Synthesis of Multifunctional Polymersomes Prepared by Polymerization-Induced Self-Assembly
Abstract
:1. Introduction
2. Results and Discussion
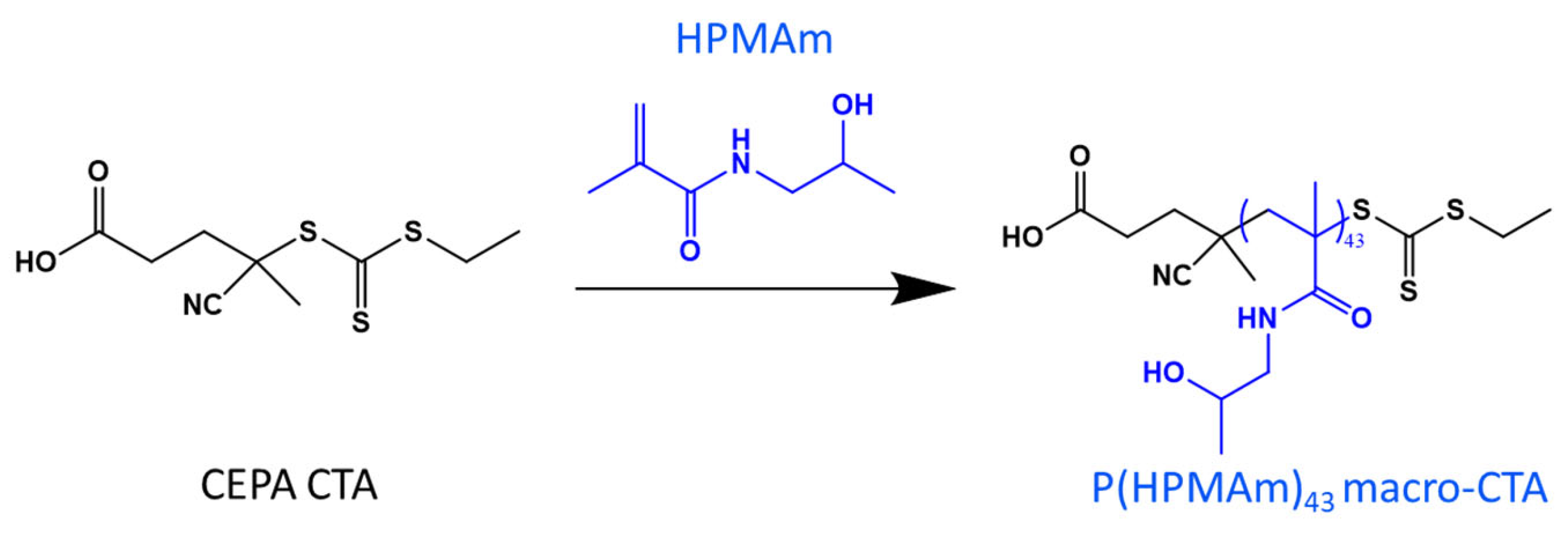
2.1. Synthesis of the P(HPMAm) Macro-CTA
2.2. Synthesis of the P(HPMAm)-b-P(MTEAM) Block Copolymer Carriers
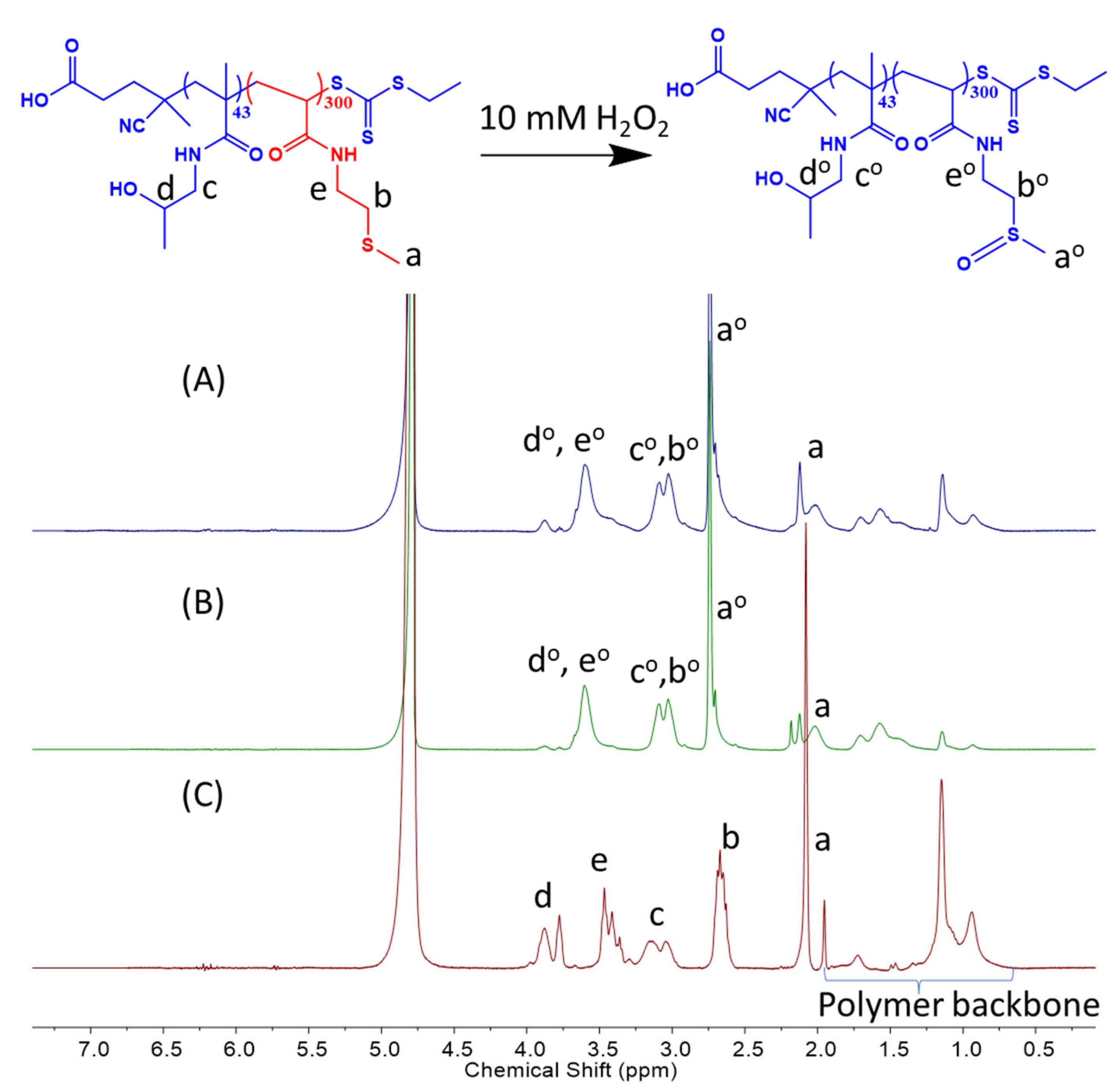
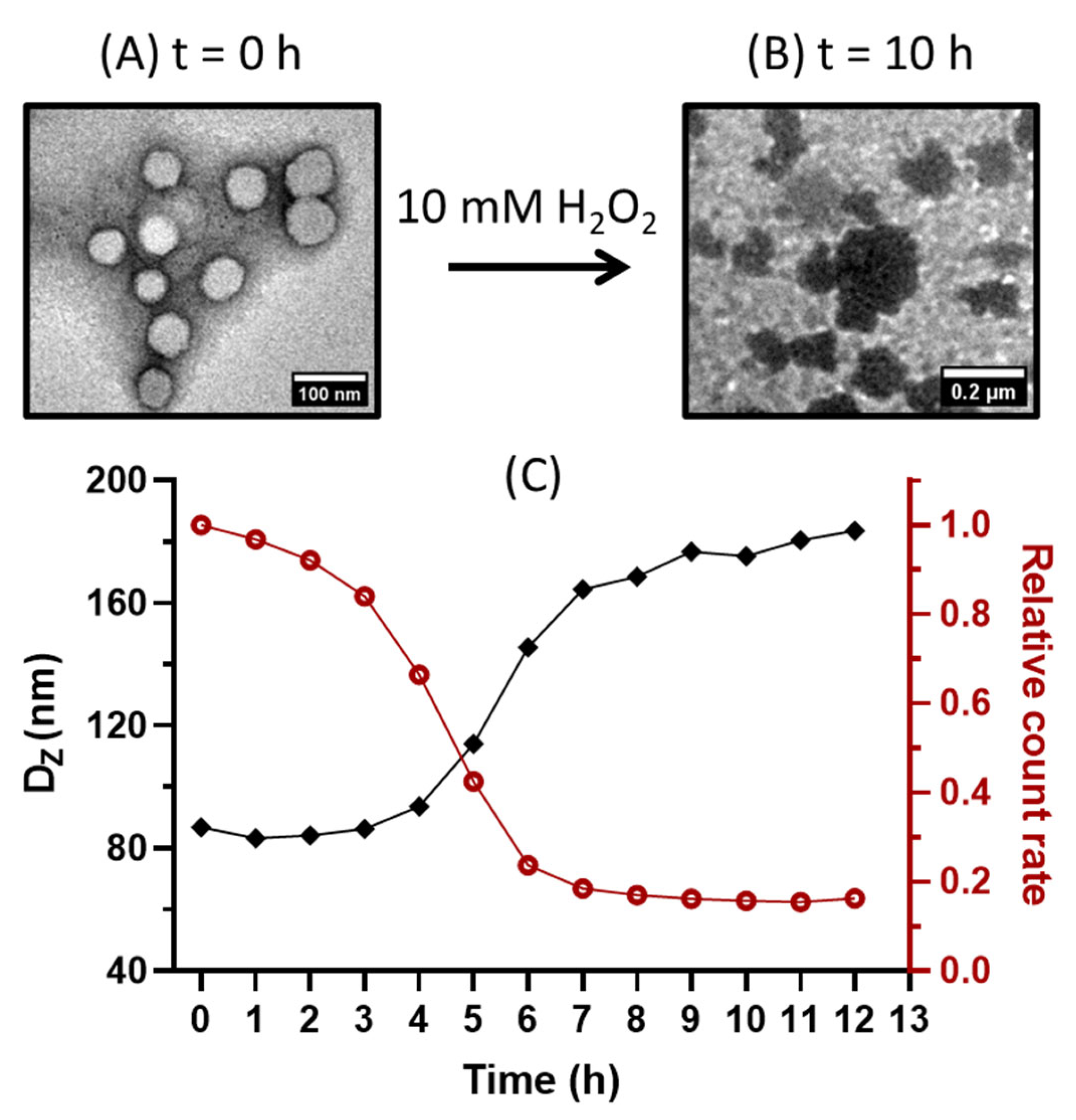
2.3. Fabrication of Vesicles and Oxidative Behavior in H2O2 Solutions
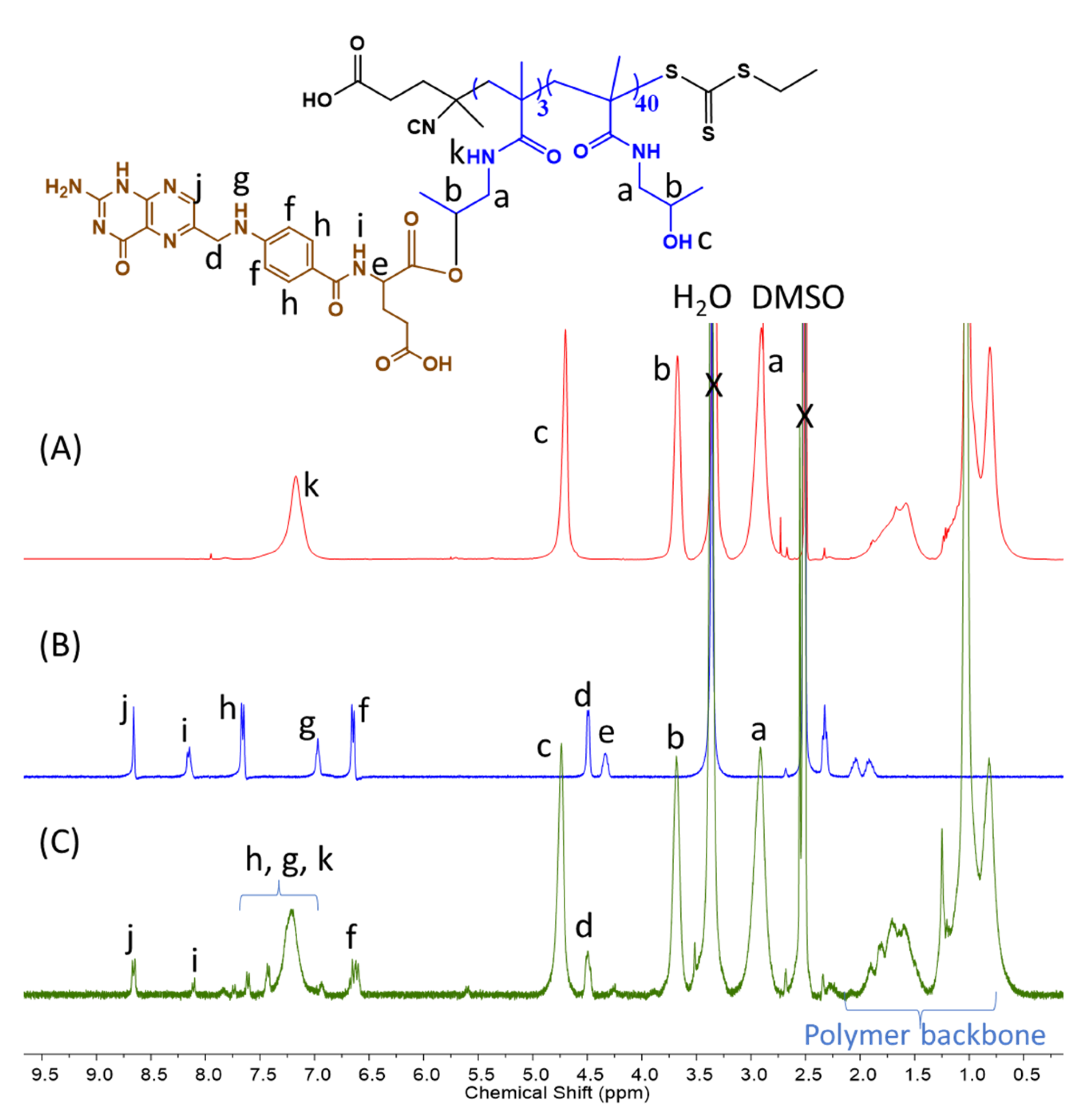
2.4. FA-Functionalized P(HPMAm)43 Macro-CTA and Diblock Copolymer Synthesis via the PISA Process with the MTEAM Monomer
2.5. Encapsulation and Release Studies
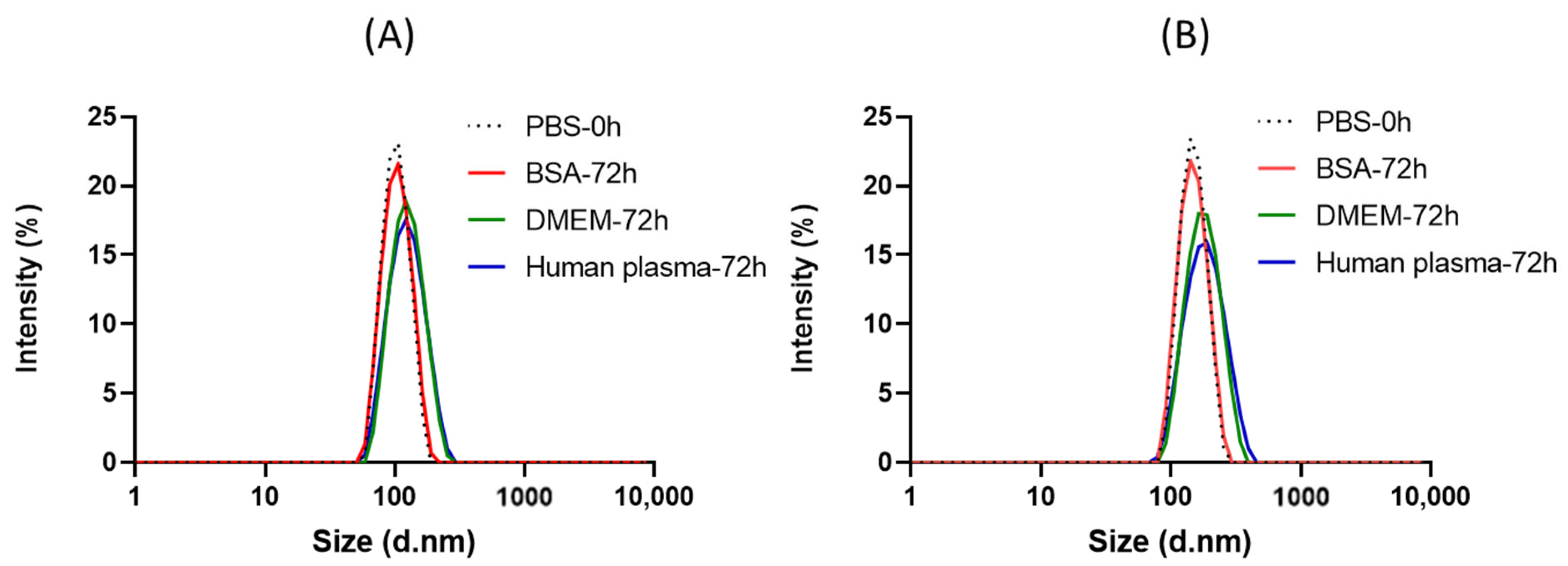
2.6. Stability in Bio-Relevant Conditions
2.7. Cytotoxicity
3. Conclusions and Perspectives
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of N-2-Hydroxypropyl Methacrylamide (HPMAm)
4.2.2. Synthesis of the N-(2-(Methylthio)ethyl)acrylamide (MTEAM) Monomer
4.2.3. Synthesis of the P(HPMAm) Macro-CTA
4.2.4. Synthesis of P(HPMAm)n-b-P(MTEAM)300 Block Copolymer NPs (n = 16, 43)
4.2.5. Attachment of Folic Acid (FA) to the P(HPMAm)43 Macro-CTA
4.2.6. Preparation of FA-Functionalized NPs
4.2.7. Encapsulation of Model Compounds into the FA-Functionalized Vesicles
4.2.8. Cytotoxicity
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberts, B.; Lewis, J. The Lipid Bilayer. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2013; pp. 6–11. ISBN 0815332181. [Google Scholar]
- Honigmann, A.; Pralle, A. Compartmentalization of the Cell Membrane. J. Mol. Biol. 2016, 428, 4739–4748. [Google Scholar] [CrossRef] [PubMed]
- Derry, M.J.; Fielding, L.A.; Armes, S.P. Polymerization-induced self-assembly of block copolymer nanoparticles via RAFT non-aqueous dispersion polymerization. Prog. Polym. Sci. 2016, 52, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Peyret, A.; Ibarboure, E.; Pippa, N.; Lecommandoux, S. Liposomes in Polymersomes: Multicompartment System with Temperature-Triggered Release. Langmuir 2017, 33, 7079–7085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, H.; Lee, H. Recent developments in microfluidic synthesis of artificial cell-like polymersomes and liposomes for functional bioreactors. Biomicrofluidics 2021, 15, 021301. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ramezani, M.; Abnous, K.; Alibolandi, M. Biocompatible polymersomes-based cancer theranostics: Towards multifunctional nanomedicine. Int. J. Pharm. 2017, 519, 287–303. [Google Scholar] [CrossRef]
- Albuquerque, L.J.; Sincari, V.; Jäger, A.; Kucka, J.; Humajova, J.; Pankrac, J.; Paral, P.; Heizer, T.; Janouškova, O.; Davidovich, I.; et al. pH-responsive polymersome-mediated delivery of doxorubicin into tumor sites enhances the therapeutic efficacy and reduces cardiotoxic effects. J. Control. Release 2021, 332, 529–538. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer Vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Le Meins, J.F.; Sandre, O.; Lecommandoux, S. Recent trends in the tuning of polymersomes’ membrane properties. Eur. Phys. J. E 2011, 34, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Marguet, M.; Edembe, L.; Lecommandoux, S. Polymersomes in Polymersomes: Multiple Loading and Permeability Control. Angew. Chem. 2012, 124, 1199–1202. [Google Scholar] [CrossRef]
- Zheng, C.; Qiu, L.; Zhu, K. Novel polymersomes based on amphiphilic graft polyphosphazenes and their encapsulation of water-soluble anti-cancer drug. Polymer 2009, 50, 1173–1177. [Google Scholar] [CrossRef]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef]
- Messager, L.; Gaitzsch, J.; Chierico, L.; Battaglia, G. Novel aspects of encapsulation and delivery using polymersomes. Curr. Opin. Pharmacol. 2014, 18, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Dolmazon, E.; Moylan, K.; Fowley, C.; McHale, A.P.; Callan, J.F.; Callan, B. A charge neutral, size tuneable polymersome capable of high biological encapsulation efficiency and cell permeation. Int. J. Pharm. 2015, 481, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.H.; Ghoroghchian, P.P.; Freudenberg, J.; Zhang, G.; Therien, M.J.; Greene, M.I.; Hammer, D.A.; Murali, R. Polymersomes: A new multi-functional tool for cancer diagnosis and therapy. Methods 2008, 46, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Walvekar, P.; Gannimani, R.; Salih, M.; Makhathini, S.; Mocktar, C.; Govender, T. Self-assembled oleylamine grafted hyaluronic acid polymersomes for delivery of vancomycin against methicillin resistant Staphylococcus aureus (MRSA). Colloids Surfaces B Biointerfaces 2019, 182, 110388. [Google Scholar] [CrossRef]
- Lefley, J.; Waldron, C.; Becer, C.R. Macromolecular design and preparation of polymersomes. Polym. Chem. 2020, 11, 7124–7136. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.C.; Varlas, S.; Couturaud, B.; Jones, J.R.; Keogh, R.; Mathers, R.T.; O’Reilly, R.K. Predicting Monomers for Use in Polymerization-Induced Self-Assembly. Angew. Chem. Int. Ed. 2018, 57, 15733–15737. [Google Scholar] [CrossRef]
- Couturaud, B.; Georgiou, P.; Varlas, S.; Jones, J.; Arno, M.C.; Foster, J.; O’Reilly, R.K. Poly(Pentafluorophenyl Methacrylate)-Based Nano-Objects Developed by Photo-PISA as Scaffolds for Post-Polymerization Functionalization. Macromol. Rapid Commun. 2019, 40, 1800460. [Google Scholar] [CrossRef]
- Khor, S.Y.; Quinn, J.F.; Whittaker, M.R.; Truong, N.P.; Davis, T.P. Controlling Nanomaterial Size and Shape for Biomedical Applications via Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2019, 40, 1800438. [Google Scholar] [CrossRef]
- Phan, H.; Cossutta, M.; Houppe, C.; Le Cœur, C.; Prevost, S.; Cascone, I.; Courty, J.; Penelle, J.; Couturaud, B. Polymerization-Induced Self-Assembly (PISA) for in situ drug encapsulation or drug conjugation in cancer application. J. Colloid Interface Sci. 2022, 618, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Liu, D.; Zhang, X.; Huang, C.; He, J.; Xu, Q.; Li, X.; Zhang, L. Facile preparation of hybrid vesicles loaded with silica nanoparticles via aqueous photoinitiated polymerization-induced self-assembly. RSC Adv. 2017, 7, 23114–23121. [Google Scholar] [CrossRef] [Green Version]
- Varlas, S.; Foster, J.C.; Georgiou, P.G.; Keogh, R.; Husband, J.T.; Williams, D.S.; O’Reilly, R.K. Tuning the membrane permeability of polymersome nanoreactors developed by aqueous emulsion polymerization-induced self-assembly. Nanoscale 2019, 11, 12643–12654. [Google Scholar] [CrossRef] [Green Version]
- Phan, H.; Taresco, V.; Penelle, J.; Couturaud, B. Polymerisation-induced self-assembly (PISA) as a straightforward formulation strategy for stimuli-responsive drug delivery systems and biomaterials: Recent advances. Biomater. Sci. 2021, 9, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhong, Z.; Feijen, J. Stimuli-Responsive Polymersomes for Programmed Drug Delivery. Biomacromolecules 2009, 10, 197–209. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Xie, Z.; Jing, X.; Bellotti, A.; Gu, Z. Stimuli-Responsive Polymersomes for Biomedical Applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ray, D.; Aswal, V.K.; Ghosh, S. Multi-Stimuli-Responsive Directional Assembly of an Amphiphilic Donor-Acceptor Alternating Supramolecular Copolymer. Chem. A Eur. J. 2018, 24, 16379–16387. [Google Scholar] [CrossRef]
- Ferrero, C.; Casas, M.; Caraballo, I. Redox-Responsive Polymersomes as Smart Doxorubicin Delivery Systems. Pharmaceutics 2022, 14, 1724. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Kembro, J.M.; Cortassa, S.; Aon, M.A. Mitochondrial Reactive Oxygen Species (ROS) and Arrhythmias. In Systems Biology of Free Radicals and Antioxidants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1047–1076. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Ke, W.; Li, J.; Mohammed, F.; Wang, Y.; Tou, K.; Liu, X.; Wen, P.; Kinoh, H.; Anraku, Y.; Chen, H.; et al. Therapeutic Polymersome Nanoreactors with Tumor-Specific Activable Cascade Reactions for Cooperative Cancer Therapy. ACS Nano 2019, 13, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, J.; Qian, C.; Lu, Y.; Kahkoska, A.R.; Xie, Z.; Jing, X.; Buse, J.B.; Gu, Z. H2O2-Responsive Vesicles Integrated with Transcutaneous Patches for Glucose-Mediated Insulin Delivery. ACS Nano 2017, 11, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Wang, Y.; Li, X.; Lu, N.; Ge, Z. Polymersome Nanoreactor-Mediated Combination Chemodynamic-Immunotherapy via ROS Production and Enhanced STING Activation. Adv. Ther. 2021, 4, 2100130. [Google Scholar] [CrossRef]
- Phan, H.; Cavanagh, R.; Destouches, D.; Vacherot, F.; Brissault, B.; Taresco, V.; Penelle, J.; Couturaud, B. H2O2-Responsive Nanocarriers Prepared by RAFT-Mediated Polymerization-Induced Self-Assembly of N-(2-(Methylthio)ethyl)acrylamide for Biomedical Applications. ACS Appl. Polym. Mater. 2022, 4, 7778–7789. [Google Scholar] [CrossRef]
- Julyan, P.J.; Seymour, L.W.; Ferry, D.R.; Daryani, S.; Boivin, C.M.; Doran, J.; David, M.; Anderson, D.; Christodoulou, C.; Young, A.M.; et al. Preliminary clinical study of the distribution of HPMA copolymers bearing doxorubicin and galactosamine. J. Control. Release 1999, 57, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Šubr, V. Structural and chemical aspects of HPMA copolymers as drug carriers. Adv. Drug Deliv. Rev. 2010, 62, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Anane-Adjei, A.B.; Fletcher, N.L.; Cavanagh, R.J.; Houston, Z.H.; Crawford, T.; Pearce, A.K.; Taresco, V.; Ritchie, A.A.; Clarke, P.; Grabowska, A.M.; et al. Synthesis, characterisation and evaluation of hyperbranched N-(2-hydroxypropyl) methacrylamides for transport and delivery in pancreatic cell lines in vitro and in vivo. Biomater. Sci. 2022, 10, 2328–2344. [Google Scholar] [CrossRef]
- Scales, C.W.; Vasilieva, Y.A.; Convertine, A.J.; Lowe, A.B.; McCormick, C.L. Direct, Controlled Synthesis of the Nonimmunogenic, Hydrophilic Polymer, Poly(N-(2-hydroxypropyl)methacrylamide) via RAFT in Aqueous Media. Biomacromolecules 2005, 6, 1846–1850. [Google Scholar] [CrossRef]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Sega, E.; Leamon, C.P.; Low, P.S. Folate receptor-targeted immunotherapy of cancer: Mechanism and therapeutic potential. Adv. Drug Deliv. Rev. 2004, 56, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A. From the “Magic Bullet” to Advanced Nanomaterials for Active Targeting in Diagnostics and Therapeutics; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780323508797. [Google Scholar]
- Bellotti, E.; Cascone, M.G.; Barbani, N.; Rossin, D.; Rastaldo, R.; Giachino, C.; Cristallini, C. Targeting Cancer Cells Overexpressing Folate Receptors with New Terpolymer-Based Nanocapsules: Toward a Novel Targeted DNA Delivery System for Cancer Therapy. Biomedicines 2021, 9, 1275. [Google Scholar] [CrossRef] [PubMed]
- Jawahar, N.; De, A.; Jubee, S.; Reddy, E.S. Folic acid-conjugated raloxifene hydrochloride carbon nanotube for targeting breast cancer cells. Drug Dev. Res. 2020, 81, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Bauri, K.; Narayanan, A.; Haldar, U.; De, P. Polymerization-induced self-assembly driving chiral nanostructured materials. Polym. Chem. 2015, 6, 6152–6162. [Google Scholar] [CrossRef]
- Lowe, A.B. RAFT alcoholic dispersion polymerization with polymerization-induced self-assembly. Polymer 2016, 106, 161–181. [Google Scholar] [CrossRef]
- Blanazs, A.; Ryan, A.J.; Armes, S.P. Predictive Phase Diagrams for RAFT Aqueous Dispersion Polymerization: Effect of Block Copolymer Composition, Molecular Weight, and Copolymer Concentration. Macromolecules 2012, 45, 5099–5107. [Google Scholar] [CrossRef]
- Blanazs, A.; Madsen, J.; Battaglia, G.; Ryan, A.J.; Armes, S.P. Mechanistic Insights for Block Copolymer Morphologies: How Do Worms Form Vesicles? J. Am. Chem. Soc. 2011, 133, 16581–16587. [Google Scholar] [CrossRef]
- Figg, C.A.; Carmean, R.N.; Bentz, K.C.; Mukherjee, S.; Savin, D.A.; Sumerlin, B.S. Tuning Hydrophobicity to Program Block Copolymer Assemblies from the Inside Out. Macromolecules 2017, 50, 935–943. [Google Scholar] [CrossRef]
- Baddam, V.; Välinen, L.; Tenhu, H. Thermoresponsive Polycation-Stabilized Nanoparticles through PISA. Control of Particle Morphology with a Salt. Macromolecules 2021, 54, 4288–4299. [Google Scholar] [CrossRef]
- Cunningham, V.J.; Alswieleh, A.M.; Thompson, K.L.; Williams, M.; Leggett, G.J.; Armes, S.P.; Musa, O.M. Poly(glycerol monomethacrylate)–Poly(benzyl methacrylate) Diblock Copolymer Nanoparticles via RAFT Emulsion Polymerization: Synthesis, Characterization, and Interfacial Activity. Macromolecules 2014, 47, 5613–5623. [Google Scholar] [CrossRef]
- de la Haye, J.L.; Zhang, X.; Chaduc, I.; Brunel, F.; Lansalot, M.; D’Agosto, F. The Effect of Hydrophile Topology in RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem. Int. Ed. 2016, 55, 3739–3743. [Google Scholar] [CrossRef]
- Blackman, L.D.; Varlas, S.; Arno, M.C.; Fayter, A.; Gibson, M.I.; O’reilly, R.K. Permeable Protein-Loaded Polymersome Cascade Nanoreactors by Polymerization-Induced Self-Assembly. ACS Macro Lett. 2017, 6, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Pearce, A.K.; Foster, J.C.; O’Reilly, R.K. Probing and Tuning the Permeability of Polymersomes. ACS Central Sci. 2021, 7, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ng, G.; Xu, J.; Kuchel, R.P.; Yeow, J.; Boyer, C. 2-(Methylthio)ethyl Methacrylate: A Versatile Monomer for Stimuli Responsiveness and Polymerization-Induced Self-Assembly in the Presence of Air. ACS Macro Lett. 2017, 6, 1237–1244. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, H.; Tao, X.; Cao, F.; Trépout, S.; Ling, J.; Li, M.-H. Oxidation-Sensitive Polymersomes Based on Amphiphilic Diblock Copolypeptoids. Biomacromolecules 2019, 20, 3435–3444. [Google Scholar] [CrossRef]
- Sobotta, F.H.; Kuchenbrod, M.T.; Gruschwitz, F.V.; Festag, G.; Bellstedt, P.; Hoeppener, S.; Brendel, J.C. Tuneable Time Delay in the Burst Release from Oxidation-Sensitive Polymersomes Made by PISA. Angew. Chem. Int. Ed. 2021, 60, 24716–24723. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, M.; Gao, W.; Yang, Y.; Zhang, J.; Pu, Y.; He, B. Polymeric nanoparticles responsive to intracellular ROS for anticancer drug delivery. Colloids Surfaces B Biointerfaces 2019, 181, 252–260. [Google Scholar] [CrossRef]
- Yoo, J.; Rejinold, N.S.; Lee, D.Y.; Jon, S.; Kim, Y.-C. Protease-activatable cell-penetrating peptide possessing ROS-triggered phase transition for enhanced cancer therapy. J. Control. Release 2017, 264, 89–101. [Google Scholar] [CrossRef] [PubMed]
- de Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible Polymeric Nanoparticles Degrade and Release Cargo in Response to Biologically Relevant Levels of Hydrogen Peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef] [Green Version]
- Truong, N.P.; Dussert, M.V.; Whittaker, M.R.; Quinn, J.F.; Davis, T.P. Rapid synthesis of ultrahigh molecular weight and low polydispersity polystyrene diblock copolymers by RAFT-mediated emulsion polymerization. Polym. Chem. 2015, 6, 3865–3874. [Google Scholar] [CrossRef]
- Larnaudie, S.C.; Brendel, J.C.; Romero-Canelón, I.; Sanchez-Cano, C.; Catrouillet, S.; Sanchis, J.; Coverdale, J.P.C.; Song, J.-I.; Habtemariam, A.; Sadler, P.J.; et al. Cyclic Peptide–Polymer Nanotubes as Efficient and Highly Potent Drug Delivery Systems for Organometallic Anticancer Complexes. Biomacromolecules 2018, 19, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulbrich, K.; Šubr, V.; Strohalm, J.; Plocová, D.; Jelínková, M.; Říhová, B. Polymeric drugs based on conjugates of synthetic and natural macromolecules: I. Synthesis and physico-chemical characterisation. J. Control. Release 2000, 64, 63–79. [Google Scholar] [CrossRef]
- Chytil, P.; Etrych, T.; Kříž, J.; Šubr, V.; Ulbrich, K. N-(2-Hydroxypropyl)methacrylamide-based polymer conjugates with pH-controlled activation of doxorubicin for cell-specific or passive tumour targeting. Synthesis by RAFT polymerisation and physicochemical characterisation. Eur. J. Pharm. Sci. 2010, 41, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Q.; Wen, C.; Gong, T.; Li, J.; Liang, W.; Li, M.; Wang, Y.; Guo, R. Folic acid and deoxycholic acid derivative modified Fe3O4 nanoparticles for efficient pH-dependent drug release and multi-targeting against liver cancer cells. RSC Adv. 2021, 11, 39804–39812. [Google Scholar] [CrossRef]
- Lee, S.J.; Shim, Y.-H.; Oh, J.-S.; Jeong, Y.-I.; Park, I.-K.; Lee, H.C. Folic-acid-conjugated pullulan/poly(DL-lactide-co-glycolide) graft copolymer nanoparticles for folate-receptor-mediated drug delivery. Nanoscale Res. Lett. 2015, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]




| Time (min) | Conv. % | Theoretical DP a | Experimental Mn b (×10−3) | Experimental Mn c (×10−3) | Đ | Size (nm) | PDI |
|---|---|---|---|---|---|---|---|
| 60 | 14 | 43 | 12.7 | 29.2 | 1.33 | 90.44 | 0.55 |
| 90 | 52 | 154 | 35.1 | 60.1 | 1.88 | 111.1 | 0.44 |
| 105 | 67 | 202 | 64.6 | 69.1 | 1.86 | 109.2 | 0.31 |
| 120 | 80 | 240 | 99.5 | 65.3 | 1.66 | 113.1 | 0.26 |
| 135 | 90 | 271 | 138.9 | 85.0 | 2.03 | 113.7 | 0.25 |
| 150 | 96 | 287 | 180.7 | 89.0 | 2.12 | 114.3 | 0.24 |
| 180 | 96 | 287 | 180.6 | 84.1 | 2.26 | 117.3 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, H.; Cavanagh, R.; Jacob, P.; Destouches, D.; Vacherot, F.; Brugnoli, B.; Howdle, S.; Taresco, V.; Couturaud, B. Synthesis of Multifunctional Polymersomes Prepared by Polymerization-Induced Self-Assembly. Polymers 2023, 15, 3070. https://doi.org/10.3390/polym15143070
Phan H, Cavanagh R, Jacob P, Destouches D, Vacherot F, Brugnoli B, Howdle S, Taresco V, Couturaud B. Synthesis of Multifunctional Polymersomes Prepared by Polymerization-Induced Self-Assembly. Polymers. 2023; 15(14):3070. https://doi.org/10.3390/polym15143070
Chicago/Turabian StylePhan, Hien, Robert Cavanagh, Philippa Jacob, Damien Destouches, Francis Vacherot, Benedetta Brugnoli, Steve Howdle, Vincenzo Taresco, and Benoit Couturaud. 2023. "Synthesis of Multifunctional Polymersomes Prepared by Polymerization-Induced Self-Assembly" Polymers 15, no. 14: 3070. https://doi.org/10.3390/polym15143070
APA StylePhan, H., Cavanagh, R., Jacob, P., Destouches, D., Vacherot, F., Brugnoli, B., Howdle, S., Taresco, V., & Couturaud, B. (2023). Synthesis of Multifunctional Polymersomes Prepared by Polymerization-Induced Self-Assembly. Polymers, 15(14), 3070. https://doi.org/10.3390/polym15143070







