Chitosan Derivative-Based Microspheres Loaded with Fibroblast Growth Factor for the Treatment of Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biosynthesis and Characterization of the NA-FGF
2.3. Establishment of the Diabetic Mouse Model
2.4. Activity Analysis of the NA-FGF3
2.5. Preparation and Characterization of NA-FGF-N-2-HACC/CMCS MPs
2.6. Oral Stability of NA-FGF-N-2-HACC/CMCS MPs
2.7. Safety of NA-FGF-N-2-HACC/CMCS MPs
2.8. Pharmacodynamic Analysis of NA-FGF-N-2-HACC/CMCS MPs
2.9. Analysis of the Blood Glucose Regulation by NA-FGF-N-2-HACC/CMCS MPs via Oral Administration
2.10. In Vitro and In Vivo Release Capacity of NA-FGF-N-2-HACC/CMCS MPs
2.11. Statistical Analysis
3. Results
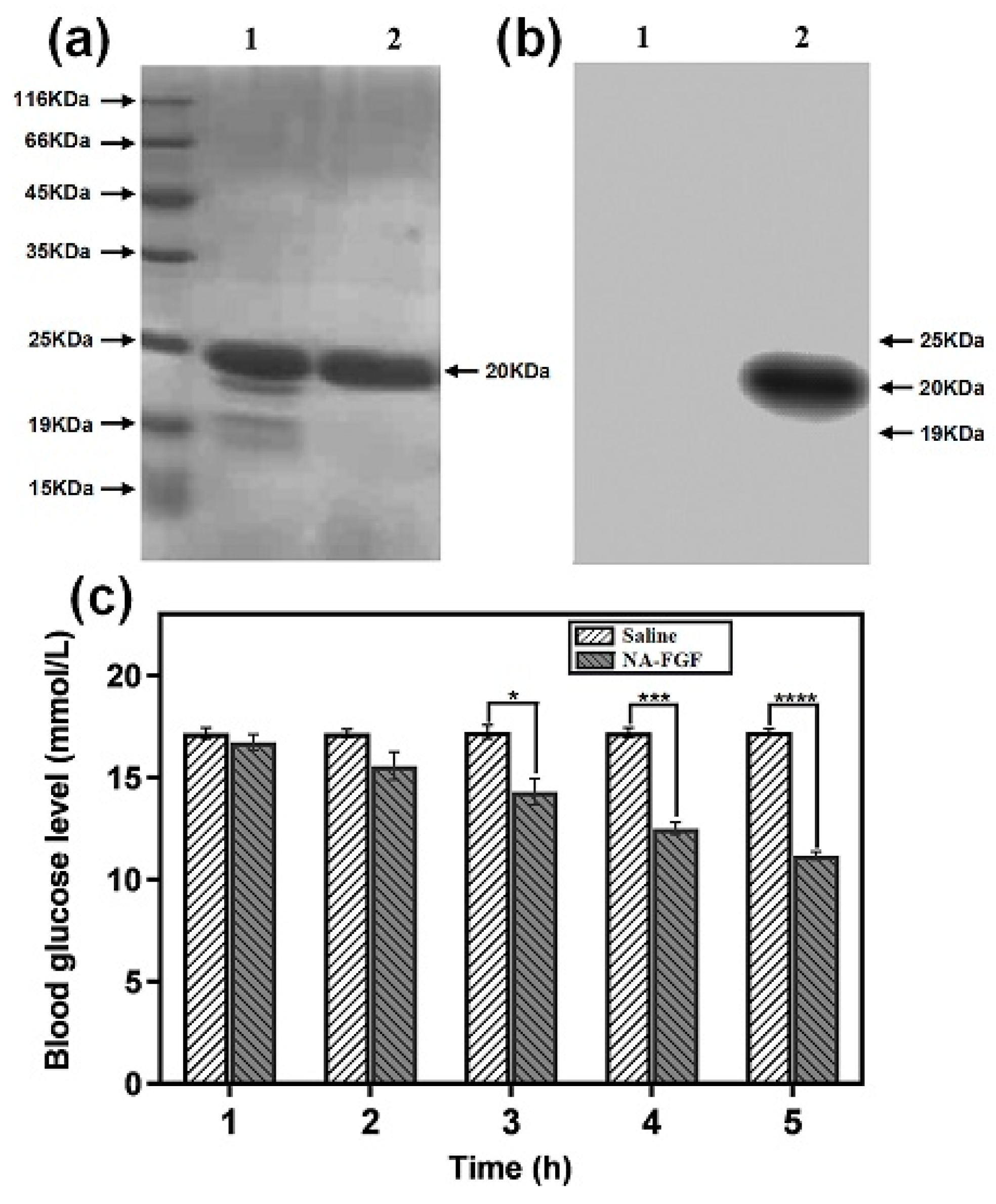
3.1. Detection and Biological Activity of the NA-FGF
3.2. Preparation and Optimization of NA-FGF-N-2-HACC/CMCS MPs
3.3. Characterization of NA-FGF-N-2-HACC/CMCS MPs
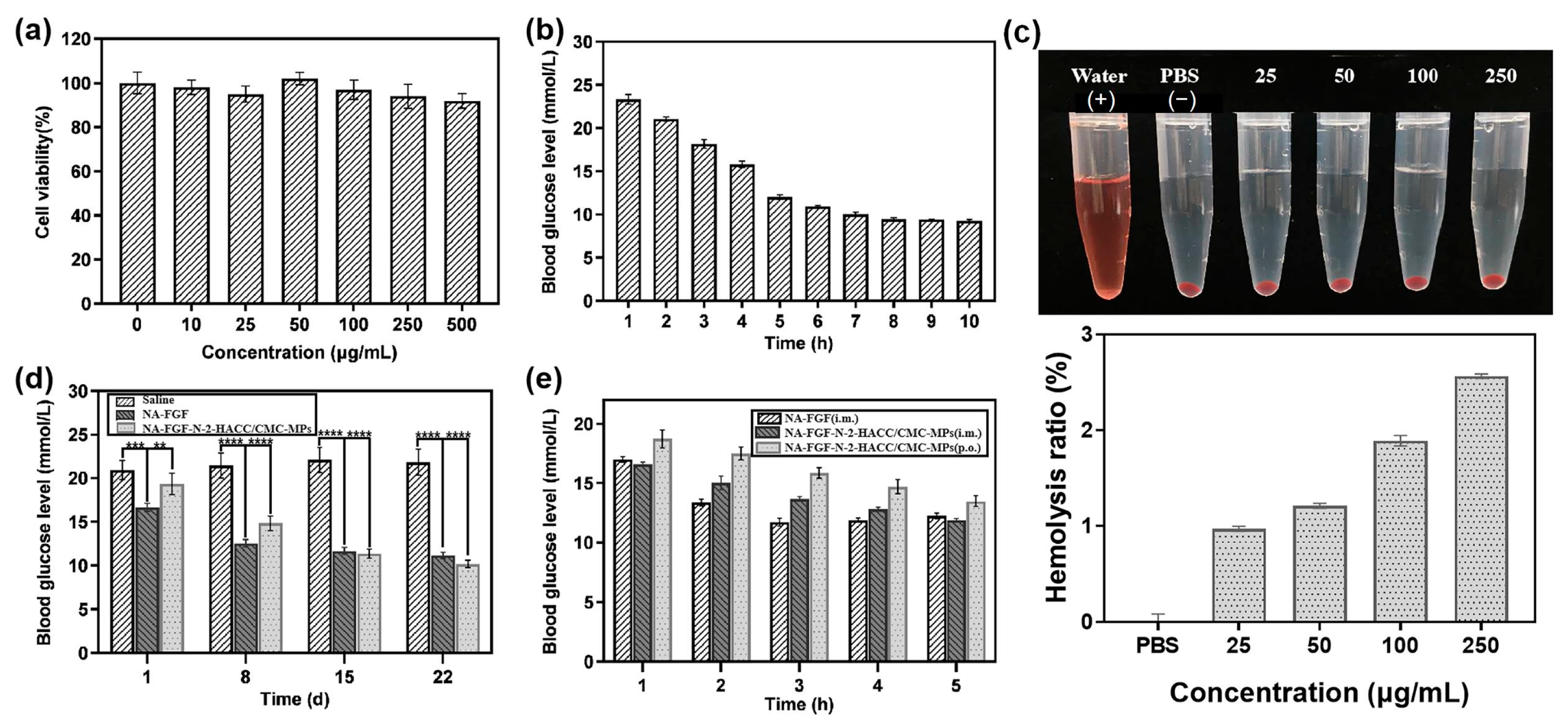
3.4. Oral Stability Analysis of NA-FGF-N-2-HACC/CMCS MPs
3.5. Biosafety Analysis of NA-FGF-N-2-HACC/CMCS MPs
3.6. Pharmacodynamic Analysis of NA-FGF-N-2-HACC/CMCS MPs
3.7. Blood Glucose Regulation of NA-FGF-N-2-HACC/CMCS MPs via Oral Administration
3.8. In Vitro and In Vivo Release Analysis of the NA-FGF-N-2-HACC/CMCS MPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, W.; Ehlerding, E.B.; Lan, X.; Luo, Q.Y.; Cai, W. Molecular Imaging of β-Cells: Diabetes and Beyond. Adv. Drug Deliv. Rev. 2019, 139, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Shpakov, A.O.; Derkach, K.V.; Surkova, E.V.; Bespalov, A.I. Perspectives of Application of Intranasally Administered Insulin for Correction of Metabolic and Hormonal Disorders in Diabetes Mellitus and Metabolic Syndrome. Probl. Endokrinol. 2019, 65, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.L.; Wang, X.Z.; Xie, W.; Chen, X.W.; Zhu, Y.L.; Li, X.G. Macrophage migration inhibitory factor may contribute to hypertrophy of lumbar ligamentum flavum in type 2 diabetes mellitus. Chin. Med. J. 2020, 133, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Sun, J.W.; Shao, D.; Zhang, H.H.; Bai, C.F.; Cao, F.L. The Association between Diabetes Complications, Diabetes Distress, and Depressive Symptoms in Patients with Type 2 Diabetes Mellitus. Clin. Nurs. Res. 2021, 30, 293–301. [Google Scholar] [CrossRef]
- Romero, R.; Erez, O.; Hüttemann, M.; Maymon, E.; Panaitescu, B.; Conde-Agudelo, A.; Pacora, P.; Yoon, B.H.; Grossman, L.I. Metformin, the Aspirin of the 21st Century: Its Role in Gestational Diabetes Mellitus, Prevention of Preeclampsia and Cancer, and The Promotion of Longevity. Am. J. Obstet. Gynecol. 2017, 217, 282–302. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Juan, J.; Xu, Q.Q.; Su, R.N.; Hirst, J.E.; Yang, H.X. Increasing Insulin Resistance Predicts Adverse Pregnancy Outcomes in Women with Gestational Diabetes Mellitus. J. Diabetes 2020, 12, 438–446. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, S.; Lu, H.; Ying, J.Y. Acid-Resistant and Physiological pH-Responsive DNA Hydrogel Composed of A-Motif and i-Motif toward Oral Insulin Delivery. J. Am. Chem. Soc. 2022, 144, 5461–5470. [Google Scholar] [CrossRef]
- Sheweita, S.A.; ElHady, S.A.; Hammoda, H.M. Trigonella Stellata Reduced the Deleterious Effects of Diabetes Mellitus through Alleviation of Oxidative Stress, Antioxidant- and Drug-Metabolizing Enzymes Activities. J. Ethnopharmacol. 2020, 256, 112821. [Google Scholar] [CrossRef]
- Szkudelski, T.; Konieczna, K.; Szkudelska, K. Regulatory Effects of Metformin, An Antidiabetic Biguanide Drug, on the Metabolism of Primary Rat Adipocytes. Molecules 2022, 27, 5250. [Google Scholar] [CrossRef]
- Kostev, K.; Jacob, L. Association Between Depression and Persistence with Oral Antihyperglycemic Drugs in Type 2 Diabetes Mellitus Patients in Germany. Psychiatry Res. 2018, 261, 90–93. [Google Scholar] [CrossRef]
- Gomez, P.R.D.; Romero, M.D.T.; Portillo, C.M.; Olmedo, I.S.; Brocca, M.A.M. Diabetes Mellitus Associated with Immune Checkpoint Inhibitors Treatment: A Clinical Case by Atezolizumab. Endocrinol. Diabetes Nutr. 2021, 68, 363–365. [Google Scholar]
- Baik, S.H.; McDonald, C.J. Independent Effects of 15 Commonly Prescribed Drugs on All-Cause Mortality Among US Elderly Patients with Type 2 Diabetes Mellitus. BMJ Open Diabetes Res. Care 2020, 8, e000940. [Google Scholar] [CrossRef] [PubMed]
- Coppage, A.L.; Heard, K.R.; Dimare, M.T.; Liu, Y.; Wu, W.; Lai, J.H.; Bachovchin, W.W. Human FGF-21 Is A Substrate of Fibroblast Activation Protein. PLoS ONE 2017, 11, e0151269. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.I.; Hecht, R.; Li, Y.S.; Sun, J.; Belouski, E.; Hall, M.; Hager, T.; Yie, J.; Wang, W.; Winters, D.; et al. Rationale-Based Engineering of a Potent Long-Acting FGF21 Analog for the Treatment of Type 2 Diabetes. PLoS ONE 2012, 7, e49345. [Google Scholar]
- Chapman, L.E.; Darling, A.L.; Brown, J.E. The Association Between the Biguanide Drug Metformin and Vitamin B12 Deficiency in Diabetic Patients: A Systematic Review. Proc. Nutr. Soc. 2015, 74, E128. [Google Scholar] [CrossRef]
- Rigato, M.; Avogaro, A.; Kreutzenberg, S.V.; Fadini, G.P. Effects of Basal Insulin on Lipid Profile Compared to Other Classes of Antihyperglycemic Agents in Type 2 Diabetic Patients. J. Clin. Endocr. Metab. 2020, 105, 2464–2474. [Google Scholar] [CrossRef]
- Ihana-Sugiyama, N.; Sugiyama, T.; Tanaka, H.; Ueki, K.; Kobayashi, Y.; Ohsugi, M. Comparison of Effectiveness and Drug Cost Between Dipeptidyl Peptidase-4 Inhibitor and Biguanide as The First-Line Anti-Hyperglycaemic Medication Among Japanese Working Generation with Type 2 Diabetes. J. Eval. Clin. Pract. 2020, 26, 299–307. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, S.E.; Lee, H.B.; Song, M.W.; Kim, Y.H.; Jeong, J.Y.; Kang, Y.; Kim, H.J.; Kim, T.H.; Jeon, J.Y.; et al. A Class I Histone Deacetylase Inhibitor Attenuates Insulin Resistance and Inflammation in Palmitate-Treated C2C12 Myotubes and Muscle of HF/HFr Diet Mice. Front. Pharmacol. 2020, 11, 601448. [Google Scholar] [CrossRef]
- Dua, K.; Bebawy, M.; Awasthi, R.; Tekade, R.K.; Tekade, M.; Gupta, G.; Hansbro, P.M. Application of Chitosan and Its Derivatives in Nanocarrier Based Pulmonary Drug Delivery Systems. Pharm. Nanotechnol. 2017, 5, 243–249. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, D.; Liu, D.; Su, J.; Jin, Y.; Wang, D.; Han, B.; Jiang, Z.; Liu, B. Applications of Chitosan and Its Derivatives in Skin and Soft Tissue Diseases. Front. Bioeng. Biotechnol. 2022, 10, 894667. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes Coated by Chitosan for the Controlled Release of Khellin. Polymers 2020, 12, 1766. [Google Scholar] [CrossRef] [PubMed]
- Ying, R.; Wang, H.; Sun, R.; Chen, K. Preparation and properties of a highly dispersed nano-hydroxyapatite colloid used as a reinforcing filler for chitosan. Mater. Sci. Eng. C 2020, 110, 110689. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Soad, A.M.; Lazzara, G.; Abd El-Magied, M.O.; Cavallaro, G.; Al-Otaibi, J.S.; Sayyed, M.I.; Kovaleva, E.G. Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media. Int. J. Mol. Sci. 2022, 23, 2396. [Google Scholar] [CrossRef] [PubMed]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Bin Arifin, M.A.; Ahmed, S. A Review on Chitosan and Chitosan-Based Bionanocomposites: Promising Material for Combatting Global Issues and Its Applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.; Zhang, S.; Zhang, X.; Wan, L.; Yun, Z.; Ji, S.; Gong, F.; Huang, M.; Wang, L.; et al. GRGDS-Functionalized Chitosan Nanoparticles as A Potential Intravenous Hemostat for Traumatic Hemorrhage Control in an Animal Model. Nanomedicine 2018, 14, 2531–2540. [Google Scholar] [CrossRef]
- Wang, J.; Chin, D.; Poon, C.; Mancino, V.; Pham, J.; Li, H.; Ho, P.Y.; Hallows, K.R.; Chung, E.J. Oral Delivery of Metformin by Chitosan Nanoparticles for Polycystic Kidney Disease. J. Control. Release 2021, 329, 1198–1209. [Google Scholar] [CrossRef]
- Shim, S.; Yoo, H.S. The Application of Mucoadhesive Chitosan Nanoparticles in Nasal Drug Delivery. Mar. Drugs 2020, 18, 605. [Google Scholar] [CrossRef]
- Ding, Y.F.; Li, S.; Liang, L.; Huang, Q.; Yuwen, L.; Yang, W.; Wang, R.; Wang, L.H. Highly Biocompatible Chlorin e6-Loaded Chitosan Nanoparticles for Improved Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9980–9987. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in Vitro and in Vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, G.W.; Zhao, K. Mannose-anchored quaternized chitosan/thiolated carboxymethyl chitosan composite NPs as mucoadhesive carrier for drug delivery. Carbohydr. Polym. 2022, 283, 119174. [Google Scholar] [CrossRef]
- Shelma, R.; Sharma, C.P. Submicroparticles Composed of Amphiphilic Chitosan Derivative for Oral Insulin and Curcumin Release Applications. Colloids Surf. B 2011, 88, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Q.; Meng, Q.Y.; Li, Q.; Liu, J.B.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Sun, Y.W.; Chen, G.; Rong, G.Y.; Kang, H.; Jin, Z.; Wang, X.H. Biological Evaluation of N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan as a Carrier for the Delivery of Live Newcastle Disease Vaccine. Carbohydr. Polym. 2016, 149, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Ding, F.; Fu, J.W.; Tao, C.; Yu, Y.H.; He, X.R.; Gao, Y.G.; Zhang, Y.I.I. Recent Advances of Chitosan and Its Derivatives in Biomedical Applications. Curr. Med. Chem. 2020, 27, 3023–3045. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Jin, Z.; Li, W.; Cao, H.W.; Zhang, X.; Chen, G.; Wu, H.; Guo, C.; Zhang, Y.; Kang, H.; Wang, Y.F.; et al. Antimicrobial Activity and Cytotoxicity of N-2-HACC and Characterization of Nanoparticles with N-2-HACC and CMC as a Vaccine Carrier. Chem. Eng. J. 2013, 221, 331–341. [Google Scholar] [CrossRef]
- Trucillo, P. Discrete and Continuous Glucose Monitoring Systems: The Point of View of a Patient Affected by Type-1 Diabetes. Processes 2022, 10, 2706. [Google Scholar] [CrossRef]
- Logith Kumar, R.; Keshav Narayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A Review of Chitosan and Its Derivatives in Bone Tissue Engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Ren, S.; Pan, J.; Wang, Y.; Shen, Y.; Zeng, Z.; Cui, H.; Zhao, X. Versatile Oral Insulin Delivery Nanosystems: From Materials to Nanostructures. Int. J. Mol. Sci. 2022, 23, 3362. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Z.S.; Zhao, T.B.; Ye, M.L.; Hu, C.J.; Zhou, H.M.; Yin, Z.H.; Chen, Y.; Zhang, Y.; Wang, S.F.; et al. Targeting Insulin Resistance in Type 2 Diabetes via Immune Modulation of Cord Blood-Derived Multipotent Stem Cells (CB-SCs) in Stem Cell Educator Therapy: Phase I/II Clinical Trial. BMC Med. 2013, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.; Kerr, D. Skin-Related Complications of Insulin Therapy. Am. J. Clin. Dermatol. 2003, 4, 661–667. [Google Scholar] [CrossRef] [PubMed]




| N-2-HACC Concentration (mg/mL) | CMCS Concentration (mg/mL) | N-2-HACC:NA-FGF (v:v) | EE (%) | LC (%) |
|---|---|---|---|---|
| 1.5 | 0.8 | 4:4 | 71.1 ± 2.2 | 40.2 ± 1.1 |
| 1.5 | 1.0 | 4:8 | 72.4 ± 1.8 | 39.8 ± 1.2 |
| 1.5 | 1.2 | 4:5 | 89.4 ± 2.8 | 48.8 ± 1.4 |
| 2.0 | 0.8 | 4:8 | 82.2 ± 1.4 | 43.4 ± 1.5 |
| 2.0 | 1.0 | 4:5 | 80.4 ± 1.6 | 42.7 ± 1.8 |
| 2.0 | 1.2 | 4:4 | 84.6 ± 2.5 | 44.4 ± 1.3 |
| 3.0 | 0.8 | 4:5 | 76.7 ± 2.0 | 38.8 ± 1.0 |
| 3.0 | 1.0 | 4:4 | 79.5 ± 2.4 | 41.1 ± 1.2 |
| 3.0 | 1.2 | 4:8 | 80.6 ± 2.1 | 37.8 ± 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Chen, Q.; Wang, W.; Lin, Y.; Kang, H.; Jin, Z.; Zhao, K. Chitosan Derivative-Based Microspheres Loaded with Fibroblast Growth Factor for the Treatment of Diabetes. Polymers 2023, 15, 3099. https://doi.org/10.3390/polym15143099
Wu J, Chen Q, Wang W, Lin Y, Kang H, Jin Z, Zhao K. Chitosan Derivative-Based Microspheres Loaded with Fibroblast Growth Factor for the Treatment of Diabetes. Polymers. 2023; 15(14):3099. https://doi.org/10.3390/polym15143099
Chicago/Turabian StyleWu, Jue, Qian Chen, Wenfei Wang, Yuhong Lin, Hong Kang, Zheng Jin, and Kai Zhao. 2023. "Chitosan Derivative-Based Microspheres Loaded with Fibroblast Growth Factor for the Treatment of Diabetes" Polymers 15, no. 14: 3099. https://doi.org/10.3390/polym15143099
APA StyleWu, J., Chen, Q., Wang, W., Lin, Y., Kang, H., Jin, Z., & Zhao, K. (2023). Chitosan Derivative-Based Microspheres Loaded with Fibroblast Growth Factor for the Treatment of Diabetes. Polymers, 15(14), 3099. https://doi.org/10.3390/polym15143099







