A Sustainable Gel Polymer Electrolyte for Solid-State Electrochemical Devices
Abstract
:1. Introduction
2. Materials and Methods
3. Results
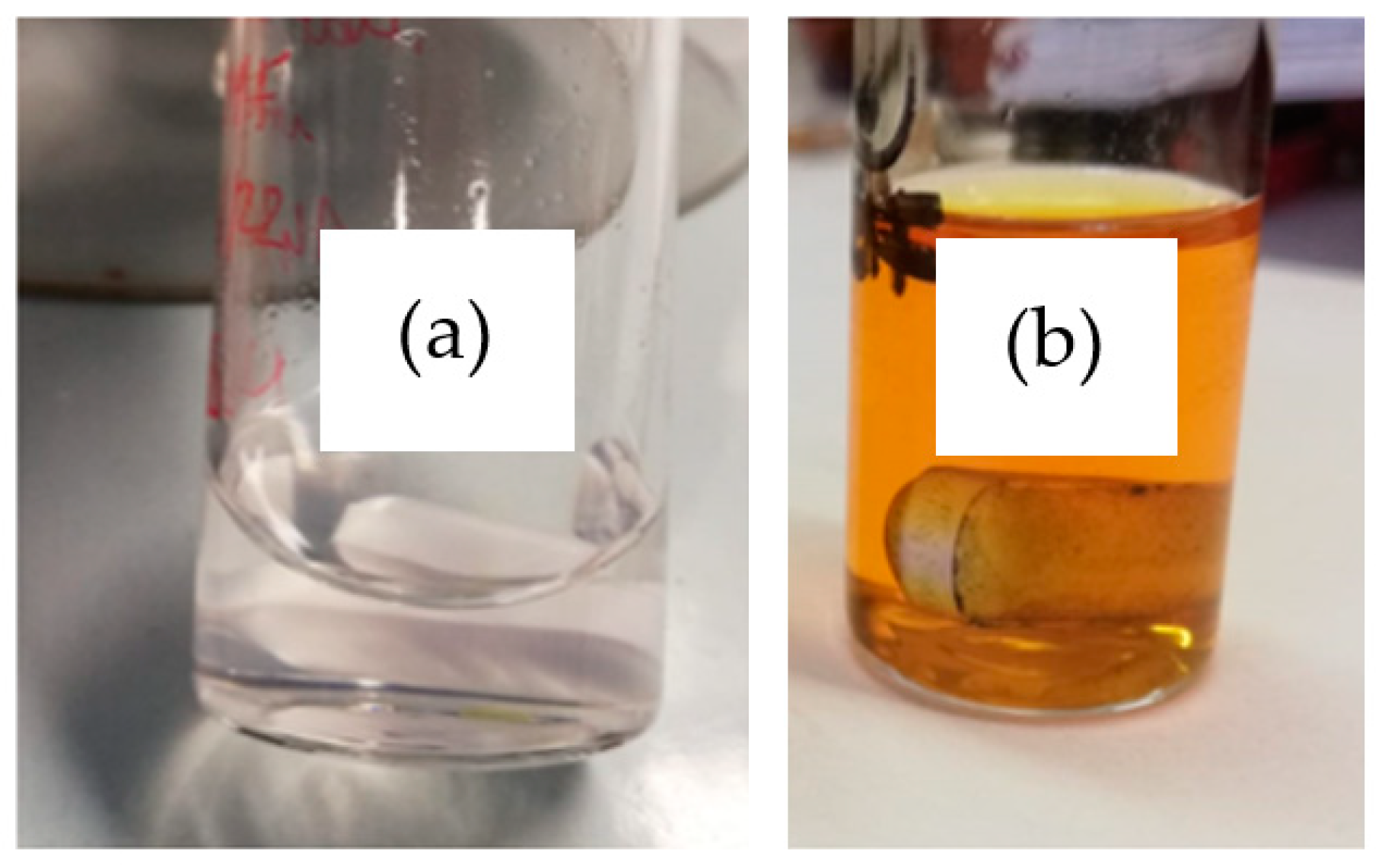
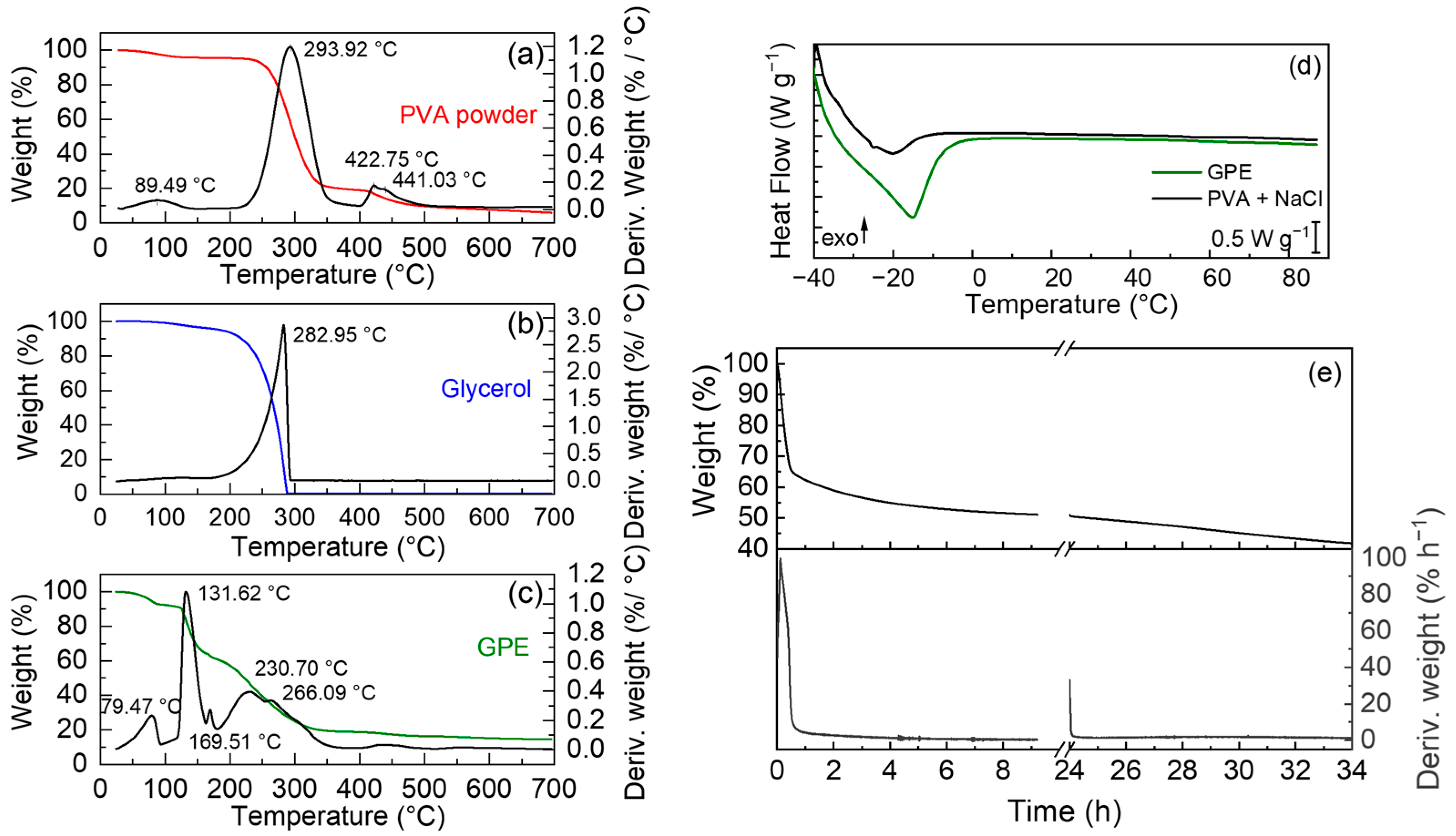
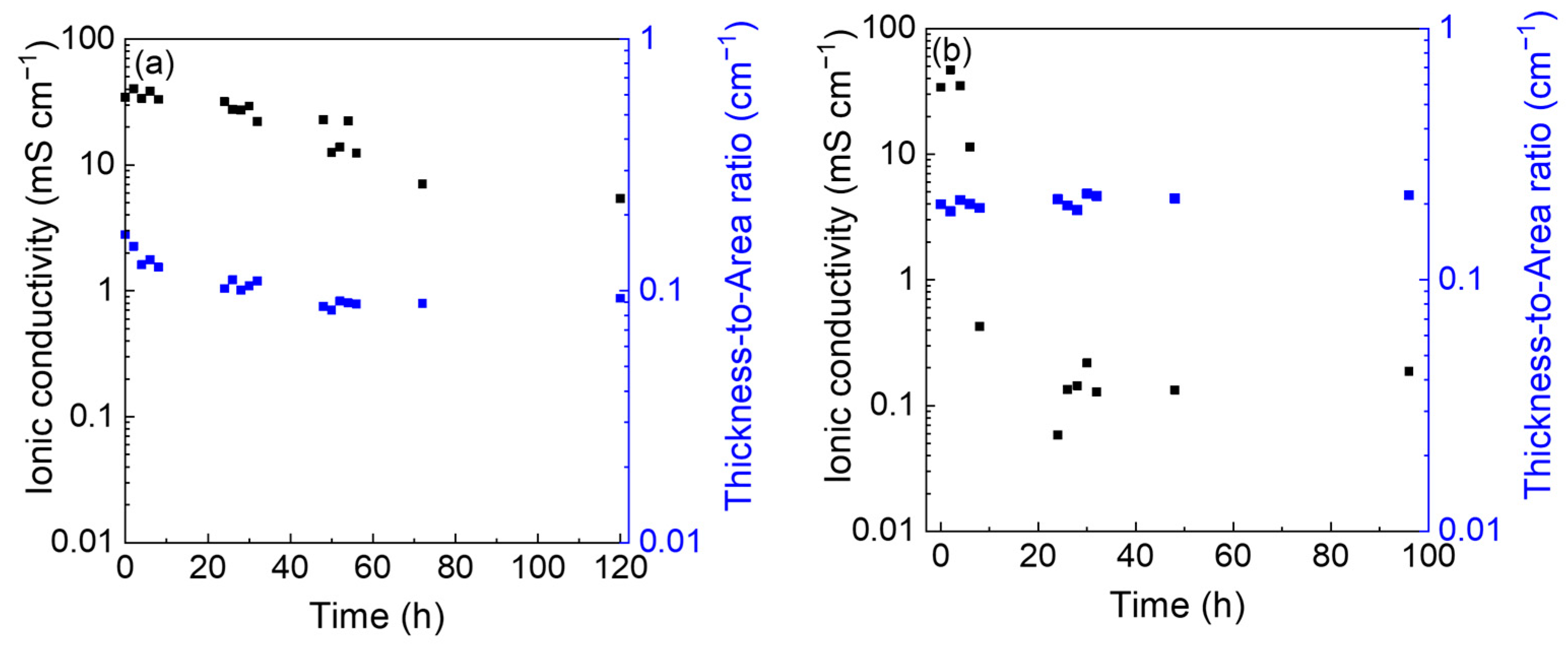
3.1. Physicochemical Characterization
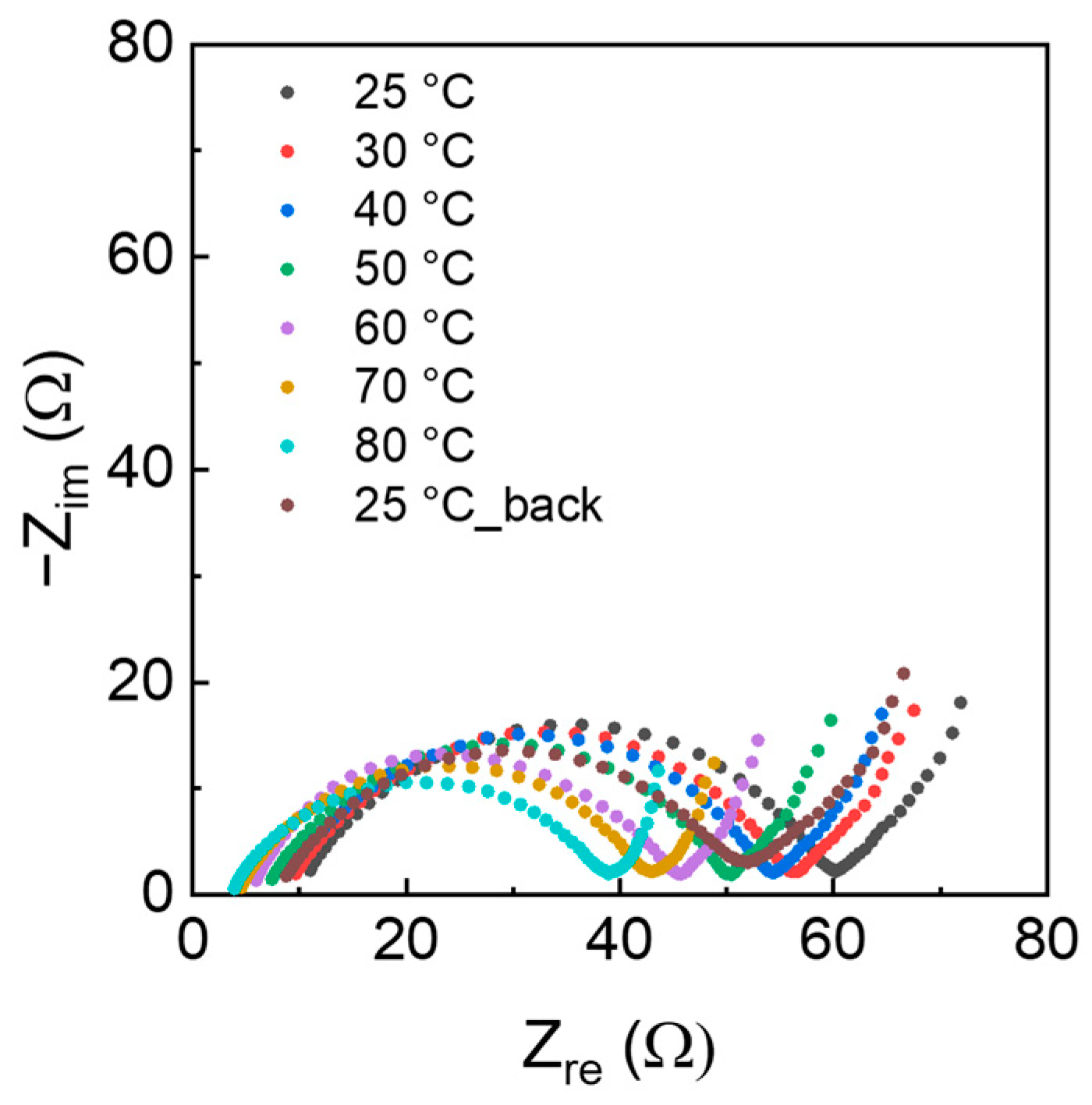
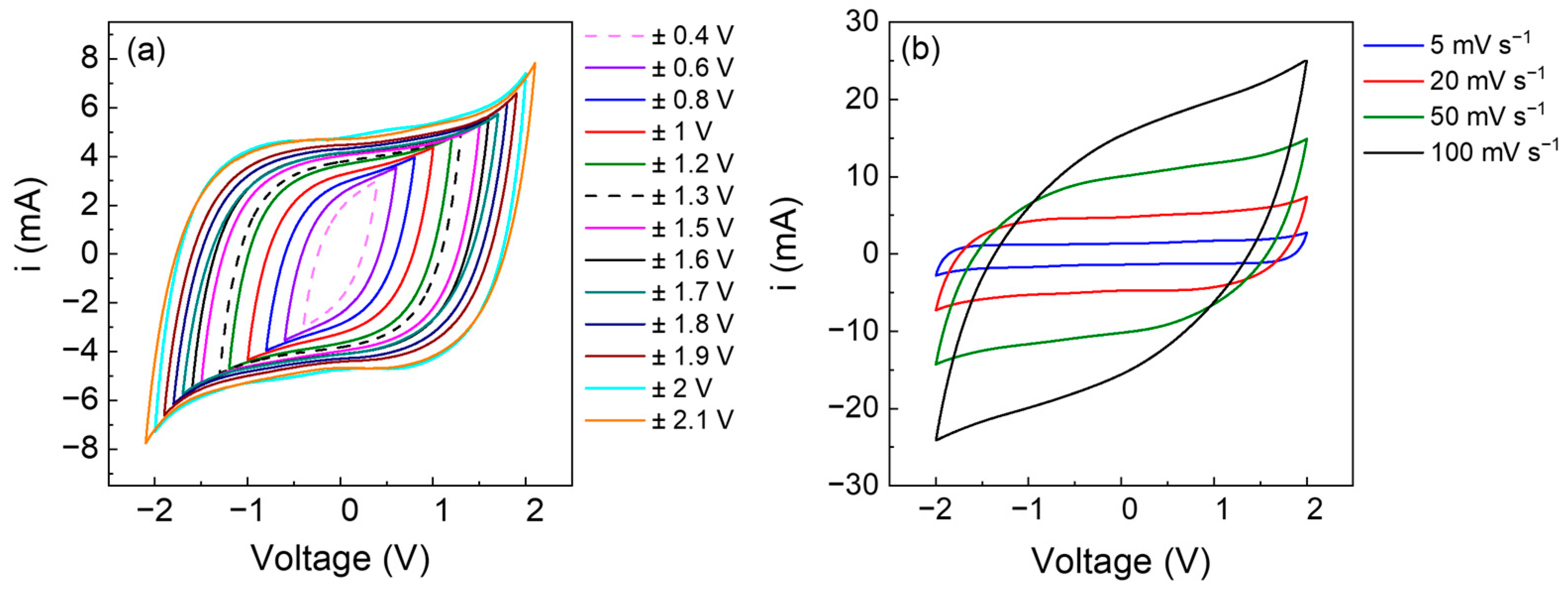
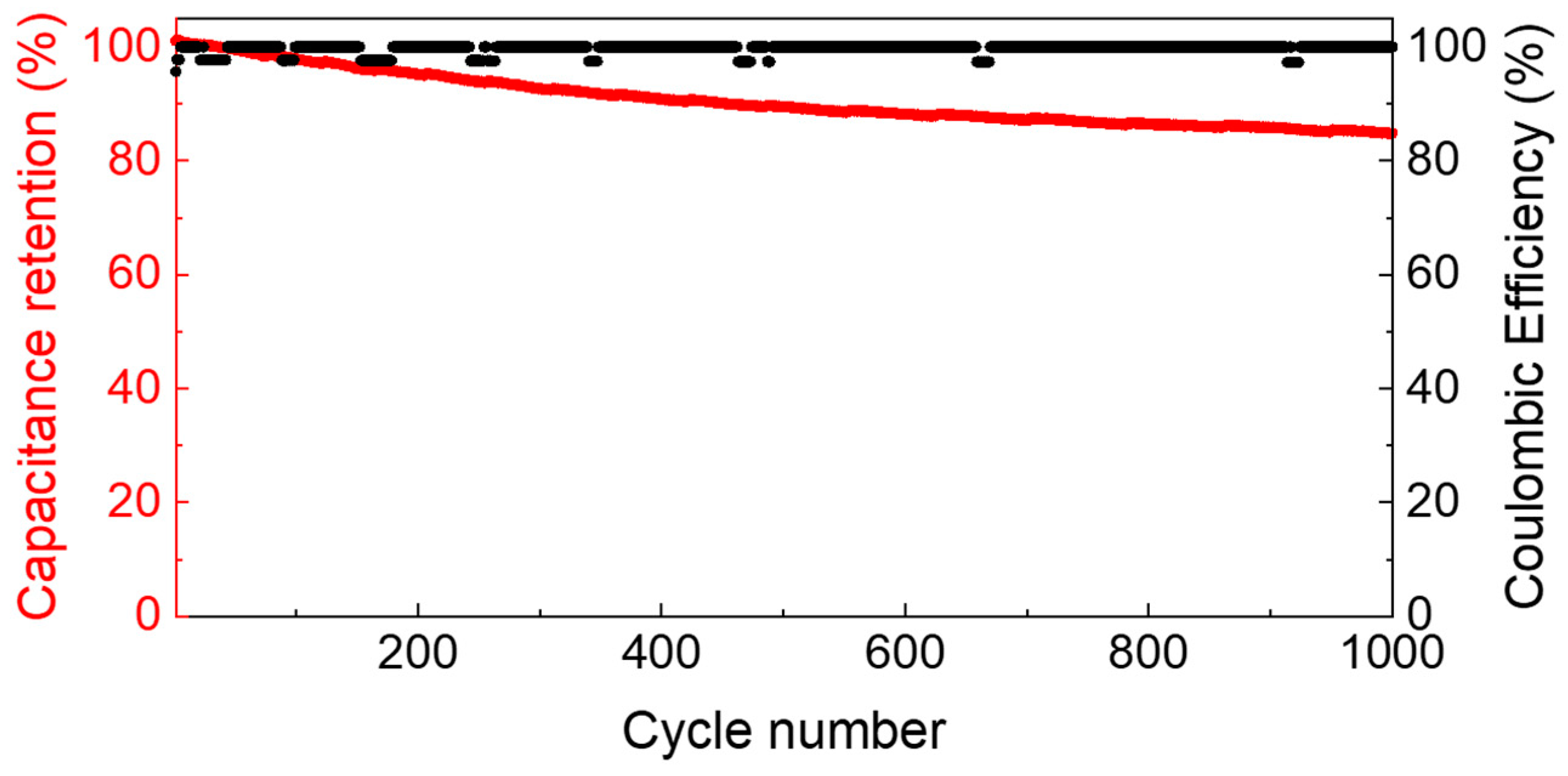
3.2. Electrochemical Tests with AC Electrodes
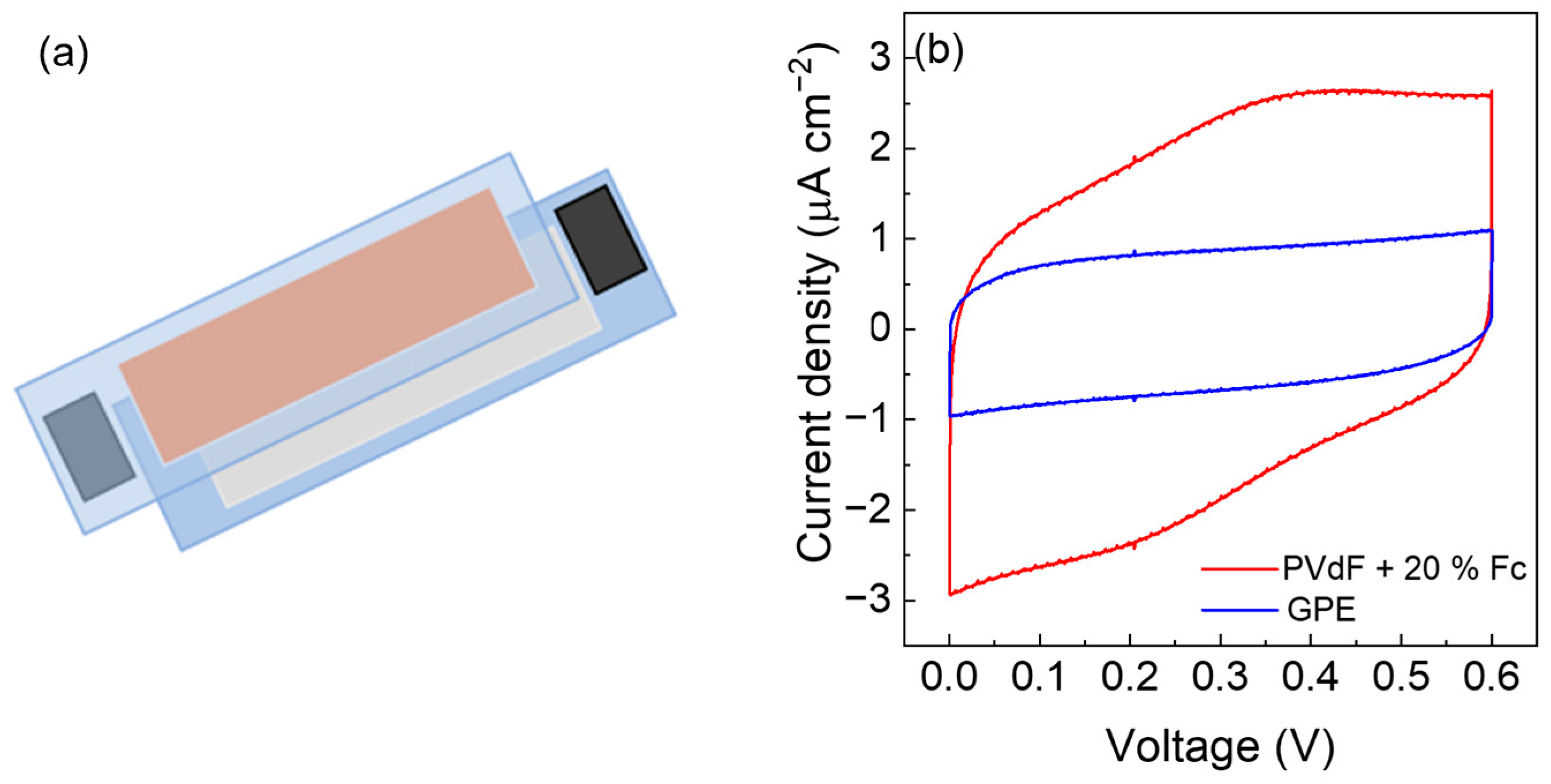
3.3. Electrochemical Tests with PVdF + 20% wt. Fc Electrode
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

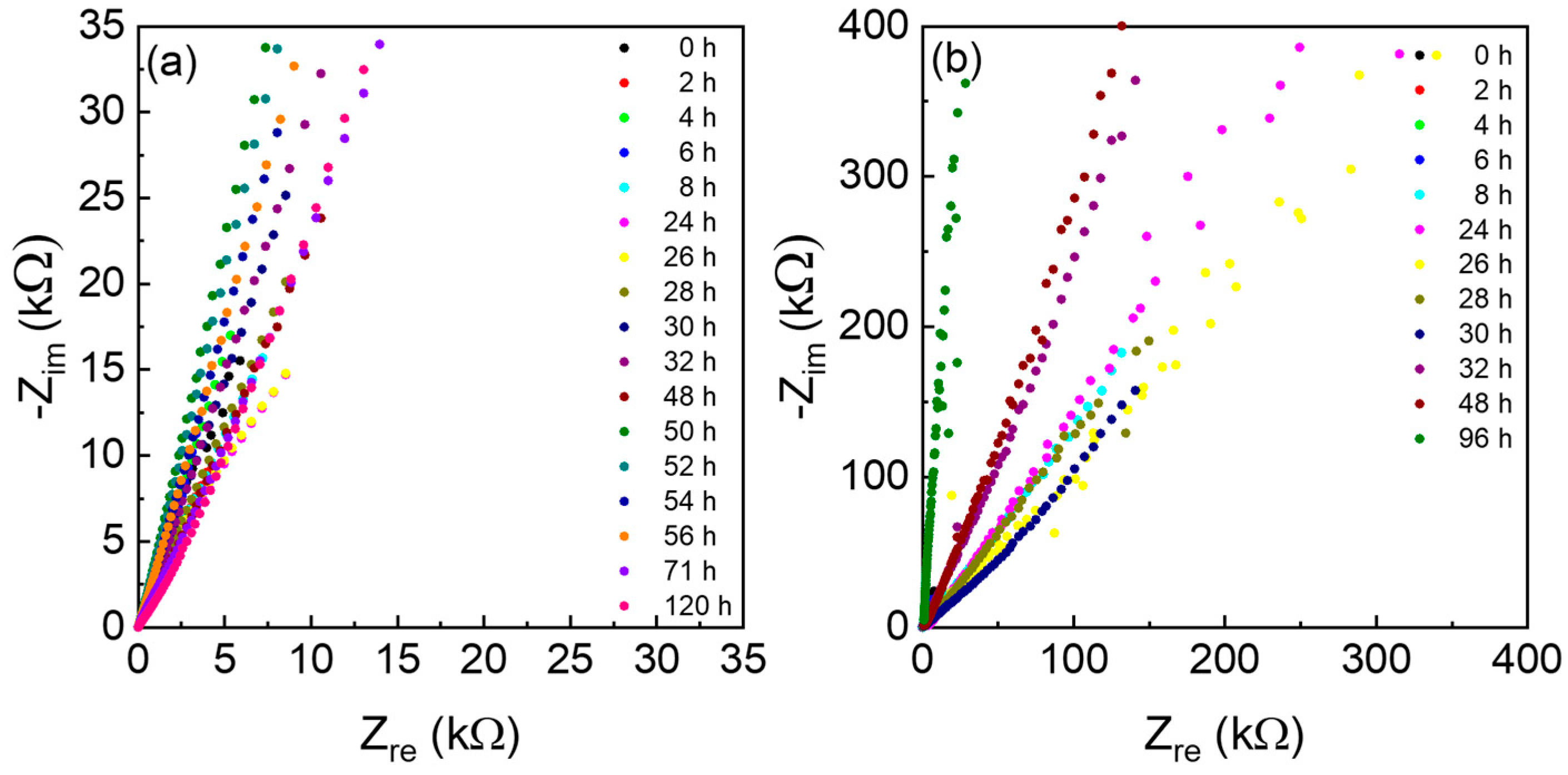
| Time (h) | Resistance (Ω) | Thickness (µm) | Diameter (mm) | Conductivity (mS cm−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Sealed | Open | Sealed | Open | Sealed | Open | Sealed | Open | |
| 0 | 4.9 | 5.9 | 1419 | 1568 | 1.04 | 1.00 | 35 | 34 |
| 2 | 3.7 | 4.0 | 1252 | 1441 | 1.03 | 0.99 | 40 | 47 |
| 4 | 3.7 | 5.9 | 1184 | 1320 | 1.09 | 0.90 | 34 | 35 |
| 6 | 3.4 | 18 | 1127 | 1137 | 1.04 | 0.85 | 38 | 11 |
| 8 | 3.7 | 454 | 1055 | 1046 | 1.04 | 0.83 | 33 | 0.43 |
| 24 | 3.2 | 3588 | 901 | 1025 | 1.06 | 0.79 | 32 | 0.06 |
| 26 | 4.0 | 1467 | 868 | 1018 | 1.00 | 0.81 | 28 | 0.13 |
| 28 | 3.7 | 1318 | 870 | 1000 | 1.05 | 0.82 | 27 | 0.14 |
| 30 | 3.6 | 1009 | 822 | 972 | 1.00 | 0.75 | 29 | 0.22 |
| 32 | 4.9 | 1679 | 776 | 1004 | 0.95 | 0.77 | 22 | 0.13 |
| 48 | 3.8 | 1587 | 736 | 982 | 1.04 | 0.77 | 23 | 0.13 |
| 50 | 6.7 | - | 739 | - | 1.06 | - | 12 | - |
| 52 | 6.6 | - | 729 | - | 1.01 | - | 14 | - |
| 54 | 4.0 | - | 731 | - | 1.02 | - | 22 | - |
| 56 | 7.1 | - | 724 | - | 1.02 | - | 12 | - |
| 72 | 12.6 | - | 715 | - | 1.01 | - | 7 | - |
| 96 | - | 1156 | - | 960 | - | 0.75 | - | 0.19 |
| 120 | 17.3 | - | 704 | - | 0.98 | - | 5 | - |
References
- Singh, R.; Reddy Polu, A.; Bhattacharya, B.; Rhee, H.W.; Varlikli, C.; Singh, P.K. Perspectives for solid biopolymer electrolytes in dye sensitized solar cell and battery application. Renew. Sustain. Energy Rev. 2016, 65, 1098–1117. [Google Scholar] [CrossRef] [Green Version]
- Teo, L.P.; Buraidah, M.H.; Arof, A.K. Development on Solid Polymer Electrolytes for Electrochemical Devices. Molecules 2021, 26, 6499. [Google Scholar] [CrossRef] [PubMed]
- Nyuk, C.M.; Isa, M.; Nizam, M.I. Solid biopolymer electrolytes based on carboxymethyl cellulose for use in coin cell proton batteries. J. Sustain. Sci. Manag. Spec. Issue 2017, 42–48. Available online: https://jssm.umt.edu.my/wp-content/uploads/sites/51/2020/05/Chapter-6-SI2.pdf (accessed on 12 June 2023).
- Liew, C.W.; Ramesh, S.; Arof, A.K. Good prospect of ionic liquid based-poly(vinyl alcohol) polymer electrolytes for supercapacitors with excellent electrical, electrochemical and thermal properties. Int. J. Hydrogen Energy 2014, 39, 2953–2963. [Google Scholar] [CrossRef]
- Samui, A.B.; Sivaraman, P. Solid polymer electrolytes for supercapacitors. Polym. Electrolytes 2010, 431–470. [Google Scholar] [CrossRef]
- Zhao, W.; Yi, J.; He, P.; Zhou, H. Solid-State Electrolytes for Lithium-Ion Batteries: Fundamentals, Challenges and Perspectives. Electrochem. Energ. Rev. 2019, 2, 574–605. [Google Scholar] [CrossRef] [Green Version]
- Flamme, B.; Rodriguez Garcia, G.; Weil, M.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidala, V.; Chagnes, A. Guidelines to design organic electrolytes for lithium-ion batteries: Environmental impact, physicochemical and electrochemical properties. Green Chem. 2017, 19, 1828. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2017, 8, 1702184. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q. Strategic Structural Design of a Gel Polymer Electrolyte toward a High Efficiency Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 3937–3971. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Cai, H.; Chen, Z.; Guo, S.; Ma, D.; Wang, J. Polyacrylamide gel electrolyte for high-performance quasi-solid-state electrochromic devices. Sol. Energy Mater. Sol. Cells 2023, 256, 112310. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, D.; Yue, J.; Zhang, X.; Wu, Z.; Zhang, T.; Chen, C.; Fei, T. Optical Waveguide Sensors for Measuring Human Temperature and Humidity with Gel Polymer Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 60384–60392. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Hyeon, J.S.; Kim, H.; Mun, T.J.; Haines, C.S.; Li, N.; Baughman, R.H.; Kim, S.J. Enhancing the Work Capacity of Electrochemical Artificial Muscles by Coiling Plies of Twist-Released Carbon Nanotube Yarns. ACS Appl. Mater. Interfaces 2019, 11, 13533–13537. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Yang, H.X.; Liu, Z.K.; Wang, Y.; Li, N.W.; Yu, L. Multiscale Structural Gel Polymer Electrolytes with Fast Li+ Transport for Long-Life Li Metal Batteries. Adv. Funct. Mater. 2023, 33, 2209837. [Google Scholar] [CrossRef]
- Sohn, J.-Y.; Choi, J.H.; Kim, P.-W.; Hwang, I.T.; Shin, J.; Jung, C.-H.; Lee, Y.-M. In-situ preparation of chemically-crosslinked polyvinylpyrrolidone gel polymer electrolyte for lithium ion battery via room-temperature electron beam-induced gelation. Radiat. Phys. Chem. 2023, 211, 111047. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Xia, Y.; Liang, Y.; Xia, X.; Tu, J. A polyacrylonitrile (PAN)-based double-layer multifunctional gel polymer electrolyte for lithium-sulfur batteries. J. Membr. Sci. 2019, 582, 37–47. [Google Scholar] [CrossRef]
- Zhou, Z.; Pei, X.; Zhang, T.; Wang, L.; Hong, J.; Lu, Y.; He, G. A Gel Polymer Electrolyte with 2D Filler-reinforced for Dendrite Suppression Li-Ion Batteries. Electroanalysis 2023, 35, 2200306. [Google Scholar] [CrossRef]
- Dennis, J.O.; Shukur, M.F.; Aldaghri, O.A.; Ibnaouf, K.H.; Adam, A.A.; Usman, F.; Hassan, Y.M.; Alsadig, A.; Danbature, W.L.; Abdulkadir, B.A. A Review of Current Trends on Polyvinyl Alcohol (PVA)-Based Solid Polymer Electrolytes. Molecules 2023, 28, 1781. [Google Scholar] [CrossRef]
- Marchiori, C.F.N.; Carvalho, R.P.; Ebadi, M.; Brandell, D.; Moyses Araujo, C. Understanding the Electrochemical Stability Window of Polymer Electrolytes in Solid-State Batteries from Atomic-Scale Modeling: The Role of Li-Ion Salts. Chem. Mater. 2020, 32, 7237–7246. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent Advances in Innovative Polymer Electrolytes based on Poly (ionic liquid)s. Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Cho, Y.G.; Hwang, C.; Cheong, D.S.; Kim, Y.-S.; Son, H.-K. Gel/Solid Polymer Electrolytes Characterized by In Situ Gelation or Polymerization for Electrochemical Energy Systems. Adv. Mater. 2019, 31, 1804909. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, S.; Sun, Y.; Xiang, N.; Jiang, X.; Hou, L. Facile preparation and characterization of poly(vinyl alcohol)-NaCl-glycerol. Eur. Polym. J. 2018, 106, 206–213. [Google Scholar] [CrossRef]
- Shi, S.; Peng, X.; Liu, T.; Chen, Y.-N.; He, C.; Wang, H. Facile preparation of hydrogen-bonded supramolecular polyvinylalcohol-glycerol gels with excellent thermoplasticity and mechanical properties. Polymer 2017, 111, 168–176. [Google Scholar] [CrossRef]
- Sun, Y.; Xiang, N.; Jiang, X.; Hou, L. Preparation of high tough poly(vinyl alcohol) hydrogel by soaking in NaCl aqueous solution. Mater. Lett. 2017, 194, 34–37. [Google Scholar] [CrossRef]
- Di, X.; Ma, Q.; Xu, Y.; Yang, M.; Wu, G.; Sun, P. High-performance ionic conductive poly(vinyl alcohol) hydrogels for flexible strain sensors based on a universal soaking strategy. Mater. Chem. Front. 2021, 5, 315–323. [Google Scholar] [CrossRef]
- Wu, G.M.; Lin, S.J.; Yang, C.C. Preparation and characterization of PVA/PAA membranes for solid polymer electrolytes. J. Membr. Sci. 2006, 275, 127–133. [Google Scholar] [CrossRef]
- Abdulkadir, B.A.; Dennis, J.O.; Al-Hadeethi, Y.; Shukur, M.F.; Mkawi, E.M.; Al-Harbi, N.; Ibnaouf, K.H.; Aldaghri, O.; Usman, F.; Adam, A.A. Optimization of the Electrochemical Performance of a Composite Polymer Electrolyte Based on PVA-K2CO3-SiO2 Composite. Polymers 2021, 13, 92. [Google Scholar] [CrossRef]
- Mojet, B.L.; Ebbesenz, S.D.; Lefferts, L. Light at the interface: The potential of attenuated total reflection infrared spectroscopy for understanding heterogeneous catalysis in water. Chem. Soc. Rev. 2010, 39, 4643–4655. [Google Scholar] [CrossRef]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate methods for evaluating the efficiency and capacitive behavior of different types of supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Yang, H.; Xu, S.; Jiang, L.; Dan, Y. Thermal Decomposition Behavior of Poly (Vinyl Alcohol) with Different Hydroxyl Content. J. Macromol. Sci. 2012, 51, 464–480. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, L.; Zhang, C.; Li, L. Water states and thermal processability of boric acid modified poly(vinyl alcohol). J. Appl. Polym. Sci. 2016, 133, 43246. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, Y.; Dai, Z. Gel Electrolytes for Electrochemical Actuators and Sensors Applications. Chem. Asian J. 2023, 18, e202201160. [Google Scholar] [CrossRef] [PubMed]




| Humidity | Conductivity (mS cm−1) |
|---|---|
| 10% | 0.24 |
| 50% | 3.65 |
| 90% | 35.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tombolesi, S.; Zanieri, N.; Bargnesi, L.; Mernini, M.; Lacarbonara, G.; Arbizzani, C. A Sustainable Gel Polymer Electrolyte for Solid-State Electrochemical Devices. Polymers 2023, 15, 3087. https://doi.org/10.3390/polym15143087
Tombolesi S, Zanieri N, Bargnesi L, Mernini M, Lacarbonara G, Arbizzani C. A Sustainable Gel Polymer Electrolyte for Solid-State Electrochemical Devices. Polymers. 2023; 15(14):3087. https://doi.org/10.3390/polym15143087
Chicago/Turabian StyleTombolesi, Serena, Niccolò Zanieri, Luca Bargnesi, Martina Mernini, Giampaolo Lacarbonara, and Catia Arbizzani. 2023. "A Sustainable Gel Polymer Electrolyte for Solid-State Electrochemical Devices" Polymers 15, no. 14: 3087. https://doi.org/10.3390/polym15143087
APA StyleTombolesi, S., Zanieri, N., Bargnesi, L., Mernini, M., Lacarbonara, G., & Arbizzani, C. (2023). A Sustainable Gel Polymer Electrolyte for Solid-State Electrochemical Devices. Polymers, 15(14), 3087. https://doi.org/10.3390/polym15143087












