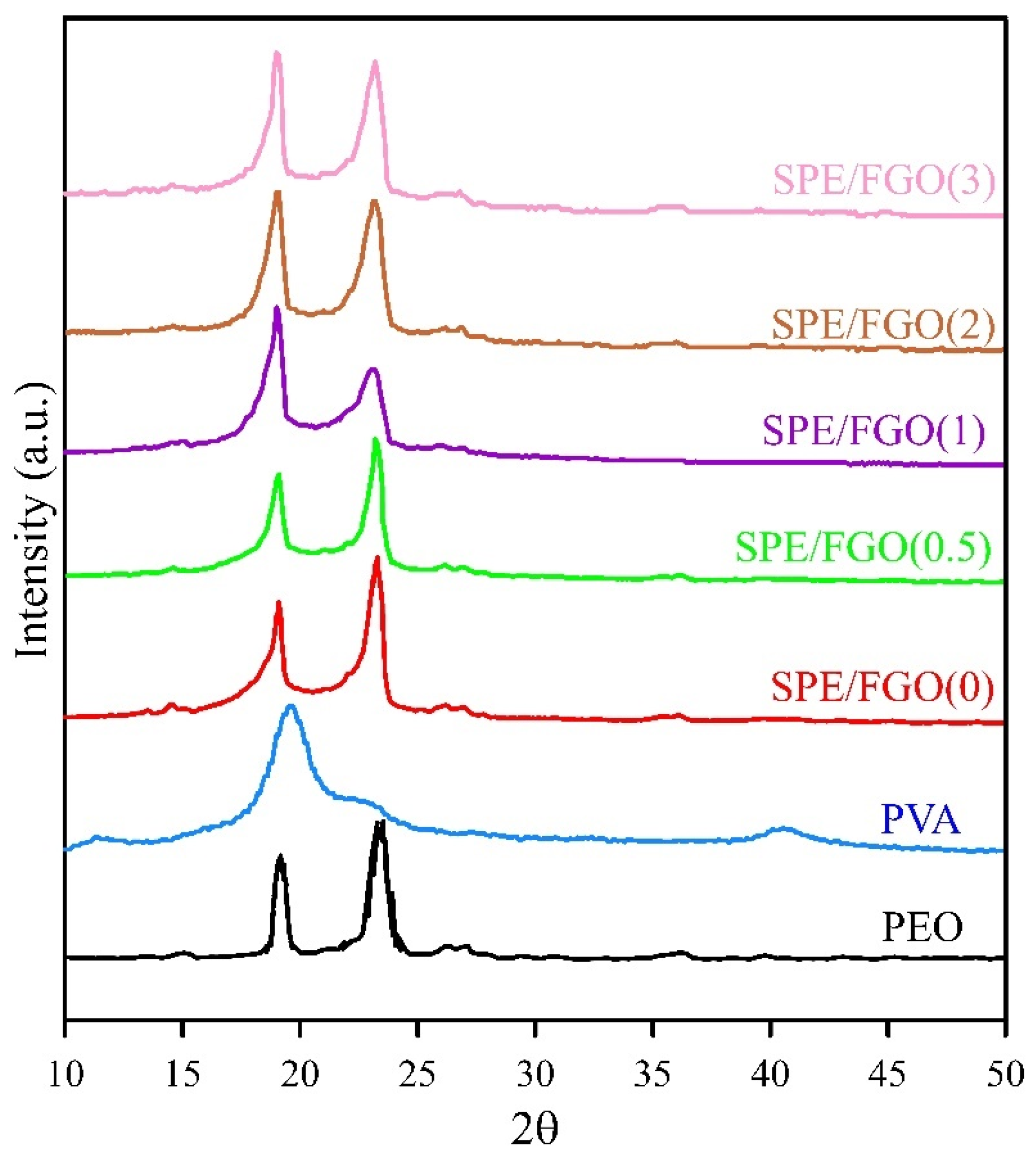
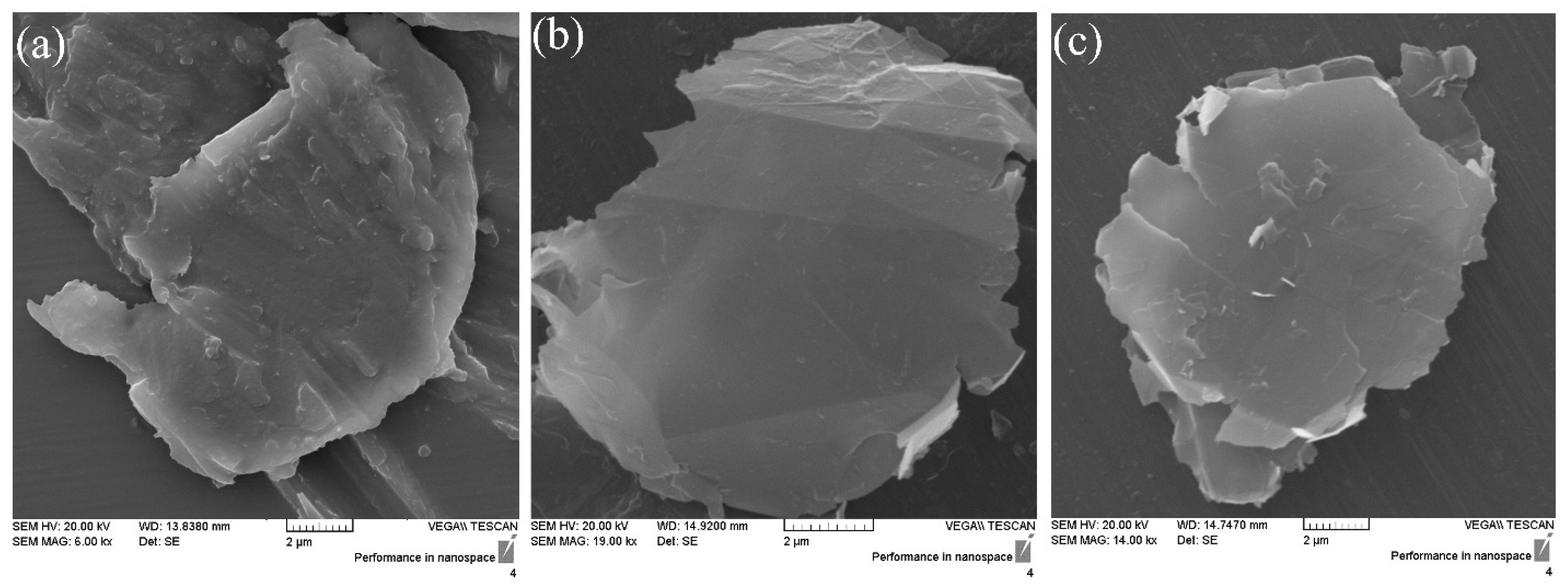
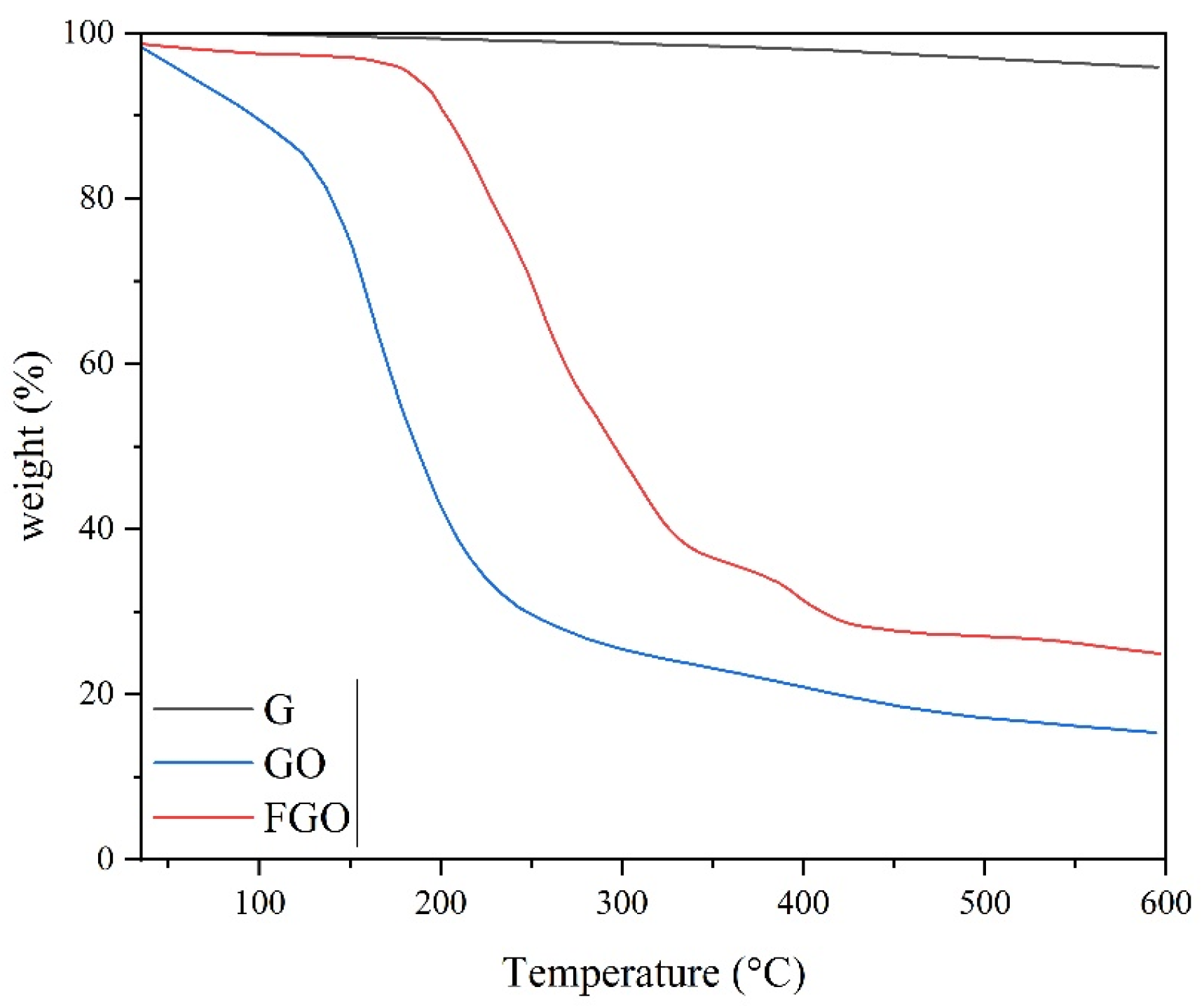

| Samples | Degree of Crystallinity (%) |
|---|---|
| PEO | 80.5 |
| PVA | 51.5 |
| SPE/FGO(0) | 48.2 |
| SPE/FGO(0.5) | 44.3 |
| SPE/FGO(1) | 39.5 |
| SPE/FGO(2) | 34.2 |
| SPE/FGO(3) | 34.3 |
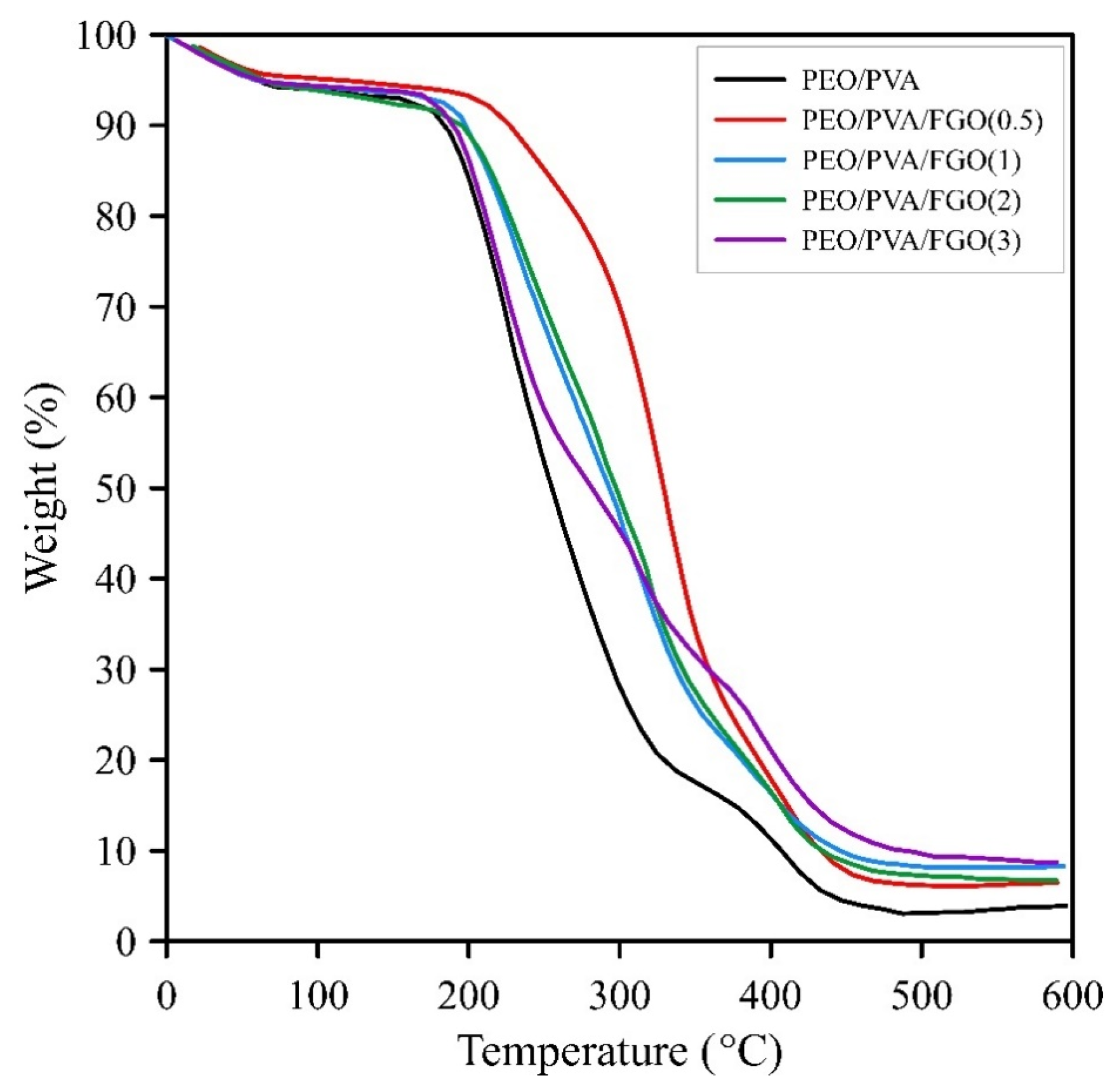
| Samples | T5% (°C) | T25% (°C) | Char Residual (%) |
|---|---|---|---|
| PEO/PVA | 182.9 | 214.6 | 4.32 |
| PEO/PVA/FGO(0.5) | 206.5 | 289.4 | 6.86 |
| PEO/PVA/FGO(1) | 188.5 | 234 | 8.82 |
| PEO/PVA/FGO(2) | 195.8 | 240.5 | 7.22 |
| PEO/PVA/FGO(3) | 187.7 | 218.9 | 9.4 |
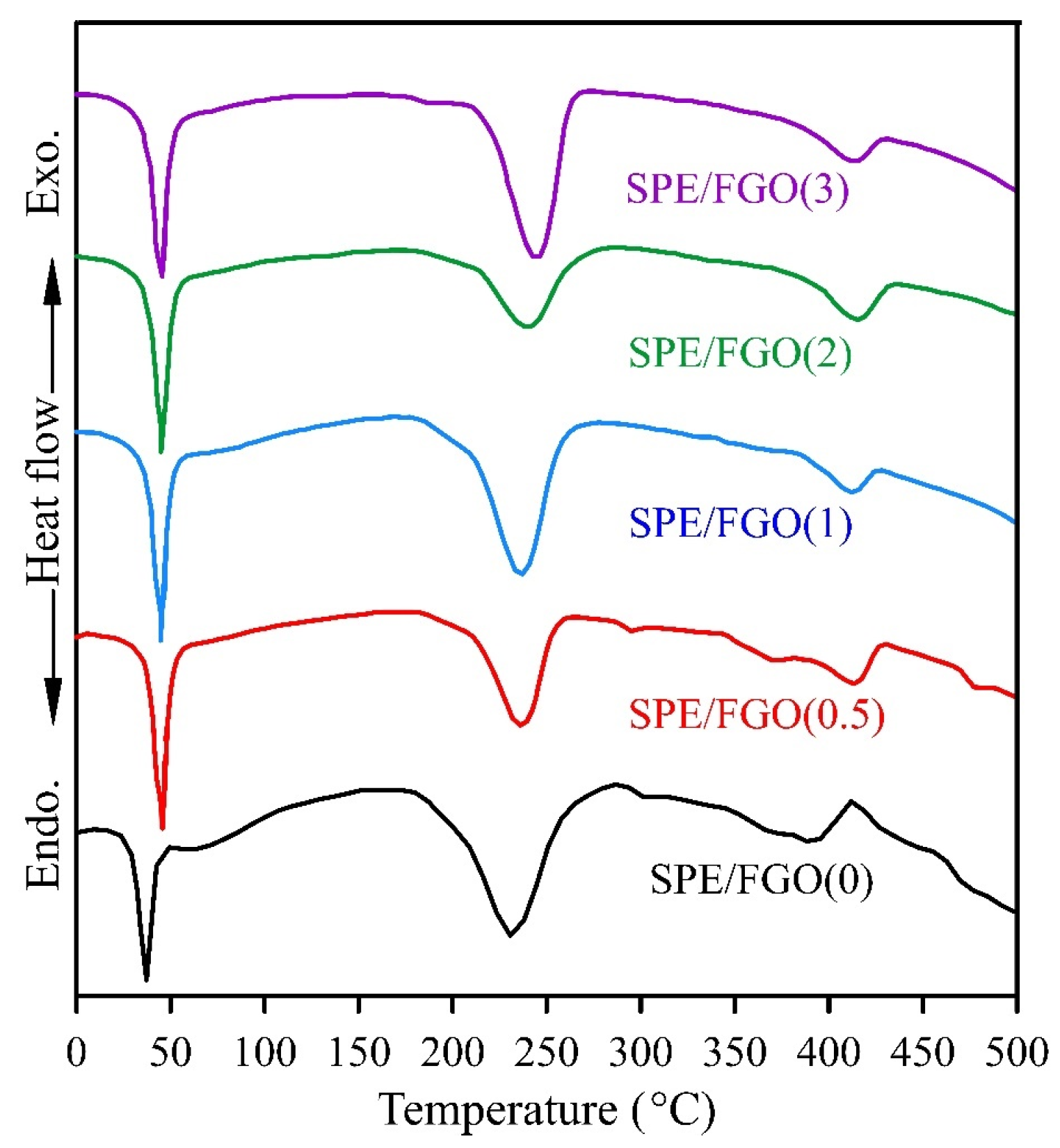
| Samples | Tg (°C) | Tm (°C) | Td (°C) | ΔHm (J/g) | χc (%) |
|---|---|---|---|---|---|
| SPE/FGO(0) | 40.1 | 233.2 | 391.2 | 56.11 | 39.52 |
| SPE/FGO(0.5) | 45.65 | 237.6 | 419.9 | 44.63 | 31.43 |
| SPE/FGO(1) | 45.7 | 237.4 | 411.1 | 42.77 | 30.12 |
| SPE/FGO(2) | 46.4 | 241.5 | 416.3 | 29.39 | 20.7 |
| SPE/FGO(3) | 46.7 | 243.8 | 415.3 | 42.04 | 29.61 |
| Samples | σ (S·cm−1) | Electrical Conductivity (S·cm−1) |
|---|---|---|
| SPE/FGO(0) | 1.2 × 10−5 | 7.1 × 10−12 |
| SPE/FGO(0.5) | 2.85 × 10−5 | 5.32 × 10−11 |
| SPE/FGO(1) | 3.61 × 10−5 | 6.2 × 10−11 |
| SPE/FGO(2) | 5.2 × 10−5 | 8.4 × 10−11 |
| SPE/FGO(3) | 4.2 × 10−5 | 9.1 × 10−11 |
| Samples | Young’s Modulus (MPa) | Yield Stress (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| PEO | 203 | 6.65 | 7.3 | 583 |
| PVA | 682 | 16.1 | 16.6 | 211 |
| PEO/PVA | 453 | 10.4 | 10.8 | 374 |
| PEO/PVA/FGO(0.5) | 573 | 12.9 | 13.4 | 465 |
| PEO/PVA/FGO(1) | 615 | 13.3 | 13.6 | 483 |
| PEO/PVA/FGO(2) | 563 | 12.7 | 13.4 | 456 |
| PEO/PVA/FGO(3) | 521 | 11.3 | 11.4 | 386 |
| SPEs | Temperature (°C) | Ionic Conductivity (S/cm) | Ref. |
|---|---|---|---|
| PEO/PVA/LiOH | 25 | 2.18 × 10−5 | [55] |
| PEO/PPC | 25 | 2.04 × 10−5 | [11] |
| PEO/PVP/LiClO4 | 30 | 0.2307 × 10−5 | [37] |
| SPE/FGnP(0.5) | 25 | 2.53 × 10−5 | [24] |
| SPE/FGO(2) | 25 | 5.2 × 10−5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eslami, B.; Ghasemi, I.; Esfandeh, M. Using Pegylated Graphene Oxide to Achieve High Performance Solid Polymer Electrolyte Based on Poly(ethylene oxide)/Polyvinyl Alcohol Blend (PEO/PVA). Polymers 2023, 15, 3063. https://doi.org/10.3390/polym15143063
Eslami B, Ghasemi I, Esfandeh M. Using Pegylated Graphene Oxide to Achieve High Performance Solid Polymer Electrolyte Based on Poly(ethylene oxide)/Polyvinyl Alcohol Blend (PEO/PVA). Polymers. 2023; 15(14):3063. https://doi.org/10.3390/polym15143063
Chicago/Turabian StyleEslami, Behnam, Ismaeil Ghasemi, and Masoud Esfandeh. 2023. "Using Pegylated Graphene Oxide to Achieve High Performance Solid Polymer Electrolyte Based on Poly(ethylene oxide)/Polyvinyl Alcohol Blend (PEO/PVA)" Polymers 15, no. 14: 3063. https://doi.org/10.3390/polym15143063
APA StyleEslami, B., Ghasemi, I., & Esfandeh, M. (2023). Using Pegylated Graphene Oxide to Achieve High Performance Solid Polymer Electrolyte Based on Poly(ethylene oxide)/Polyvinyl Alcohol Blend (PEO/PVA). Polymers, 15(14), 3063. https://doi.org/10.3390/polym15143063






