The Effect of Porosity on the Thermal Conductivity of Highly Thermally Conductive Adhesives for Advanced Semiconductor Packages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Materials
2.2. Characterization
3. Results and Discussion
3.1. Typical Properties of Highly Thermally Conductive Composites
3.2. Analysis of the Differences between the Theoretically Calculated and Experimentally Measured TC Values
3.3. Suggestion of a Modified Theoretical Model to Predict Practical Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chauhan, D.; Singvi, N.; Singh, R. Effect of geometry of filler particles on the effective thermal conductivity of two-phase systems. Int. J. Mod. Nonlinear Theory Appl. 2012, 1, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.X.; He, Z.X.; Mo, D.C.; Lu, S.S. Thermal conductivity enhancement with different fillers for epoxy resin adhesives. Appl. Therm. Eng. 2014, 66, 493–498. [Google Scholar] [CrossRef]
- Ali, A.S.; Ying, A.; Abdou, M. Experimental measurement of the effective thermal conductivity of a lithium titanate (Li2TiO3) pebbles-packed bed. J. Mater. Process. Technol. 2007, 181, 206–212. [Google Scholar]
- Choi, M.K.; Jung, H.H.; Oh, K.S.; Ryu, D.S.; Lee, S.H.; Do, W.C.; Kweon, Y.D.; Kelly, M.; Park, K.R.; Kim, J.Y.; et al. Development of an extremely high thermal conductivity TIM for large electronics packages in the 4th industrial revolution era. In IEEE 70th Electronic Components and Technology Conference (ECTC); IEEE: Piscataway, NJ, USA, 2020; pp. 1332–1337. [Google Scholar]
- Dan, B.; Sammakia, B.G.; Subbarayan, G.; Kanuparthi, S. An improved efficient network model for determining the effective thermal conductivity of particulate thermal interface materials. J. Electron. Packag. 2013, 135, 031003-1–031003-8. [Google Scholar] [CrossRef]
- Kanuparthi, S.; Subbarayan, G. An efficient network model for determining the effective thermal conductivity of particulate thermal interface materials. IEEE Trans. Compon. Pack. Tech. 2008, 31, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Vaitheeswaran, P.K.; Subbarayan, G. Estimation of effective thermal and mechanical properties of particulate thermal interface materials by a random network model. J. Electron. Packag. 2018, 140, 02090-1–02090-7. [Google Scholar] [CrossRef]
- Liao, Y.; Weng, Y.; Wan, J.; Zhou, H.; Lin, J.; He, S. Silicone rubber composites with high breakdown strength and low dielectric loss based on polydopamine coated mica. Polymers 2019, 11, 2030. [Google Scholar] [CrossRef] [Green Version]
- Engeln, I.; Meissner, M. Thermal properties of crystalline polymers at low temperatures. In Nonmetallic Materials and Composites at Low Temperature; Springer: Boston, MA, USA, 1982; Volume 2, pp. 1–25. [Google Scholar]
- Li, M.; Xiao, Y.; Zhang, Z.; Yu, J. Bimodal sintered silver nanoparticle paste with ultrahigh thermal conductivity and shear strength for high temperature thermal interface material applications. ACS Appl. Mater. Interfaces 2015, 7, 9157–9168. [Google Scholar] [CrossRef]
- Zulkarnain, M.; Lubis, M.S.Y.; Ariyanti, S.; Jaafar, M. Silver particles on the thermal and electrical characteristics of ECAs. Int. J. Recent Technol. Eng. 2019, 8, 536–542. [Google Scholar]
- Mizuuchi, K.; Inoue, K.; Agari, Y. Trend of the development of metal-based heat dissipative materials. Microelectron. Reliab. 2017, 79, 5–19. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chen, C.C.; Chang, C.H.; Wang, C.C. Using graphene-powder-based thermal interface material for high lumen LED array chip: An experimental study of heat dissipation. Sens. Mater. 2020, 32, 4277–4297. [Google Scholar] [CrossRef]
- Shtein, M.; Nadiv, R.; Buzaglo, M.; Regev, O. Graphene-based hybrid composites for efficient thermal management of electronic devices. ACS Appl. Mater. Interfaces 2015, 7, 23725–23730. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, Y.; Chatterjee, D.; Bose, S. Core-multishell heterostructure with excellent heat dissipation for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2018, 10, 30762–30773. [Google Scholar] [CrossRef]
- Nie, S.; Hao, N.; Zhang, K.; Xing, C.; Wang, S. Cellulose nanofibris-based thermally conductive composites for flexible electronics: A mini review. Cellulose 2020, 27, 4173–4187. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Yao, Y.; Pan, G.; Sun, J.; Zeng, X.; Sun, R.; Xu, J.B.; Song, B.; Wong, C.P. Polymer composite with improved thermal conductivity by constructing a hierarchically ordered three-dimensional interconnected network of BN. ACS Appl. Mater. Interfaces 2017, 9, 13544–13553. [Google Scholar] [CrossRef] [PubMed]
- Greil, P.; Kulig, M.; Hotza, D.; Lange, H.; Tischtau, R. Aluminum nitride ceramics with high thermal conductivity from gas-phase synthesized powders. J. Eur. Ceram. Soc. 1994, 13, 229–237. [Google Scholar] [CrossRef]
- Lv, R.; Liu, J.; Li, Y.; Li, S.; Kou, Z.; He, D. High pressure sintering of cubic boron nitride compacts with Al and AlN. Diam. Relat. Mater. 2008, 17, 2062–2066. [Google Scholar] [CrossRef]
- Cho, H.B.; Konno, A.; Fujihara, T.; Suzuki, T.; Tanaka, S.; Jiang, W.; Suematsu, H.; Niihara, K.; Nakayama, T. Self-assemblies of linearly aligned diamond in polysiloxane/diamond composite films with enhanced thermal conductivity. Compos. Sci. Technol. 2011, 72, 112–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Zhao, J.H.; Sheng, K.; Cannon, W.R.; Wang, X.; Fursin, L. Rheology and thermal conductivity of diamond powder-filled liquid epoxy encapsulants for electronic packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2009, 32, 716–723. [Google Scholar] [CrossRef]
- Li, J.W.; Nakamura, M.; Shirai, T.; Matsumaru, K.; Ishizaki, C.; Ishizaki, K. Hydrolysis of aluminum nitride powders in moist air. Adv. Technol. Mater. Mater. Process. 2005, 7, 37–42. [Google Scholar]
- Karayacoubian, P.; Yovanovich, M.M.; Culham, J.R. Thermal resistance-based bounds for the effective conductivity of composite thermal interface materials. In Proceedings of the 22nd Annual IEEE Semiconductor Thermal Measurement And Management Symposium, Dallas, TX, USA, 14–16 March 2006. [Google Scholar]
- Falat, T.; Wymysiowska, A.W.; Kolbe, J.; Jansen, K.M.B.; Ernst, L. Numerical approach to characterization of thermally conductive adhesive. In Proceedings of the EuroSime 2006-7th International Conference on Thermal, Mechanical and Multiphysics Simulation and Experiments in Micro-Electronics and Micro-Systems, Como, Italy, 24–26 April 2006. [Google Scholar]
- Mishra, D.; Mohapatra, L.; Satapathy, A.; Patnaik, A. Determination of thermal conductive of polymer composites filled with solid glass beads. In Proceedings of the International Conference on Advancement in Polymeric Materials, Chennai, India, 25–27 March 2011. [Google Scholar]
- Esfahan, N.N.; Toghraie, D.; Afrand, M. A new correlation for predicting the thermal conductivity of ZnO-Ag (50%–50%)/water hybrid nanofluid: An experimental study. Powder Technol. 2018, 323, 367–373. [Google Scholar] [CrossRef]
- Landauer, R. Electrical conductivity in inhomogeneous media. AIP Conf. Proc. 1978, 40, 2–45. [Google Scholar]
- Boehm, H.P. Functional groups on the surfaces of solids. Angewante Chem. 1966, 5, 533–544. [Google Scholar] [CrossRef]
- Schulman, D.S.; Arnold, A.J.; Das, S. Contact engineering for 2D materials and devices. Chem. Soc. Rev. 2018, 47, 3037–3058. [Google Scholar] [CrossRef]
- Garrett, K.W.; Rosenberg, H.M. The thermal conductivity of epoxy-resin/powder composite materials. J. Phys. D Appl. Phys. 1974, 7, 1247–1258. [Google Scholar] [CrossRef]


| Technologies | Metallic Fillers | Hybrid Fillers | High k Ceramic Fillers |
|---|---|---|---|
| Structure | 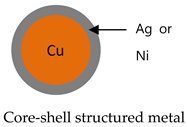 - Ag coated Cu - Ni coated Cu |  |  - High Purity Al2O3 (99.99%) - AlN, SiC, MgO |
| Merit | - High thermal conductivity | - Cost | - Electrical insulation - Cost |
| Demerit | - Cost (∵high density) - Electrically conductive | - Difficult to load - Electrically conductive | - Need surface treatment |
| Sample | Composition (wt%) | Filler wt% | Filler vol% | Remark | |||||
|---|---|---|---|---|---|---|---|---|---|
| 20 µm | 3 µm | 0.25 µm | PDMS | Total | |||||
| Al2O3-based | AO1 | 56.0 | 18.0 | 21.3 | 4.7 | 100 | 95.3 | 83.4 | High purity grade |
| AO2 | 56.0 | 18.0 | 21.3 | 4.7 | 100 | 95.3 | 83.4 | General grade | |
| AO3 | 54.0 | 17.0 | 21.0 | 6.4 | 100 | 93.6 | 78.6 | Graphene -coated | |
| AlN-based | AN1 | 54.0 (30 µm) | 17.0 (5 µm) | 24.1 (0.5 µm) | 4.9 | 100 | 95.1 | 85.4 | |
| Ag-coated Cu | AC1 | 92 | - | - | 8 | 100 | 92.0 | 54.9 | |
| Sample | Filler Composition (wt%) | Filler Content (wt%) | Filler Content (vol%) | η (Pa·s) | K (Wm−1K−1) | Rth (KW−1) | BLT (µm) | ρ (Ω•cm) | Remark | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 µm | 3 µm | 0.25 µm | |||||||||
| AO1 | 56.0 | 18.0 | 21.3 | 95.3 | 83.4 | 70 | 7.12 | 0.52 | 30 | 1.6 × 1014 | Al2O3 99.99% |
| AO2 | 56.0 | 18.0 | 21.3 | 95.3 | 83.4 | 80 | 3.83 | 1.84 | 56 | 1.2 × 1014 | Al2O3 99.8% |
| AO3 | 54.0 | 17.0 | 21.0 | 93.6 | 78.6 | 350 | 6.17 | 0.86 | 27 | 3.7 × 1010 | graphene/Al2O3 99.99% |
| AN1 | 56.0 (30 µm) | 17.6 (5 µm) | 20.5 (1 µm) | 95.1 | 85.4 | 150 | 9.83 | 0.85 | 47 | 2.2 × 1013 | AlN 99.5% |
| AC1 | 92 | - | - | 92.0 | 54.9 | 130 | 4.14 | 0.78 | 20 | 4.6 × 104 | Ag/Cu |
| Samples | AO1 | AO2 | AO3 | AN1 | AC1 |
|---|---|---|---|---|---|
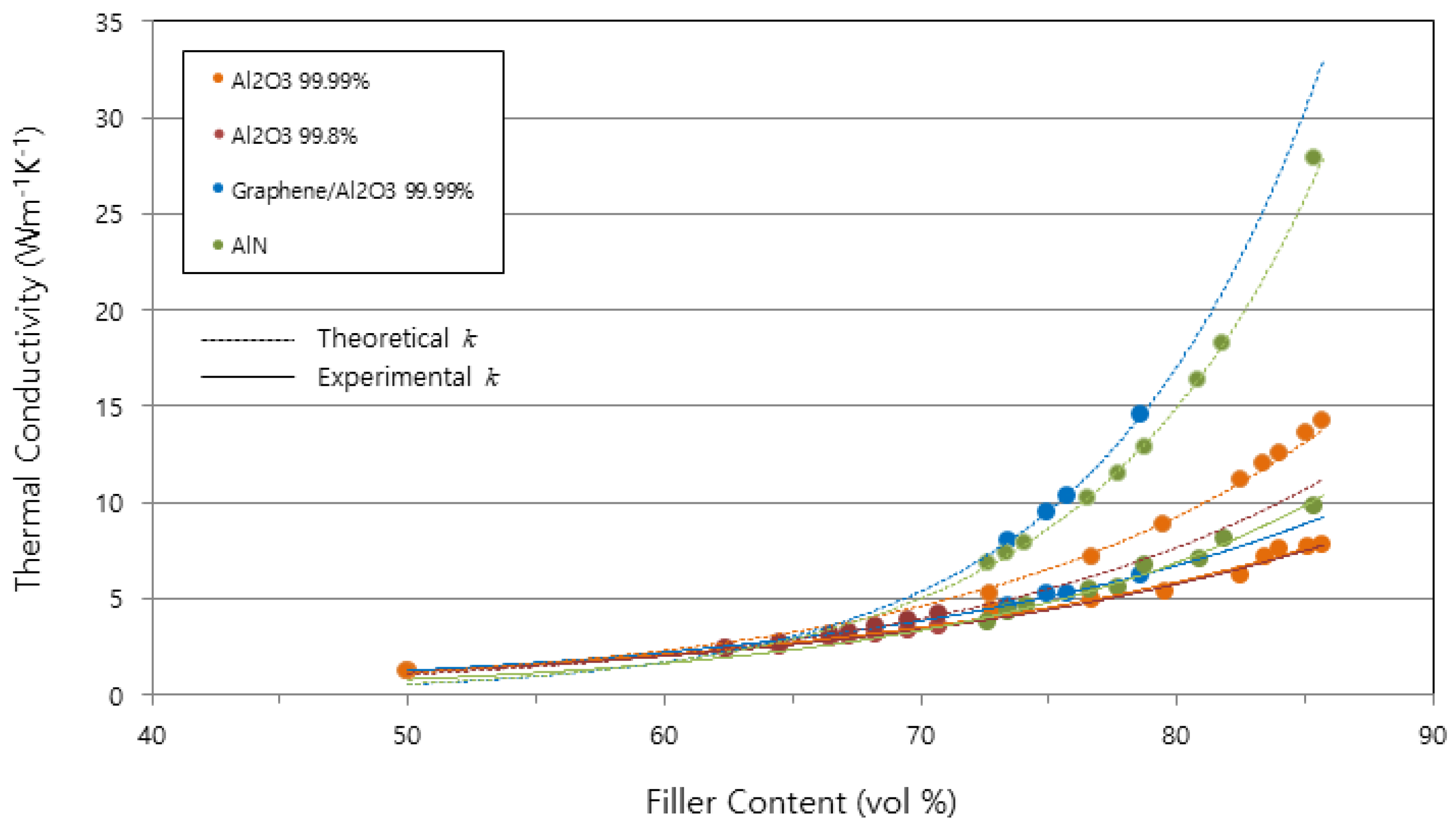
| Theoretically calculated value | 11.92 | 10.20 | 14.55 | 27.80 | 18.68 |
| Experimentally measured value | 7.80 | 3.83 | 6.17 | 9.83 | 4.14 |
| Gap | 4.12 (34.6%) | 6.37 (62.5%) | 8.38 (57.6%) | 17.97 (64.6%) | 14.54 (77.8%) |
| Estimated reason | -Interfacial micro-void -Incomplete Packing | -Purity -Interfacial micro-void -Incomplete packing | -Rough surface of filler -Interfacial micro-void -Incomplete packing | -Sphericity -Interfacial micro-void -Incomplete packing | -Small size -Interfacial micro-void -Unclose packing |
| Remark | kAl2O3 = 39 | kAl2O3 = 30 | kAl2O3 = 39, kGraphene = 390 | kAlN = 150 | kAg = 405, kCu = 400 |
| Sample | AO-1 | AO-2 | AO-3 | AO-4 | AO-5 | AO-6 | AO-7 | AO-8 | AO-9 |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 80.12 | 90.50 | 93.00 | 94.00 | 95.00 | 95.30 | 95.50 | 95.83 | 96.02 |
| vol% | 50.00 | 70.27 | 76.72 | 79.54 | 82.50 | 83.42 | 84.04 | 85.08 | 85.68 |
| Theoretical ρ (g/cm3) | 2.4649 | 3.0670 | 3.2587 | 3.3423 | 3.4302 | 3.4575 | 3.4760 | 3.5068 | 3.5248 |
| Measured ρ (g/cm3) | 2.4546 | 3.0412 | 3.2163 | 3.2915 | 3.3671 | 3.3922 | 3.3877 | 3.4195 | 3.4275 |
| Porosity * | 0.0054 | 0.0084 | 0.0130 | 0.0152 | 0.0184 | 0.0189 | 0.0254 | 0.0249 | 0.0276 |
| Theoretical k (Wm−1K−1) | 1.45 | 5.08 | 8.05 | 8.79 | 12.25 | 13.07 | 13.68 | 14.73 | 15.39 |
| Measured k (Wm−1K−1) | 1.22 | 4.40 | 4.88 | 5.40 | 6.23 | 7.12 | 7.53 | 7.66 | 7.80 |
| Adjusted k (Wm−1K−1) | 1.39 | 4.50 | 6.95 | 7.80 | 8.60 | 9.45 | 10.40 | 11.80 | 12.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-S.; Park, J.-H.; Lee, J.-H. The Effect of Porosity on the Thermal Conductivity of Highly Thermally Conductive Adhesives for Advanced Semiconductor Packages. Polymers 2023, 15, 3083. https://doi.org/10.3390/polym15143083
Choi H-S, Park J-H, Lee J-H. The Effect of Porosity on the Thermal Conductivity of Highly Thermally Conductive Adhesives for Advanced Semiconductor Packages. Polymers. 2023; 15(14):3083. https://doi.org/10.3390/polym15143083
Chicago/Turabian StyleChoi, Hyun-Seok, Jeong-Hyun Park, and Jong-Hee Lee. 2023. "The Effect of Porosity on the Thermal Conductivity of Highly Thermally Conductive Adhesives for Advanced Semiconductor Packages" Polymers 15, no. 14: 3083. https://doi.org/10.3390/polym15143083




