Unraveling the Adsorption Mechanism and Anti-Corrosion Functionality of Dextrin and Inulin as Eco-Friendly Biopolymers for the Corrosion of Reinforced Steel in 1.0 M HCl: A Thermodynamic and Kinetic Approach
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Electrochemical Methods (PDP and EIS)
2.2.2. Chemical Method (ML)
2.2.3. Spectroscopic Method (SEM)
3. Results and Discussion
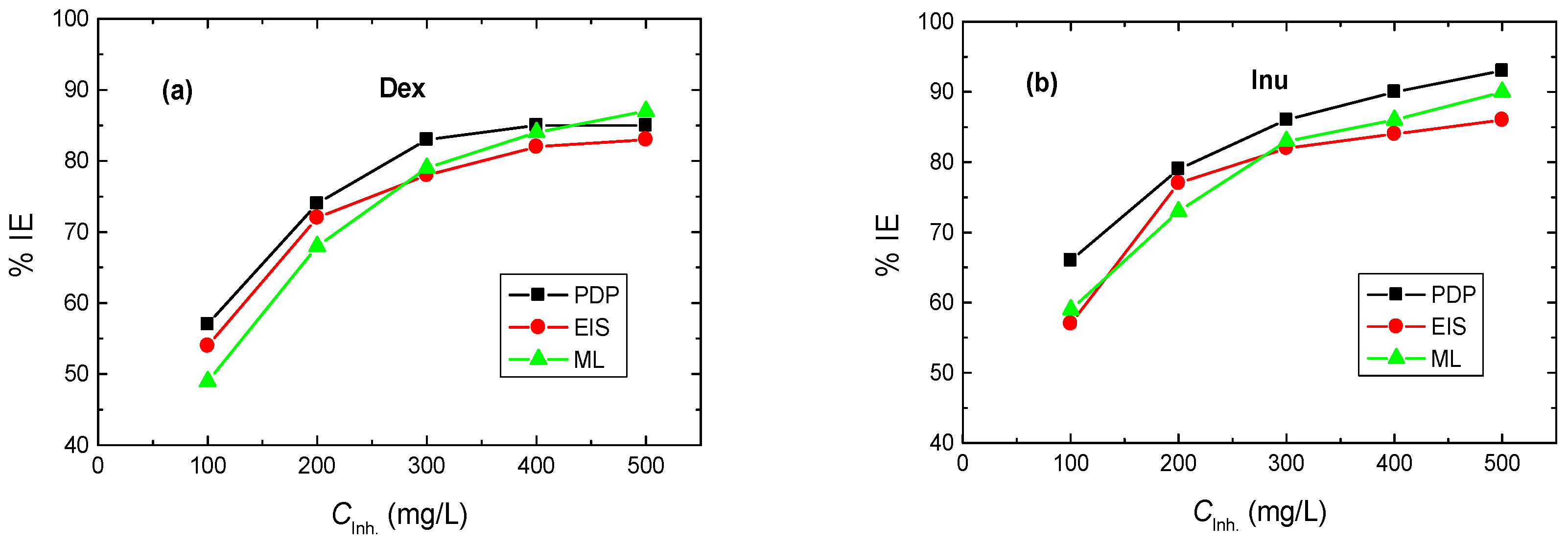
3.1. PDP Measurements
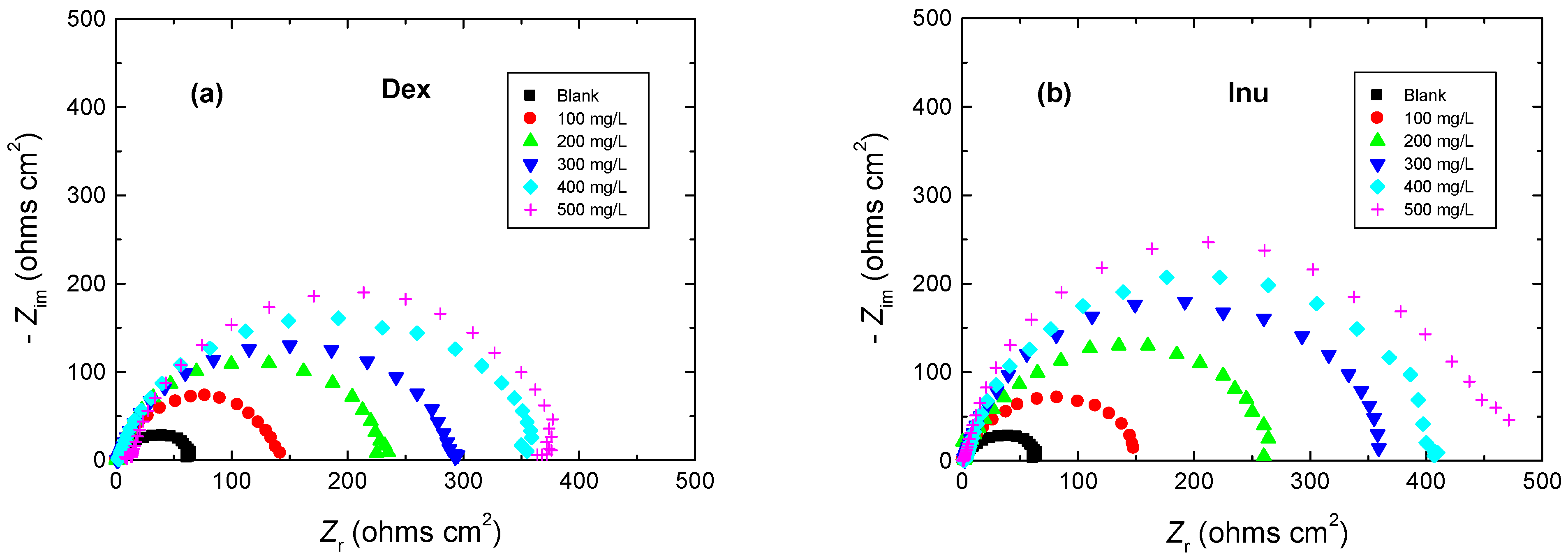
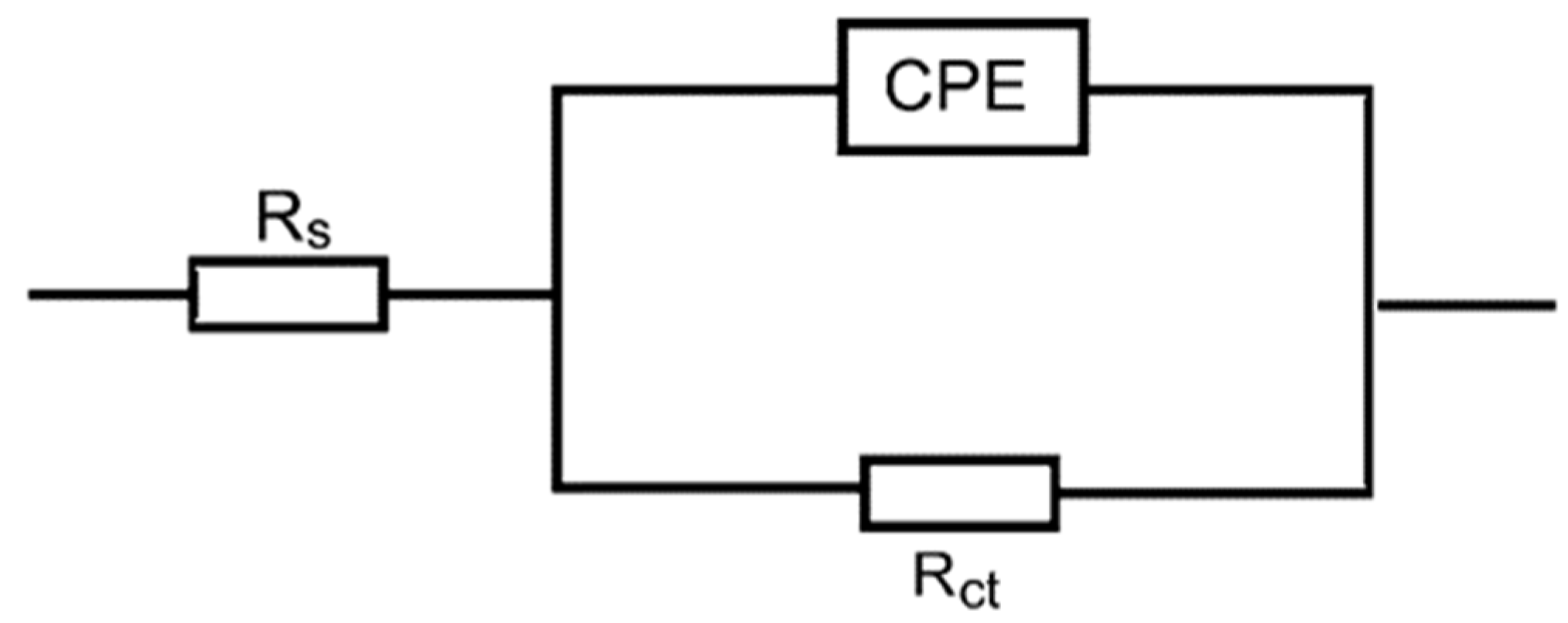
3.2. EIS Measurements
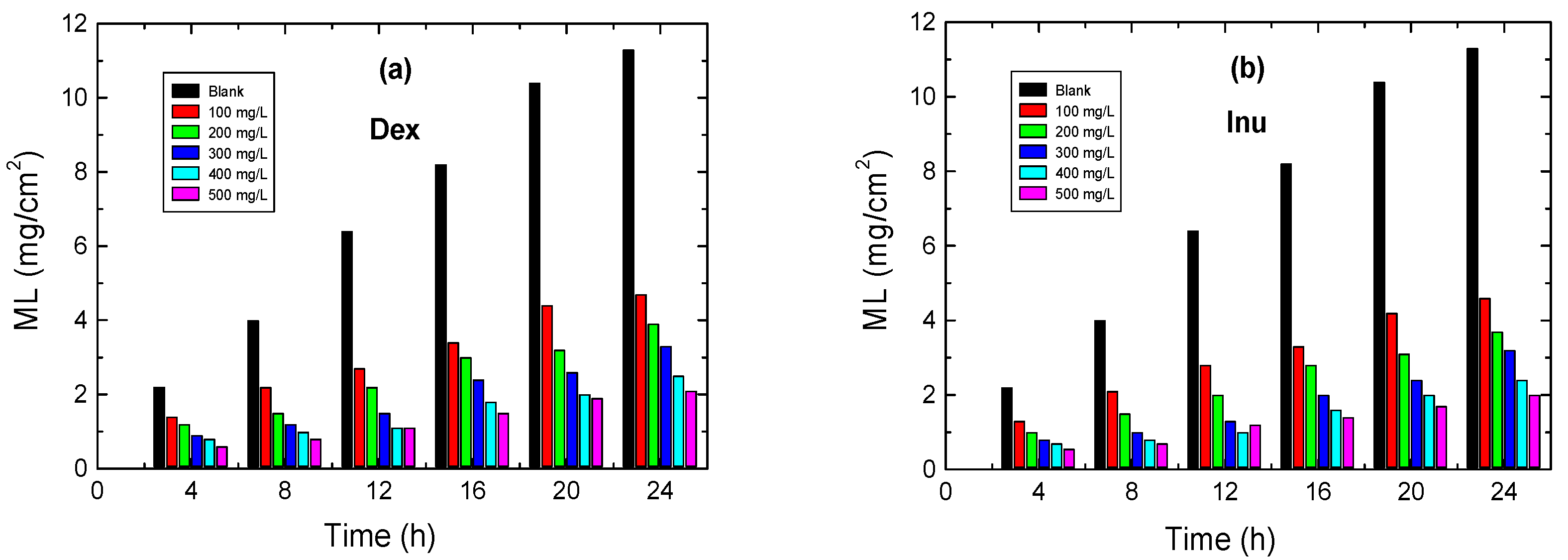
3.3. ML Measurements
3.3.1. Effect of Inhibitors’ Concentrations
3.3.2. Effect of Temperature
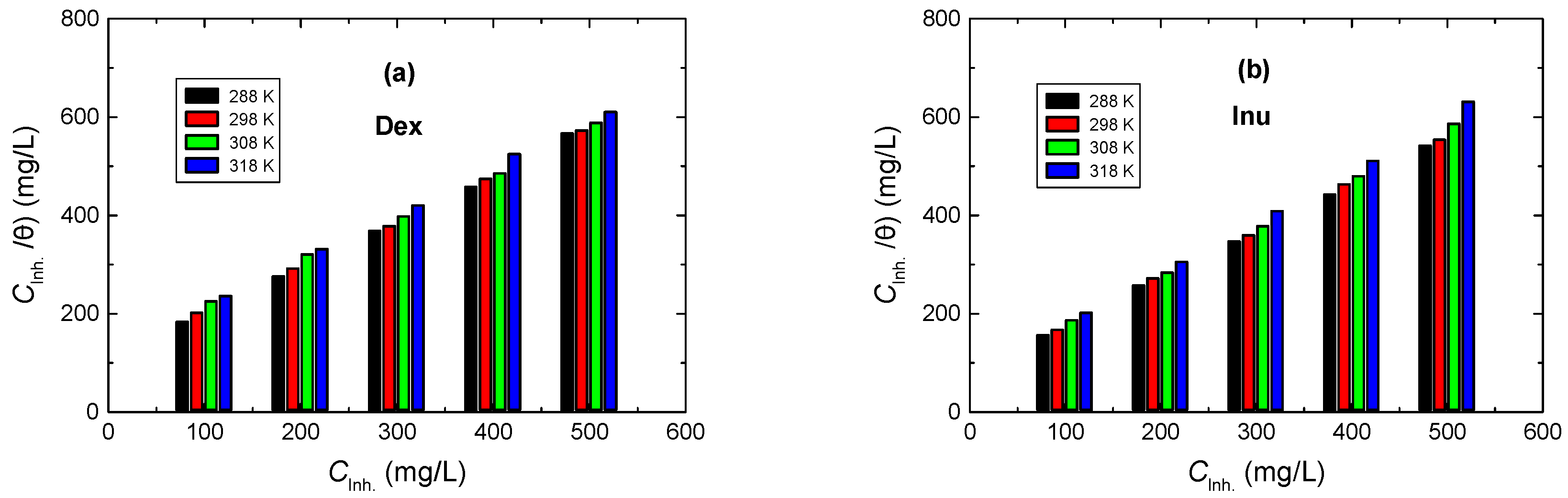
3.3.3. Adsorption Isotherms Examination
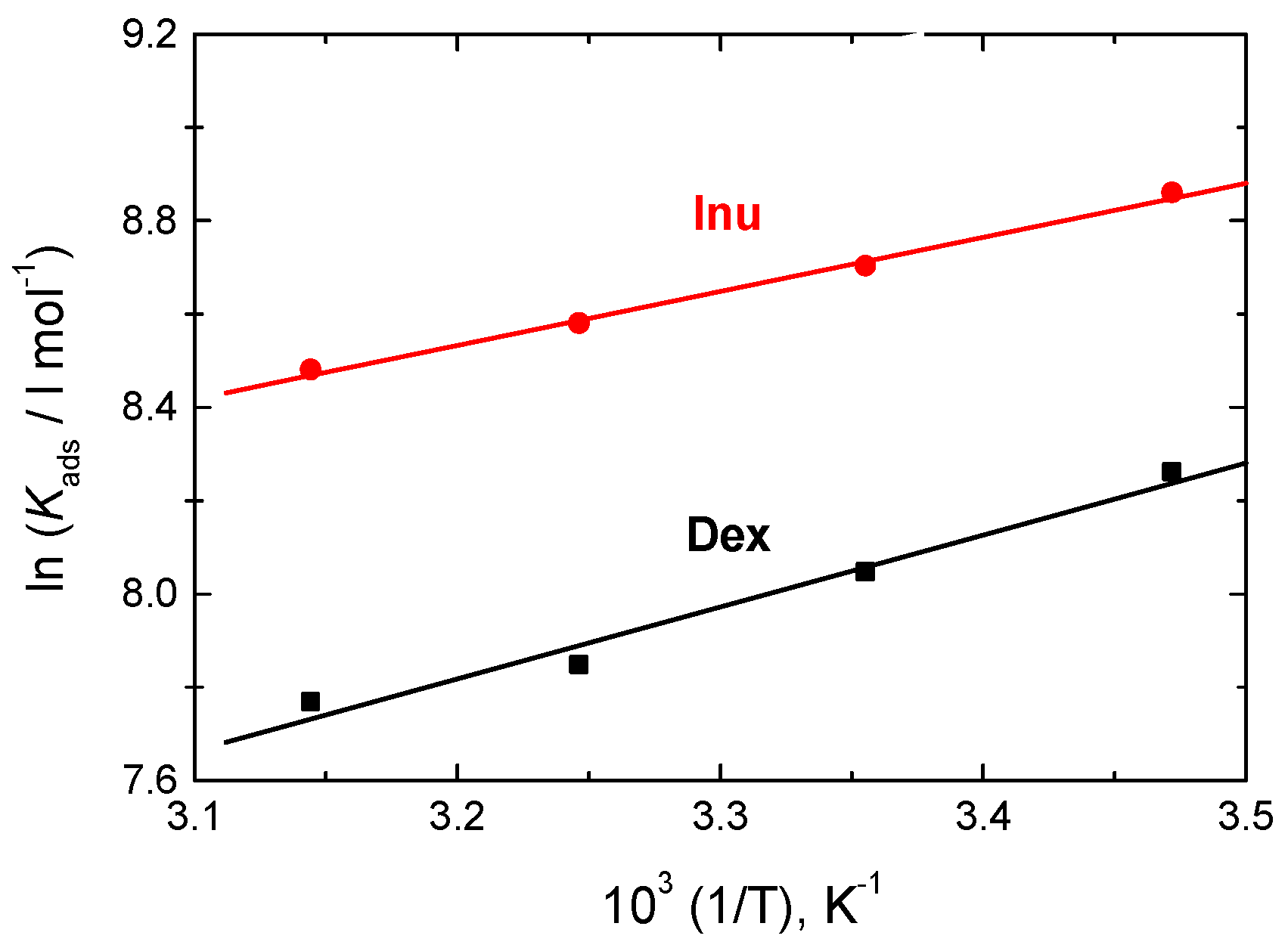
3.3.4. Thermodynamic Parameters
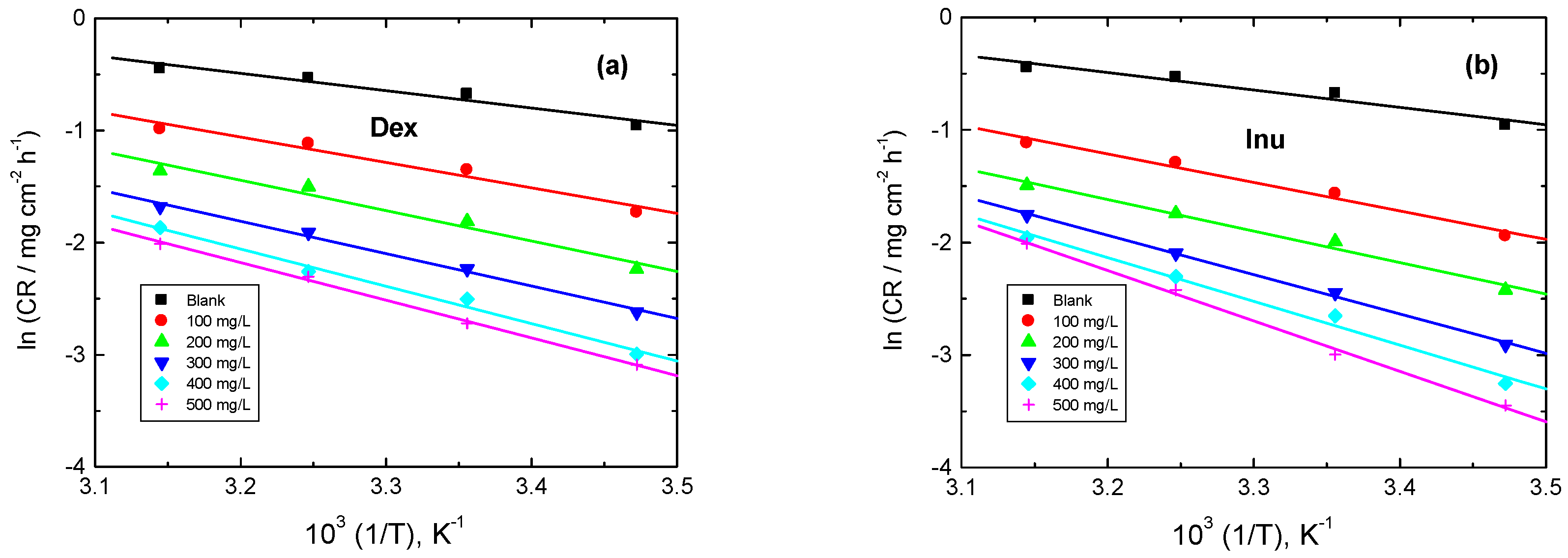
3.3.5. Kinetic Parameters
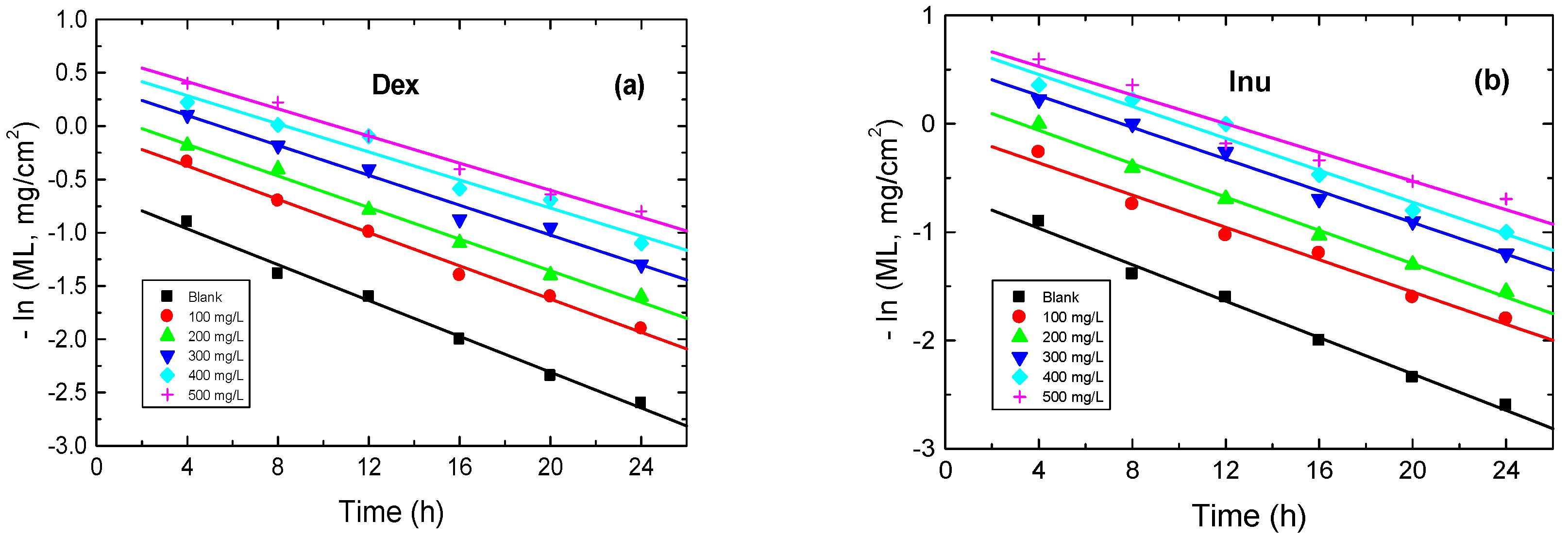
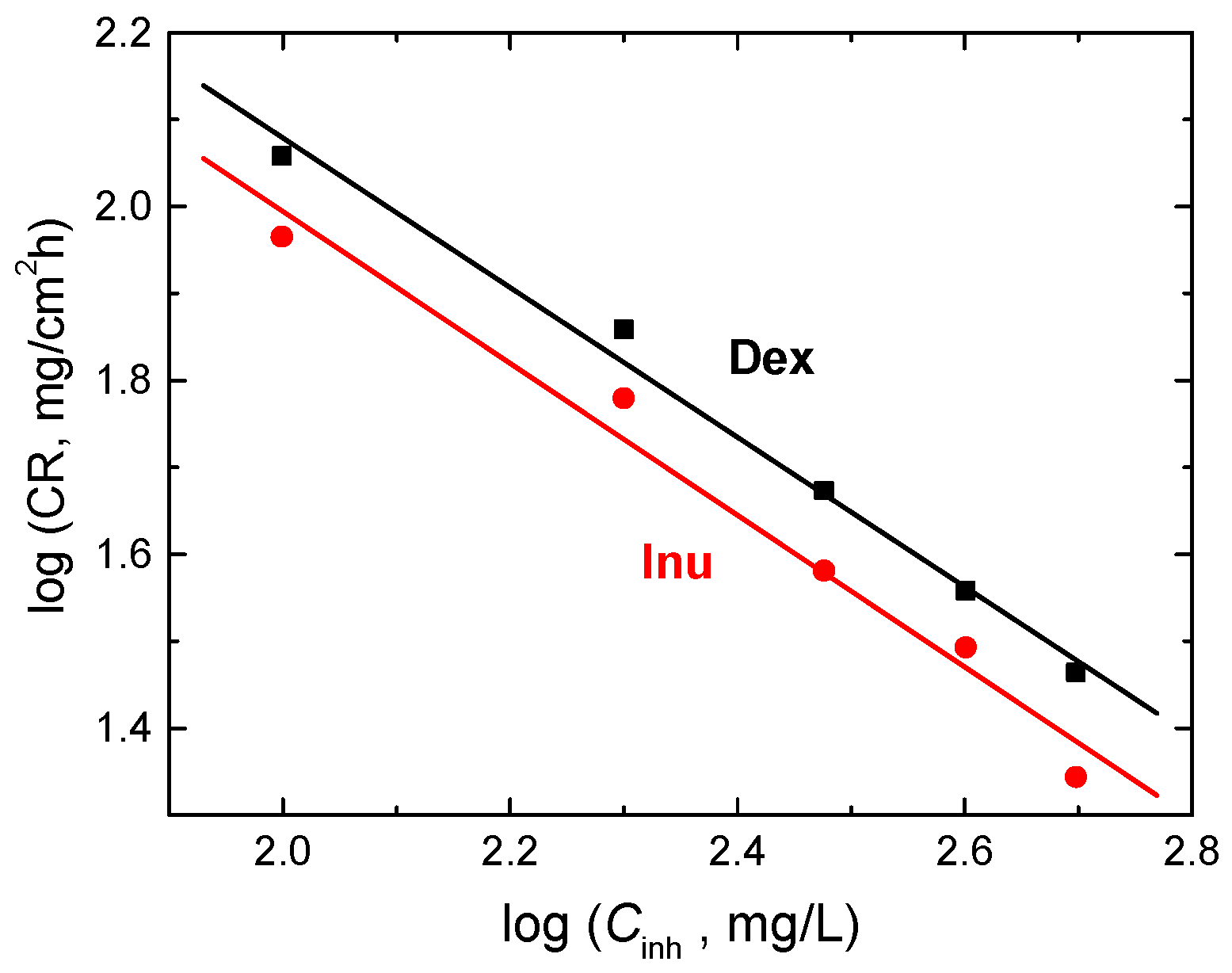
3.3.6. Kinetics of Corrosion and Its Inhibition
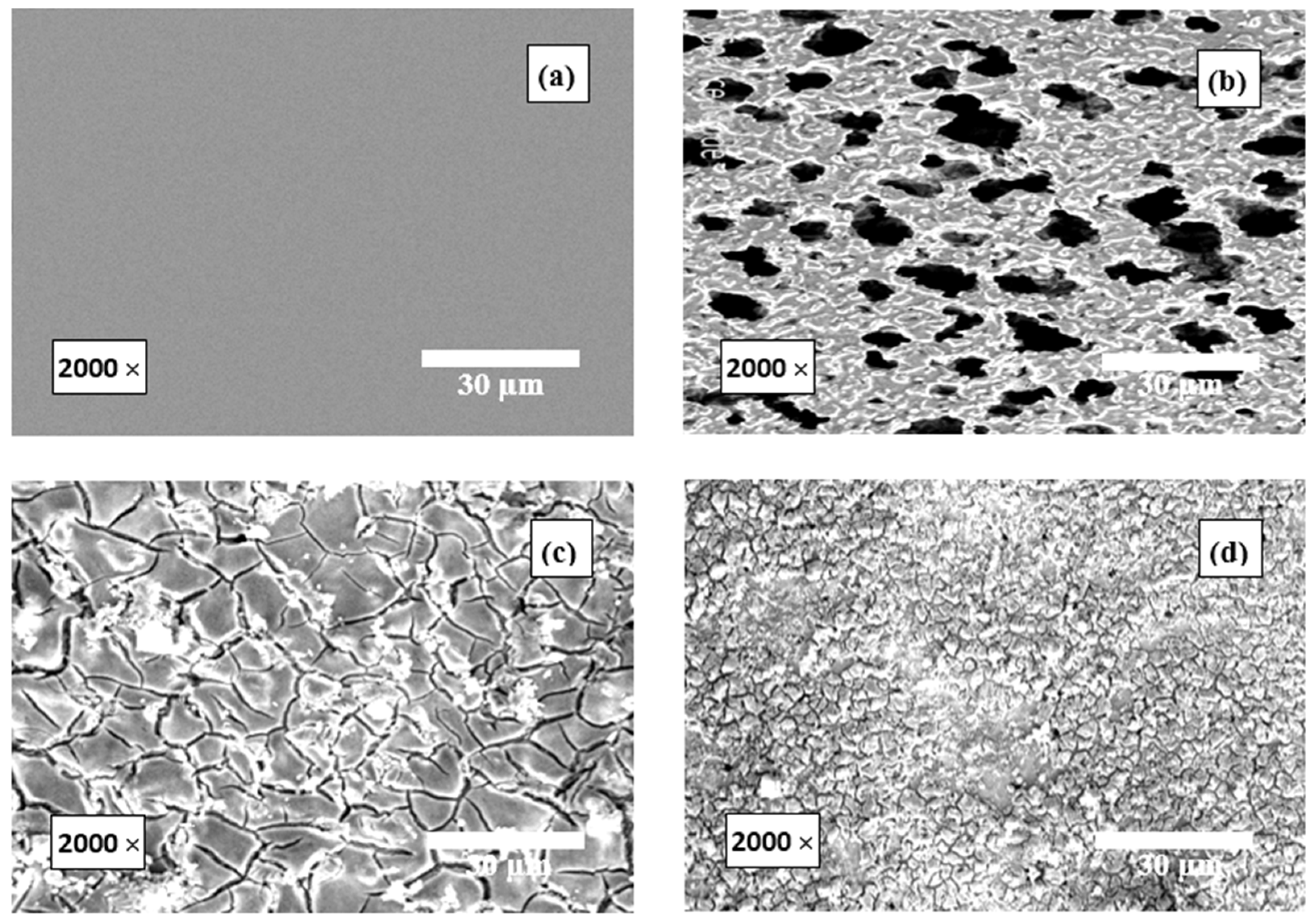
3.4. SEM Examinations
4. Conclusions
- 1.
- Two biopolymers, dextrin (Dex) and inulin (Inu), were tested as inhibitors for the corrosion of reinforcing steel (RS) in 1.0 M HCl using various experimental tools.
- 2.
- The inhibition efficiencies (% IEs) of the tested biopolymers were improved by augmenting their doses while reducing with rising temperature.
- 3.
- The % IEs of Dex and Inu at a dose of 500 mg/L reached 85% and 93%, respectively.
- 4.
- The tested biopolymers displayed mixed type with a foremost anodic one.
- 5.
- The acquired high % IEs were demonstrated by intense adsorption of Dex and Inu on the RS surface fitting the Langmuir isotherm.
- 6.
- The influence of rising temperature in the range of 288–318 K was examined.
- 7.
- Thermodynamic and kinetic parameters sustained the mechanism of physical adsorption of the inhibitors.
- 8.
- The kinetics of corrosion and its inhibition by Dex and Inu were also investigated.
- 9.
- The SEM results were set to accord with the various utilized experimental tools.
- 10.
- The results gained from all used tools were discovered to be in good agreement with each other.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heikal, M.; Ali, A.; Ibrahim, B.S.; Toghan, A. Electrochemical and Physico-mechanical Characterizations of Fly ash Composite Cements. Constr. Build. Mater. 2020, 243, 118309. [Google Scholar] [CrossRef]
- Glasser, F.P.; Eric, M.J.S. Durability of concrete: Degradation phenomena involving detrimental chemical reactions. Cem. Concr. Res. 2008, 38, 226–246. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, X.; Li, X.; Deng, P.; Wang, G. The coupled effect of temperature and carbonation on the corrosion of rebars in the simulated concrete pore solutions. J. Chem. 2015, 2015, 462605. [Google Scholar] [CrossRef]
- Murulana, L.C.; Singh, A.K.; Shukla, S.K.; Kabanda, M.M.; Ebenso, E.E. Experimental and Quantum Chemical Studies of Some Bis(trifluoromethyl-sulfonyl) Imide Imidazolium-Based Ionic Liquids as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solution. Ind. Eng. Chem. Res. 2012, 51, 13282–13299. [Google Scholar] [CrossRef]
- Olivares-Xometl, O.; López-Aguilar, C.; Herrastí-González, P.; Likhanova, N.V.; Lijanova, I.; Martínez-Palou, R.; Rivera-Márquez, J.A. Adsorption and corrosion inhibition performance by three new ionic liquids on API 5L X52 steel surface in acid media. Ind. Eng. Chem. Res. 2014, 53, 9534–9543. [Google Scholar] [CrossRef]
- Costa, E.M.; Dedavid, B.A.; Santos, C.A.; Lopes, N.F.; Fraccaro, C.; Pagartanidis, T.; Lovatto, L.P. Crevice corrosion on stainless steels in oil and gas industry: A review of techniques for evaluation, critical environmental factors and dissolved oxygen. Eng. Fail. Anal. 2023, 144, 106955. [Google Scholar] [CrossRef]
- Guzmán-Lucero, D.; Olivares-Xometl, O.; Martínez-Palou, R.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Garibay-Febles, V. Synthesis of selected vinylimidazolium ionic liquids and their effectiveness as corrosion inhibitors for carbon steel in aqueous sulfuric acid. Ind. Eng. Chem. Res. 2011, 50, 7129–7140. [Google Scholar] [CrossRef]
- Toghan, A.; Fawzy, A.; Alakhras, A.I.; Sanad, M.M.S.; Khairy, M.; Farag, A.A. Correlating experimental with theoretical studies for a new ionic liquid for inhibiting corrosion of carbon steel during oil well acidification. Metals 2023, 13, 862. [Google Scholar] [CrossRef]
- Saleh, T.A.; Haruna, K.; Alharbi, B. Diaminoalkanes functionalized graphene oxide as corrosion inhibitors against carbon steel corrosion in simulated oil/gas well acidizing environment. J. Colloid Interface Sci. 2023, 630, 591–610. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Mazumder, M.A.J.; Ali, S.A. Tipping effect of tetra-alkylammonium on the potency of N-(6-(1H-benzo[d]imidazol-1-yl)hexyl)-N, N-dimethyldodecan-1-aminium bromide (BIDAB) as corrosion inhibitor of austenitic 304L stainless steel in oil and gas acidization: Experimental and DFT approach. J. Mol. Liq. 2022, 360, 119431. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Li, Y.Y.; Lei, Y.; Wang, X.; Liu, H.F.; Zhang, G.A. Comparison of the synergistic inhibition mechanism of two eco-friendly amino acids combined corrosion inhibitors for carbon steel pipelines in oil and gas production. Appl. Surf. Sci. 2022, 583, 152559. [Google Scholar] [CrossRef]
- Farag, A.A. Oil-in-water emulsion of a heterocyclic adduct as a novel inhibitor of API X52 steel corrosion in acidic solution. Corros. Rev. 2018, 36, 575–588. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.; Sorour, A.; Saha, S.K.; Banerjee, P. Triazole-modified chitosan: A biomacromolecule as a new environmentally benign corrosion inhibitor for carbon steel in a hydrochloric acid solution. RSC Adv. 2019, 9, 14990–15003. [Google Scholar] [CrossRef] [PubMed]
- Sukul, D.; Pal, A.; Saha, S.K.; Satpati, S.; Adhikari, U.; Banerjee, P. Newly synthesized quercetin derivatives as corrosion inhibitors for MS in 1 M HCl: Combined experimental and theoretical investigation. Phys. Chem. Chem. Phys. 2018, 20, 6562–6574. [Google Scholar] [CrossRef]
- Silva, R.M.P.; Suffredini, H.B.; Bastos, I.N.; Santos, L.F.; Simões, A.M.P. Naphthenic acid corrosion of API 5L X70 steel in aqueous/oil environment using electrochemical surface-resolved and analytical techniques. Electrochim. Acta. 2022, 407, 139900. [Google Scholar] [CrossRef]
- Li, E.; Li, Y.; Liu, S.; Yao, P. Choline amino acid ionic liquids as green corrosion inhibitors of mild steel in acidic medium, Colloids Surfaces a Physicochem. Eng. Asp. 2023, 657, 130541. [Google Scholar] [CrossRef]
- Ouakki, M.; Galai, M.; Benzekri, Z.; Aribou, Z.; Ech-Chihbi, E.; Guo, L.; Dahmani, K.; Nouneh, K.; Briche, S.; Boukhris, S.; et al. A detailed investigation on the corrosion inhibition effect of by newly synthesized pyran derivative on mild steel in 1.0 M HCl: Experimental, surface morphological (SEM-EDS, DRX& AFM) and computational analysis (DFT & MD simulation). J. Mol. Liq. 2021, 344, 117777. [Google Scholar]
- Al-Gamal, A.G.; Farag, A.A.; Elnaggar, E.M.; Kabel, K.I. Comparative impact of doping nano-conducting polymer with carbon and carbon oxide composites in alkyd binder as anti-corrosive coatings. Compos. Interfaces 2018, 25, 959–980. [Google Scholar] [CrossRef]
- Fawzy, A.; Toghan, A. Inhibition evaluation of chromotrope dyes for the corrosion of mild steel in acidic environment: Thermodynamic and kinetic aspects. ACS Omega 2021, 6, 4051–4061. [Google Scholar] [CrossRef]
- Haldhar, R.; Raorane, C.J.; Mishra, V.K.; Periyasamy, T.; Berisha, A.; Kim, S.-C. Development of different chain lengths ionic liquids as green corrosion inhibitors for oil and gas industries: Experimental and theoretical investigations. J. Mol. Liq. 2023, 372, 121168. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Kholikov, A.; Akbarov, K.; Guo, L.; Kaya, S.; Katin, K.P.; Verma, D.K.; Rbaa, M.; Dagdag, O.; Haldhar, R. Novel bromide–cucurbit[7]uril supramolecular ionic liquid as a green corrosion inhibitor for the oil and gas industry. J. Electroanal. Chem. 2021, 901, 115794. [Google Scholar] [CrossRef]
- Farag, A.A.; Badr, E.A. Non-ionic surfactant loaded on gel capsules to protect downhole tubes from produced water in acidizing oil wells. Corros. Rev. 2020, 38, 151–164. [Google Scholar] [CrossRef]
- Alamry, K.A.; Khan, A.; Aslam, J.; Hussein, M.A.; Aslam, R. Corrosion inhibition of mild steel in hydrochloric acid solution by the expired Ampicillin drug. Sci. Rep. 2023, 13, 6724. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Polysaccharide from Plantago as a green corrosion inhibitor for carbon steel in 1M HCl solution. Carbohydr. Polym. 2017, 160, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Toghan, A.; Fawzy, A.; Al Bahir, A.; Alqarni, N.; Sanad, M.M.S.; Khairy, M.; Alakhras, A.I.; Farag, A.A. Computational foretelling and experimental implementation of the performance of polyacrylic acid and polyacrylamide polymers as eco-friendly corrosion inhibitors for Copper in nitric acid. Polymers 2022, 14, 4802. [Google Scholar] [CrossRef] [PubMed]
- Saad, I.R.; Abdel-Gaber, A.M.; Younes, G.O.; Nsouli, B. Corrosion Inhibition of Mild Steel in Acidic Solutions Using 1,2,4-Triazolo [1,5-a]pyrimidine. Russ. J. Appl. Chem. 2018, 91, 245–252. [Google Scholar] [CrossRef]
- Raj, A.; Kumari, P.; Lavanya, M.; Vishwanath, T.; Suvarna, A.M. Attenuation of Mild Steel-Acid Corrosion Using Exfoliated Graphite Oxide-Polymer Composite: Synthesis, Characterization, Electrochemical, and Response Surface Method Approach. Arab. J. Sci. Eng. 2023, 48, 7395–7410. [Google Scholar] [CrossRef]
- Fawzy, A.; Toghan, A.; Alqarni, N.; Morad, M.; Zaki, M.E.A.; Sanad, M.; Alakhras, A.I.; Farag, A.A. Experimental and computational exploration of chitin, pectin and amylopectin polymers as efficient eco-friendly corrosion inhibitors for mild steel in acidic environment. Kinetic, thermodynamic and mechanistic aspects. Polymers 2023, 15, 891. [Google Scholar] [CrossRef]
- Muthamma, K.; Kumari, P.; Lavanya, M.; Rao, S.A. Corrosion Inhibition of Mild Steel in Acidic Media by N-[(3,4-Dimethoxyphenyl)Methyleneamino]-4-Hydroxy-Benzamide. J. Bio. Tribo Corros. 2021, 7, 10. [Google Scholar] [CrossRef]
- Zarei, A.; Dehghani, A.; Guo, L.; Ramezanzadeh, B. Pepper extract effectiveness as a natural inhibitor against corrosion of steel samples (SS) in 1 M hydrochloric acid; Theoretical (DFT calculation—MD simulation), thermodynamic, and electrochemical-surface studies. Ind. Crops Prod. 2022, 189, 115839. [Google Scholar] [CrossRef]
- Mazumder, M.A.J. Synthesis, characterization and electrochemical analysis of cysteine modified polymers for corrosion inhibition of mild steel in aqueous 1 M HCl. RSC Adv. 2019, 9, 4277–4294. [Google Scholar] [CrossRef] [PubMed]
- Lakbaibi, Z.; Damej, M.; Molhi, A.; Benmessaoud, M.; Tighadouini, S.; Jaafar, A.; Benabbouha, T.; Ansari, A.; Driouich, A.; Tabyaoui, M. Evaluation of inhibitive corrosion potential of symmetrical hydrazine derivatives containing nitrophenyl moiety in 1M HCl for C38 steel: Experimental and theoretical studies. Heliyon 2022, 8, e09087. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Al Bahir, A.; Alqarni, N.; Toghan, A.; Khider, M.; Ibrahim, I.M.; Abulreesh, H.H.; Elbanna, K. Evaluation of synthesized biosurfactants as promising corrosion inhibitors and alternative antibacterial and antidermatophytes agents. Sci. Rep. 2023, 13, 2585. [Google Scholar] [CrossRef]
- Umoren, S.A.; Gasem, Z.M.; Obot, I.B. Natural Products for Material Protection: Inhibition of Mild Steel Corrosion by Date Palm Seed Extracts in Acidic Media. Ind. Eng. Chem. Res. 2013, 52, 14855–14865. [Google Scholar] [CrossRef]
- Salway, J.G. Medical Biochemistry at a Glance, 2nd ed.; Blackwell Publishing: Malden, MA, USA, 2006; p. 66. [Google Scholar]
- Barclay, T. Inulin—A Versatile Polysaccharide with Multiple Pharmaceutical and Food Chemical Uses; International Pharmaceutical Excipients Council: Arlington, TX, USA, 2010. [Google Scholar]
- Biswas, A.; Das, D.; Lgaz, H.; Pal, S.; Nair, U.G. Biopolymer dextrin and poly (vinyl acetate) based graft copolymer as an efficient corrosion inhibitor for mild steel in hydrochloric acid: Electrochemical, surface morphological and theoretical studies. J. Mol. Liq. 2019, 275, 867–878. [Google Scholar] [CrossRef]
- Rao, P.; Charitha, B.P. Inulin as a Potential Green Inhibitor for Corrosion Control of 6061 Al─15%(v) SiC(P) Composite—Electrochemical and Surface Studies. In Proceedings of the Manipal Research Colloquium (MRC-17), Manipal, India, 4–6 April 2017. [Google Scholar]
- Singh, A.K.; Chugh, B.; Thakur, S.; Pani, B.; Lgaz, H.; Chung, I.-M.; Pal, S.; Prakash, R. Green approach of synthesis of thiazolyl imines and their impeding behavior against corrosion of mild steel in acid medium, Colloids Surfaces a Physicochem. Eng. Asp. 2020, 599, 124824. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Molecular structural aspects of organic corrosion inhibitors: Influence of –CN and –NO2 substituents on designing of potential corrosion inhibitors for aqueous media. J. Mol. Liq. 2020, 316, 113874. [Google Scholar] [CrossRef]
- Gadow, H.S.; Fawzy, A.; Khairy, M.; Sanad, M.M.S.; Toghan, A. Experimental and Theoretical Approaches to the Inhibition of Carbon Steel Corrosion by Thiophene Derivative in 1 M HCl. Int. J. Electrochem. Sci. 2023, 18, 100174. [Google Scholar] [CrossRef]
- Emori, W.; Zhang, R.H.; Okafor, P.C.; Zheng, X.W.; He, T.; Wei, K.; Lin, X.Z.; Cheng, C.R. Adsorption and corrosion inhibition performance of multi-phytoconstituents from Dioscorea septemloba on carbon steel in acidic media: Characterization, experimental and theoretical studies, Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124534. [Google Scholar] [CrossRef]
- Akinbulumo, O.A.; Odejobi, O.J.; Odekanle, E.L. Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla extract in 1.5 M HCl. Results Mater. 2020, 5, 100074. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy, Theory, Experiment and Applications, 2nd ed.; Wiley Interscience Publications: New York, NY, USA, 2005. [Google Scholar]
- Manjula, P.; Manonmani, S.; Jayaram, P.; Rajendran, S. Corrosion behaviour of carbon steel in the presence of N-cetyl-N, N, N-trimethylammonium bromide, Zn2+ and calcium gluconate. Anti-Corros. Methods Mater. 2001, 48, 319–324. [Google Scholar] [CrossRef]
- Ouici, H.B.; Benali, O.; Harek, Y.; Larabi, L.; Hammouti, B.; Guendouzi, A. Inhibition of mild steel corrosion in 5% HCl solution by 5-(2-hydroxyphenyl)-1,2,4-triazole-3-thione. Res. Chem. Intermed. 2013, 39, 2777–2793. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, X. Facile fabrication of robust superhydrophobic coating for enhanced corrosion protection on AZ91 magnesium alloy by electroless Ni-B/GO plating. Surf. Coat. Technol. 2023, 455, 129213. [Google Scholar] [CrossRef]
- Hashem, H.E.; Farag, A.A.; Mohamed, E.A.; Azmy, E.M. Experimental and theoretical assessment of benzopyran compounds as inhibitors to steel corrosion in aggressive acid solution. J. Mol. Struct. 2022, 1249, 131641. [Google Scholar] [CrossRef]
- Toghan, A.; Gadow, H.S.; Dardeer, H.M.; Elabbasy, H.M. New promising halogenated cyclic imides derivatives as Potential Corrosion Inhibitors for Carbon Steel in Acidic Environment. J. Mol. Liq. 2021, 325, 115136. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros. Sci. 2012, 55, 407. [Google Scholar] [CrossRef]
- Benahmed, M.; Djeddi, N.; Akkal, S.; Laouer, H. Saccocalyx satureioides as corrosion inhibitor for carbon steel in acid solution. Int. J. Ind. Chem. 2016, 7, 109–120. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interf. Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef]
- Bommersbach, P.; Dumont, C.A.; Millet, J.P.; Normand, B. Hydrodynamic effect of the behavior of a corrosion inhibitor film: Characterization by electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 4011–4018. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Rawat, J. Influence of iodide ions on inhibitive performance of tetraphenyl dithiaoctaaza cyclotetradeca hexaene (PTAT) during pickling of mild steel in hot sulfuric acid. Mater. Chem. Phys. 2001, 70, 95–99. [Google Scholar] [CrossRef]
- Hsissou, R.; Azogagh, M.; Benhiba, F.; Echihi, S.; Galai, M.; Shaim, A.; Bahaj, H.; Briche, S.; Kaya, S.; Serdaroğlu, G.; et al. Insight of development of two cured epoxy polymer composite coatings as highly protective efficiency for carbon steel in sodium chloride solution: DFT, RDF, FFV and MD approaches. J. Mol. Liq. 2022, 360, 119406. [Google Scholar] [CrossRef]
- Nadi, I.; Bouanis, M.; Benhiba, F.; Nohair, K.; Nyassi, A.; Zarrouk, A.; Jama, C.; Bentiss, F. Insights into the inhibition mechanism of 2,5-bis(4-pyridyl)-1,3,4-oxadiazole for carbon steel corrosion in hydrochloric acid pickling via experimental and computational approaches. J. Mol. Liq. 2021, 342, 116958. [Google Scholar] [CrossRef]
- Abd El Rehim, S.S.; Sayyah, S.M.; El-Deeb, M.M.; Kamal, S.M.; Azooz, R.E. Adsorption and corrosion inhibitive properties of P(2-aminobenzothiazole) on mild steel in hydrochloric acid media. Int. J. Ind. Chem. 2016, 7, 39–52. [Google Scholar] [CrossRef]
- Christov, M.; Popova, A. Adsorption characteristics of corrosion inhibitors from corrosion rate measurements. Corros. Sci. 2004, 46, 1613–1620. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shaaban, S.; Khalaf, M.M.; Toghan, A.; Shalabi, K. Synthesis, experimental, and computational studies of water soluble anthranilic organoselenium compounds as safe corrosion inhibitors for J55 pipeline steel in acidic oilfield formation water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126894. [Google Scholar] [CrossRef]
- Shaban, S.M.; Badr, E.A.; Shenashen, M.A.; Farag, A.A. Fabrication and Characterization of Encapsulated Gemini Cationic Surfactant as Anticorrosion Material for Carbon Steel Protection in Down-Hole Pipelines. Environ. Technol. Innov. 2021, 23, 101603. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M.A. Cefotaxime sodium: A new and efficient corrosion inhibitor for mild steel in hydrochloric acid solution. Corros. Sci. 2009, 51, 1007–1011. [Google Scholar] [CrossRef]
- Okafor, P.C.; Zheng, Y. Synergistic inhibition behaviour of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros. Sci. 2009, 51, 850–859. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Soltani, N.; Salavati-Niasari, M.; Hamadanian, M.; Gandomi, A. Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros. Sci. 2008, 50, 2172–2181. [Google Scholar] [CrossRef]
- Zhao, T.P.; Mu, G.N. The adsorption and corrosion inhibition of anion surfactants on aluminium surface in hydrochloric acid. Corros. Sci. 1999, 41, 1937–1944. [Google Scholar] [CrossRef]
- Durnie, W.; Marco, R.D.; Jefferson, A.; Kinsella, B. Development of a structure-activity relationship for oil field corrosion inhibitors. J. Electrochem. Soc. 1999, 146, 1751–1758. [Google Scholar] [CrossRef]
- Elachouri, M.; Hajji, M.S.; Salem, M.; Kertit, S.; Aride, J.; Coudert, R.; Essassi, E. Some nonionic surfactants as inhibitors of the corrosion of iron in acid chloride solutions. Corrosion 1996, 52, 103–108. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Yin, X.; Yang, W.; Chen, Y. Experimental and theoretical study of corrosion inhibition of 3-pyridinecarbozalde thiosemicarbazone for mild steel in hydrochloric acid. Corros. Sci. 2013, 74, 206–213. [Google Scholar] [CrossRef]
- Anejjar, A.; Zarrouk, A.; Salghi, R.; Zarrok, H.; Hmamou, D.B.; Hammouti, B.; Elmahi, B.; Al-Deyab, S.S. Studies on the inhibitive effect of the ammonium iron (II) sulphate on the corrosion of carbon steel in HCl solution. J. Mater. Environ. Sci. 2013, 4, 583–592. [Google Scholar]
- Bockris, J.O.M.; Reddy, A.K.N. Modern Electrochemistry; Plenum Press: New York, NY, USA, 1977; Volume 2. [Google Scholar]
- Marsh, J. Advanced Organic Chemistry, 3rd ed.; Wiley: Eastern New Delhi, India, 1988. [Google Scholar]
- Eddy, N.O.; Patricia, A.E.; Mamza, P.A.P. Ethanol extract of Terminalia catappa as a green inhibitor for the corrosion of mild steel in H2SO4. Green Chem. Lett. Rev. 2009, 2, 223–231. [Google Scholar] [CrossRef]
- Noor, E.A. The inhibition of mild steel corrosion in phosphoric acid solutions by some N-heterocyclic compounds in the salt form. Corros. Sci. 2005, 47, 33–53. [Google Scholar] [CrossRef]
- Aoun, S.B. Highly Efficient corrosion inhibition of carbon steel in aggressive acidic media with a pyridazinium-based ionic liquid. Int. J. Electrochem. Sci. 2013, 8, 10788–10804. [Google Scholar] [CrossRef]
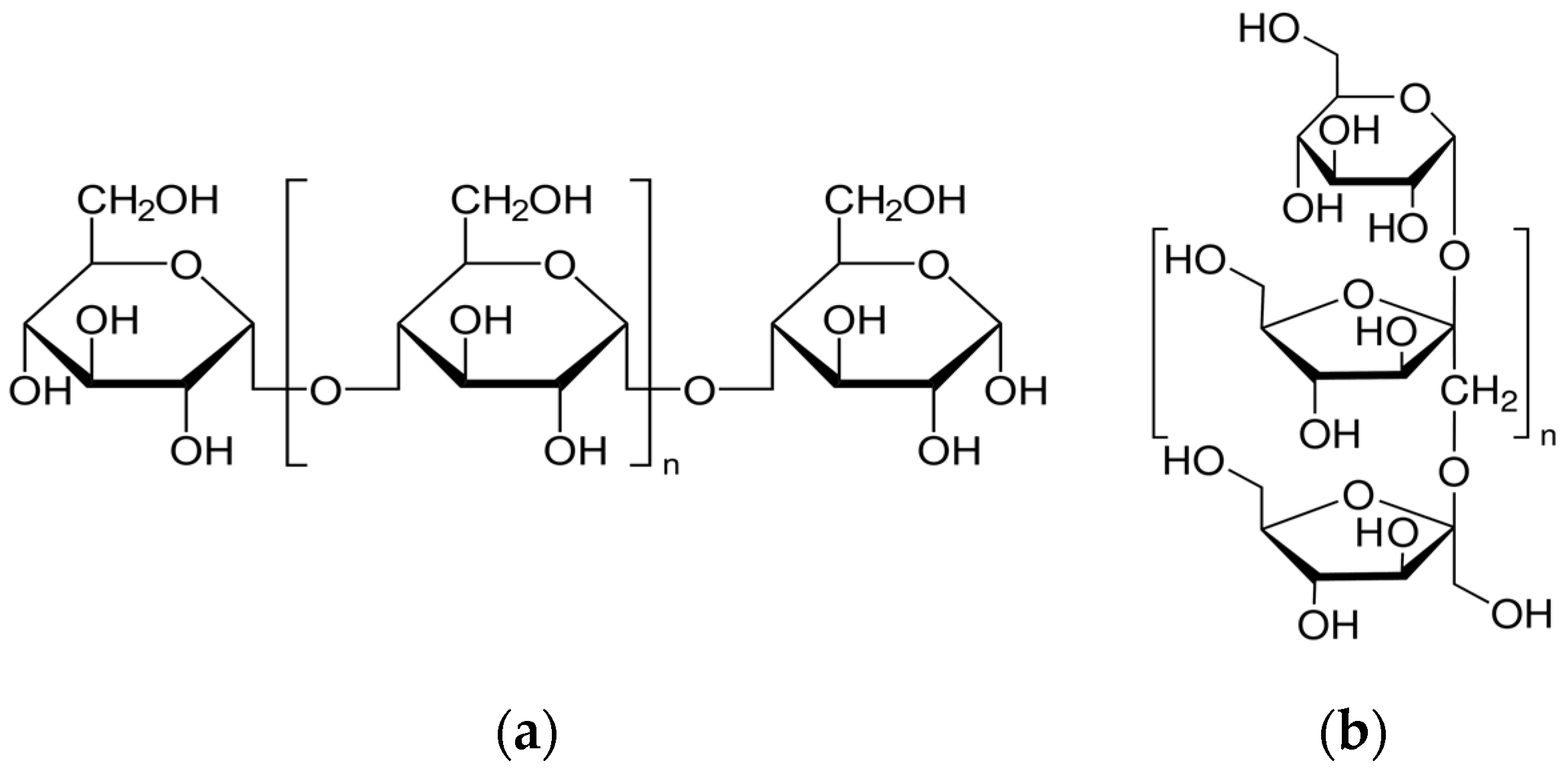
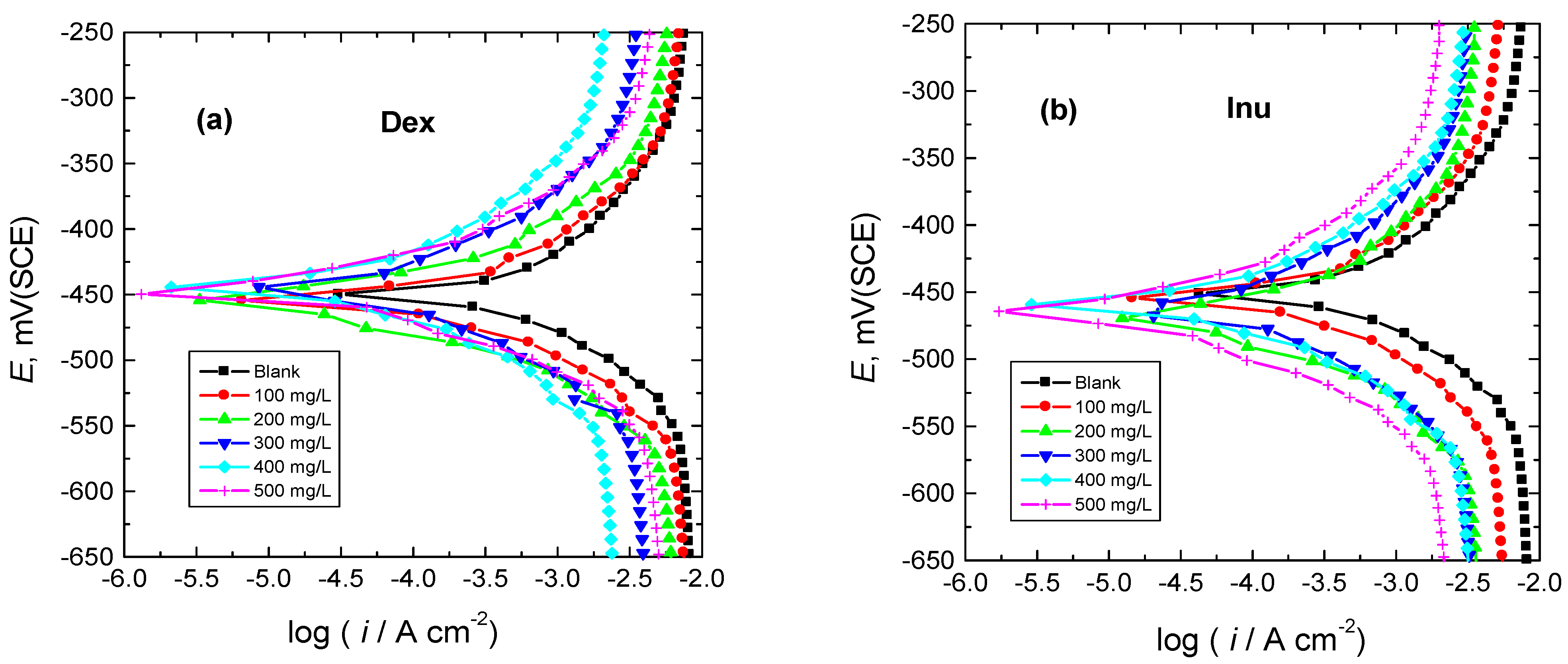
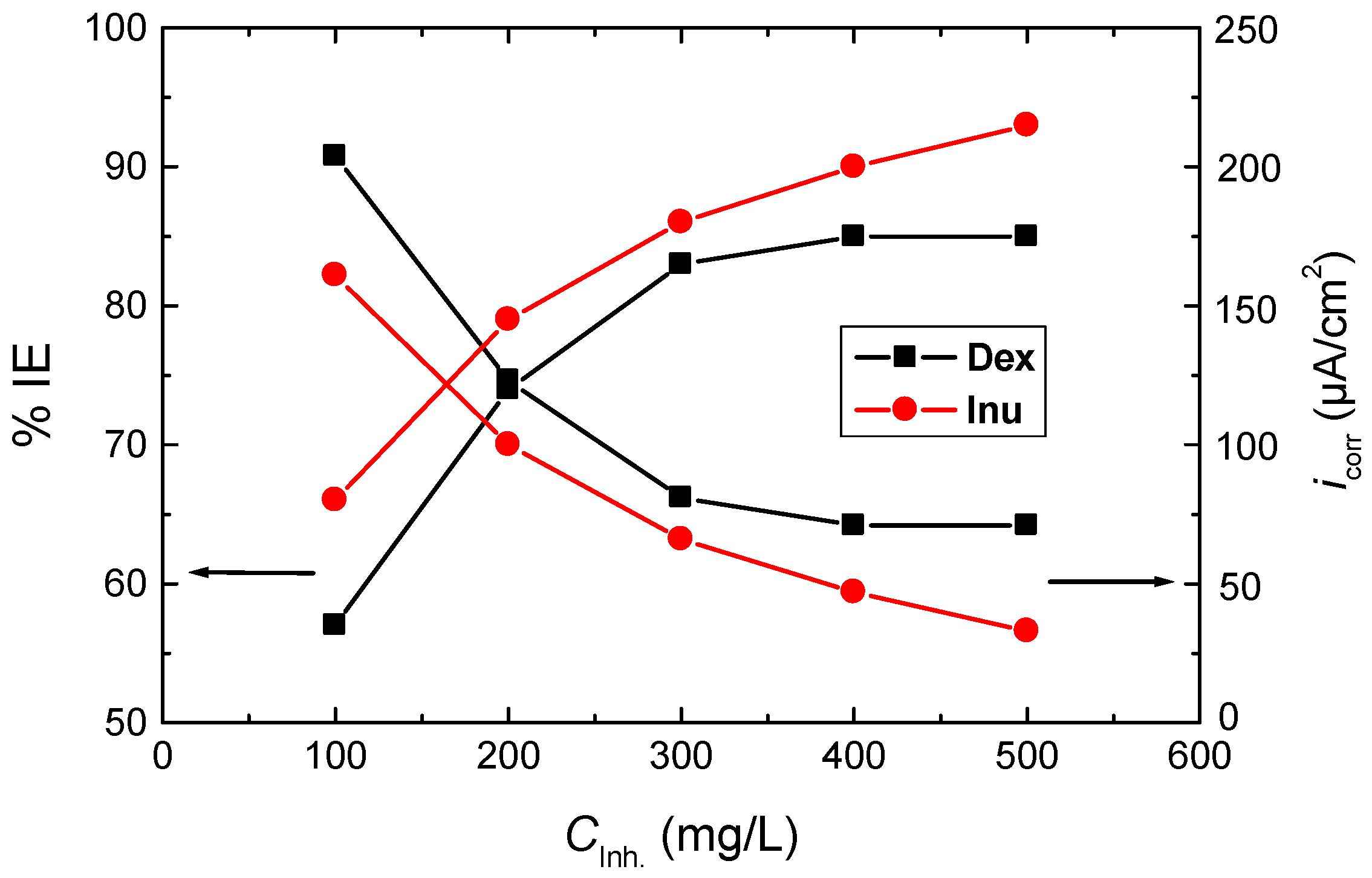


| 1.0 M HCl + | Inh. Conc. (mg/L) | −Ecorr (mV(SCE)) | βa (mV/dec.) | −βc (mV/dec.) | icorr (µA/cm2) | Rp (ohm cm2) | % IE | θ |
|---|---|---|---|---|---|---|---|---|
| - | 0 | 451 | 109 | 88 | 474 | 45 | - | - |
| Dex | 100 | 456 | 102 | 76 | 204 | 93 | 57 | 0.57 |
| 200 | 459 | 103 | 78 | 123 | 157 | 74 | 0.74 | |
| 300 | 447 | 99 | 73 | 81 | 226 | 83 | 0.83 | |
| 400 | 445 | 96 | 72 | 71 | 252 | 85 | 0.85 | |
| 500 | 450 | 95 | 81 | 71 | 268 | 85 | 0.85 | |
| Inu | 100 | 450 | 105 | 85 | 161 | 127 | 66 | 0.66 |
| 200 | 464 | 97 | 83 | 100 | 194 | 79 | 0.79 | |
| 300 | 461 | 104 | 77 | 66 | 291 | 86 | 0.86 | |
| 400 | 459 | 94 | 75 | 47 | 386 | 90 | 0.90 | |
| 500 | 468 | 97 | 79 | 33 | 567 | 93 | 0.93 |
| 1.0 M HCl + | Inh. Conc. (mg/L) | Rs (ohm cm2) | Rct (ohm cm2) | 10−2 CPE (µF/cm2) | % IE | θ |
|---|---|---|---|---|---|---|
| - | 0 | 2.73 | 66 | 29.07 | - | - |
| Dex | 100 | 1.69 | 144 | 13.15 | 54 | 0.54 |
| 200 | 2.31 | 236 | 10.34 | 72 | 0.72 | |
| 300 | 3.40 | 300 | 9.31 | 78 | 0.78 | |
| 400 | 4.97 | 367 | 8.21 | 82 | 0.82 | |
| 500 | 7.23 | 388 | 7.37 | 83 | 0.83 | |
| Inu | 100 | 3.05 | 154 | 12.76 | 57 | 0.57 |
| 200 | 2.16 | 287 | 9.66 | 77 | 0.77 | |
| 300 | 0.82 | 367 | 8.14 | 82 | 0.82 | |
| 400 | 4.07 | 413 | 7.51 | 84 | 0.84 | |
| 500 | 3.42 | 471 | 7.06 | 86 | 0.86 |
| 1.0 M HCl + | Inh. Conc. (mg/L) | Temperature (°K) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 288 | 298 | 308 | 318 | ||||||||||
| CR (mpy) | % IE | θ | CR (mpy) | % IE | θ | CR (mpy) | % IE | θ | CR (mpy) | % IE | θ | ||
| - | 0 | 169 | - | - | 224 | - | - | 258 | - | - | 282 | - | - |
| Dex | 100 | 78 | 54 | 0.54 | 114 | 49 | 0.49 | 144 | 44 | 0.44 | 164 | 42 | 0.42 |
| 200 | 47 | 72 | 0.72 | 72 | 68 | 0.68 | 98 | 62 | 0.62 | 113 | 60 | 0.60 | |
| 300 | 32 | 81 | 0.81 | 47 | 79 | 0.79 | 65 | 75 | 0.75 | 82 | 71 | 0.71 | |
| 400 | 22 | 87 | 0.87 | 36 | 84 | 0.84 | 46 | 82 | 0.82 | 68 | 76 | 0.76 | |
| 500 | 20 | 88 | 0.88 | 29 | 87 | 0.87 | 44 | 83 | 0.83 | 59 | 79 | 0.79 | |
| Inu | 100 | 63 | 63 | 0.63 | 92 | 59 | 0.59 | 121 | 53 | 0.53 | 144 | 49 | 0.49 |
| 200 | 39 | 77 | 0.77 | 60 | 73 | 0.73 | 77 | 70 | 0.70 | 99 | 65 | 0.65 | |
| 300 | 24 | 86 | 0.86 | 38 | 83 | 0.83 | 54 | 79 | 0.79 | 76 | 73 | 0.73 | |
| 400 | 17 | 90 | 0.90 | 31 | 86 | 0.86 | 44 | 83 | 0.83 | 62 | 78 | 0.78 | |
| 500 | 14 | 92 | 0.92 | 22 | 90 | 0.90 | 39 | 85 | 0.85 | 59 | 79 | 0.79 | |
| 1.0 M HCl + | Temp. (°K) | 10−3 Kads L mol−1 | ∆Goads kJ mol−1 | ∆Hoads kJ mol−1 | ∆Soads (298) J mol−1 K−1 |
|---|---|---|---|---|---|
| Dex | 288 | 3.86 | −29.39 | −12.82 | 57.53 |
| 298 | 3.12 | −29.82 | 57.05 | ||
| 308 | 2.56 | −30.32 | 56.82 | ||
| 318 | 2.36 | −31.17 | 57.70 | ||
| Inu | 288 | 7.03 | −30.82 | −9.64 | 73.54 |
| 298 | 6.01 | −31.45 | 73.19 | ||
| 308 | 5.31 | −32.19 | 73.21 | ||
| 318 | 4.81 | −33.06 | 73.65 |
| 1.0 M HCl + | Inh. Conc. (mg/L) | Ea* kJ mol−1 | ∆H* kJ mol−1 | ∆S* J mol−1 K−1 |
|---|---|---|---|---|
| - | 0 | 12.88 | 10.35 | −81.10 |
| Dex | 100 | 18.87 | 16.39 | −95.67 |
| 200 | 22.53 | 20.01 | −95.59 | |
| 300 | 24.02 | 21.55 | −94.01 | |
| 400 | 27.69 | 25.12 | −84.03 | |
| 500 | 27.93 | 25.37 | −91.10 | |
| Inu | 100 | 21.03 | 18.69 | −98.17 |
| 200 | 23.28 | 20.80 | −94.84 | |
| 300 | 29.09 | 26.54 | −79.02 | |
| 400 | 32.42 | 29.78 | −69.86 | |
| 500 | 37.07 | 34.69 | −55.29 |
| Inh. Conc. (mg/L) | Dex | Inu | ||
|---|---|---|---|---|
| 103 k1, h−1 | t1/2, h | 103 k1, h−1 | t1/2, h | |
| 0 | 84 | 8.25 | 84 | 8.25 |
| 100 | 77 | 9.01 | 74 | 9.36 |
| 200 | 74 | 9.36 | 76 | 9.12 |
| 300 | 70 | 9.90 | 73 | 9.49 |
| 400 | 66 | 10.51 | 72 | 9.63 |
| 500 | 63 | 11.02 | 66 | 10.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toghan, A.; Fawzy, A. Unraveling the Adsorption Mechanism and Anti-Corrosion Functionality of Dextrin and Inulin as Eco-Friendly Biopolymers for the Corrosion of Reinforced Steel in 1.0 M HCl: A Thermodynamic and Kinetic Approach. Polymers 2023, 15, 3144. https://doi.org/10.3390/polym15143144
Toghan A, Fawzy A. Unraveling the Adsorption Mechanism and Anti-Corrosion Functionality of Dextrin and Inulin as Eco-Friendly Biopolymers for the Corrosion of Reinforced Steel in 1.0 M HCl: A Thermodynamic and Kinetic Approach. Polymers. 2023; 15(14):3144. https://doi.org/10.3390/polym15143144
Chicago/Turabian StyleToghan, Arafat, and Ahmed Fawzy. 2023. "Unraveling the Adsorption Mechanism and Anti-Corrosion Functionality of Dextrin and Inulin as Eco-Friendly Biopolymers for the Corrosion of Reinforced Steel in 1.0 M HCl: A Thermodynamic and Kinetic Approach" Polymers 15, no. 14: 3144. https://doi.org/10.3390/polym15143144
APA StyleToghan, A., & Fawzy, A. (2023). Unraveling the Adsorption Mechanism and Anti-Corrosion Functionality of Dextrin and Inulin as Eco-Friendly Biopolymers for the Corrosion of Reinforced Steel in 1.0 M HCl: A Thermodynamic and Kinetic Approach. Polymers, 15(14), 3144. https://doi.org/10.3390/polym15143144






