Molecularly Imprinted Polymers Specific towards 4-Borono-L-phenylalanine—Synthesis Optimization, Theoretical Analysis, Morphology Investigation, Cytotoxicity, and Release Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Polymers
2.2.1. Bulk Polymer
2.2.2. Synthesis of Siloxane Particles
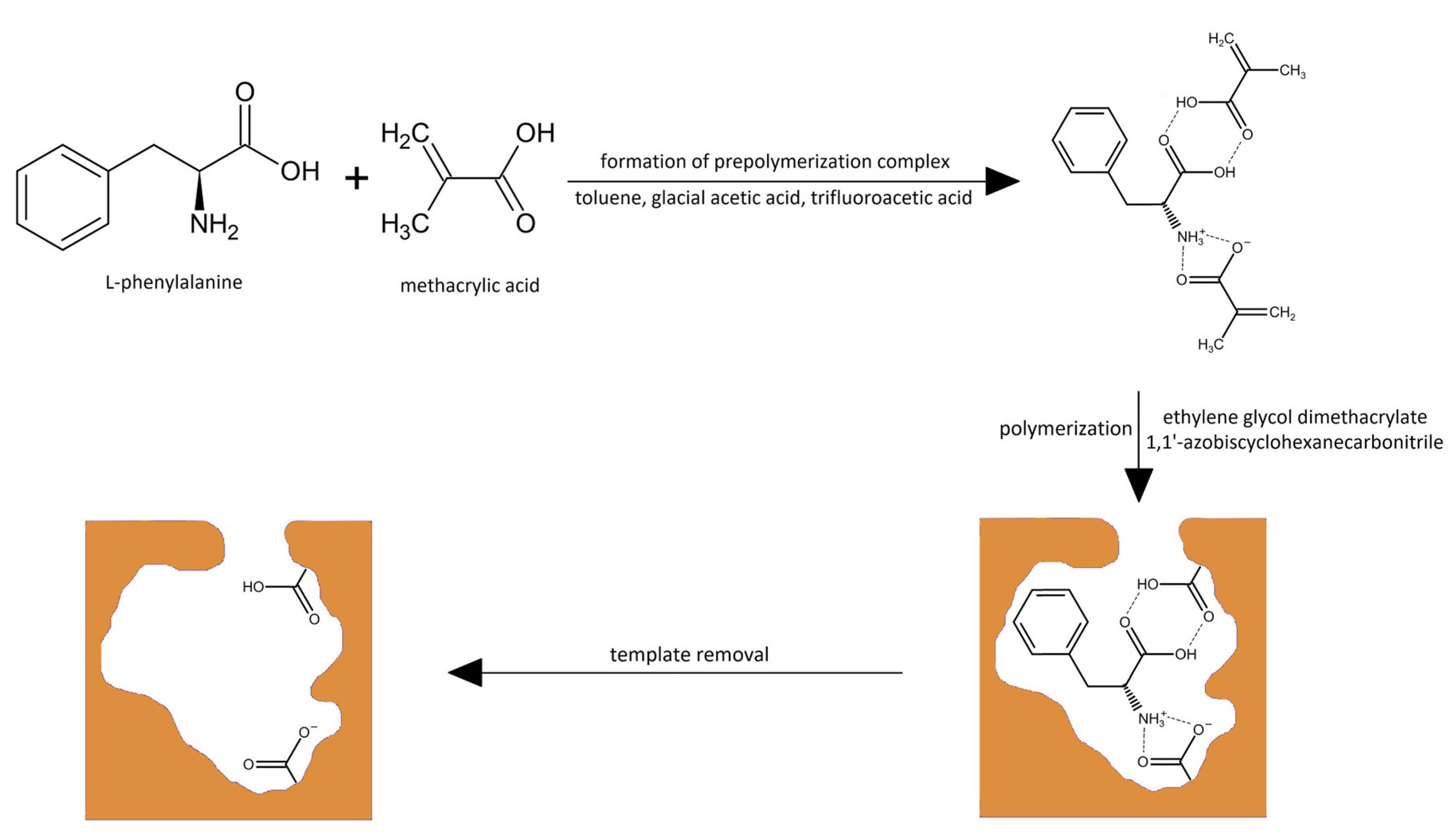
2.2.3. Honeycomb-like Polymers
2.3. Binding Studies
2.4. Release Studies
2.5. Instrumentation
2.6. Theoretical Analysis
2.7. Cytotoxicity Tests
3. Results and Discussion
3.1. Synthesis of the Materials
3.1.1. Theoretical Choice of Functional Monomer
3.1.2. Verification of Effectiveness of MIP with Bulk Polymer
3.1.3. Theoretical Explanation of Interactions in the MIP Cavity
3.1.4. Fabrication of Honeycomb-like Polymer and Adsorption Capabilities
3.2. Characterization of Honeycomb-like Polymer
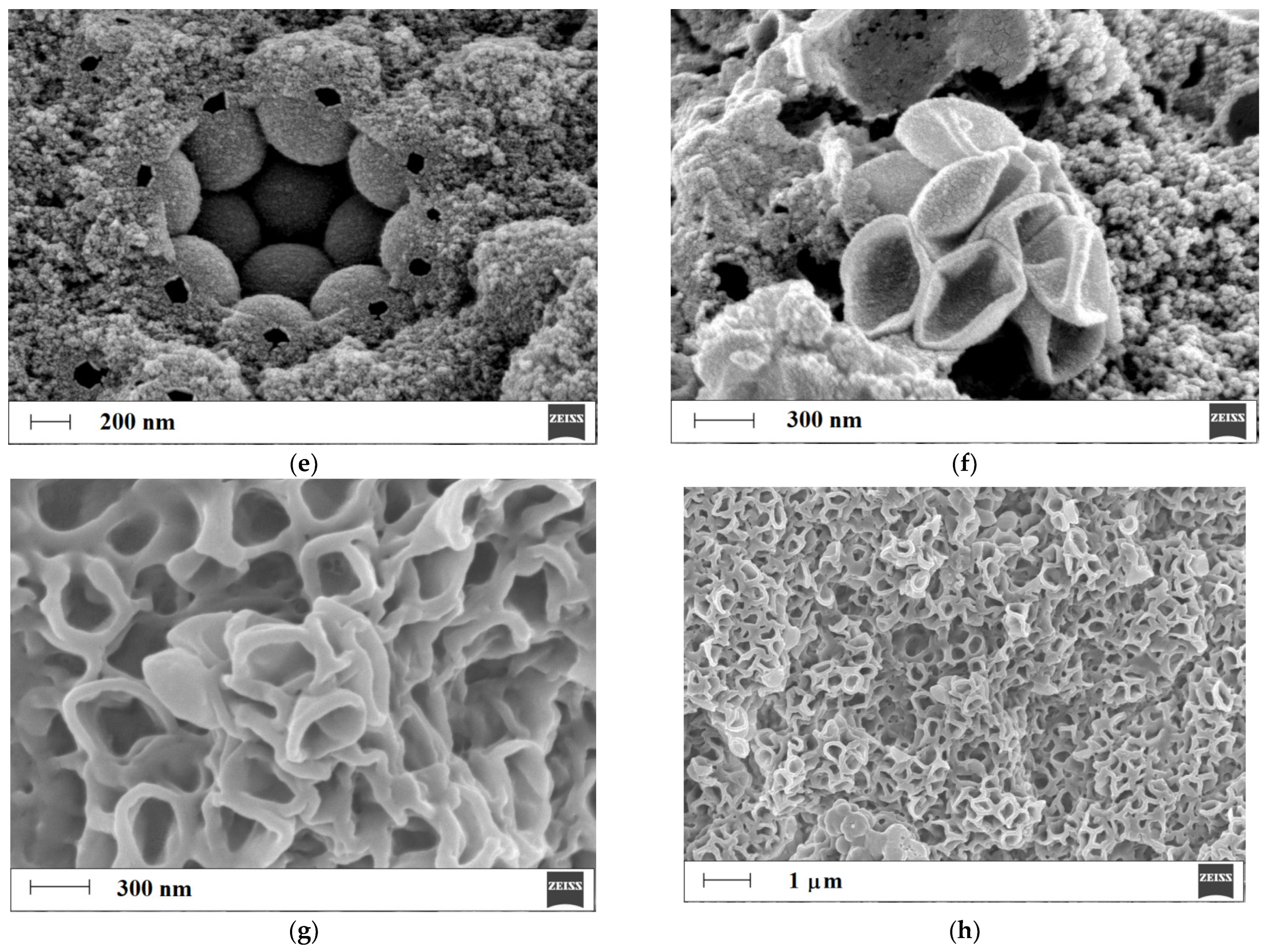
3.2.1. Morphological Studies
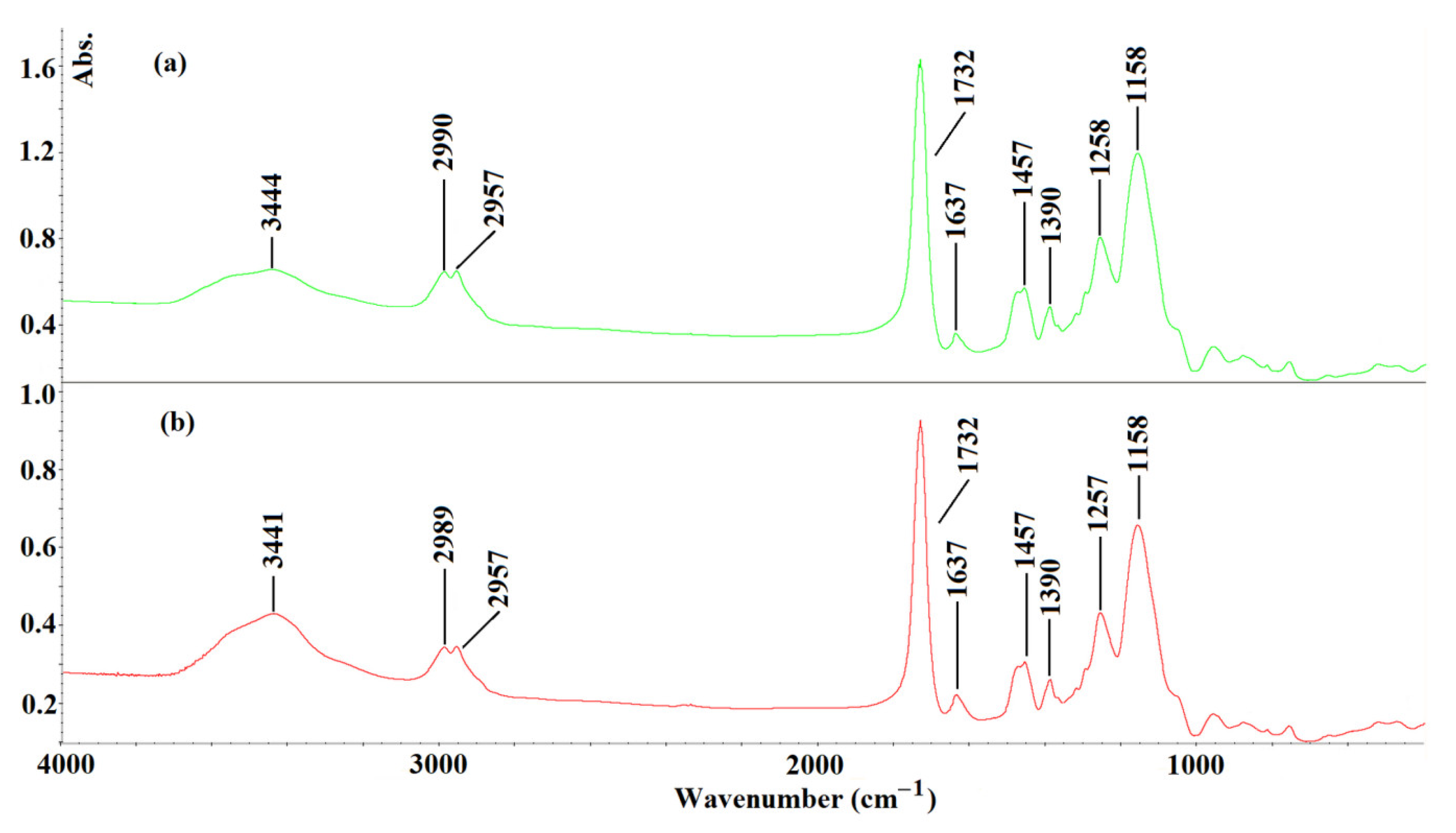
3.2.2. Structural Analysis
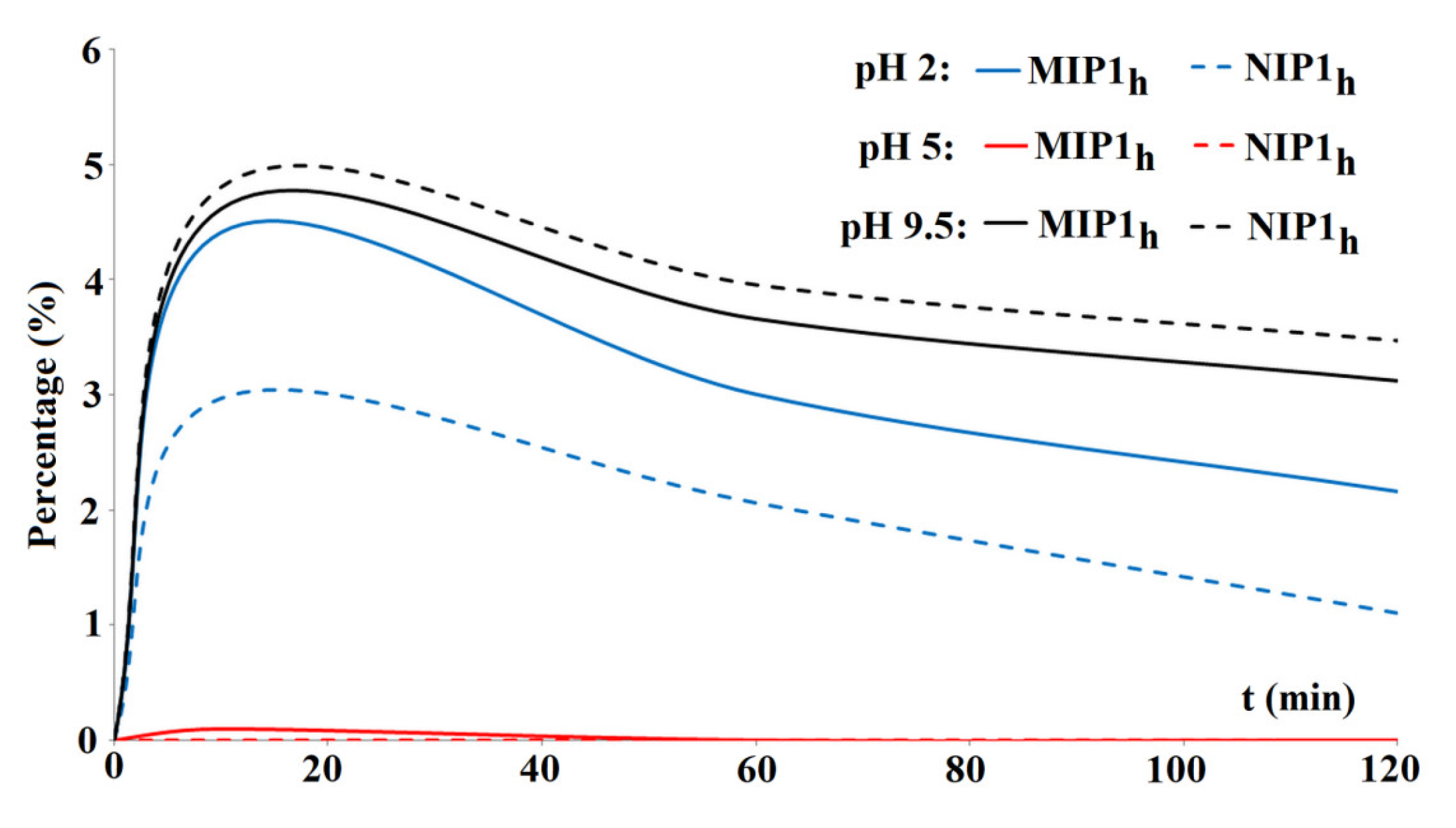
3.3. Release Studies
3.4. Cytotoxicity Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron Neutron Capture Therapy: Current Status and Future Perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Mi, P.; Yang, W. Boron Delivery Agents for Neutron Capture Therapy of Cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Bendel, P.; Wittig, A.; Basilico, F.; Mauri, P.L.; Sauerwein, W. Metabolism of Borono-Phenylalanine–Fructose Complex (BPA–Fr) and Borocaptate Sodium (BSH) in Cancer Patients—Results from EORTC trial 11001. J. Pharm. Biomed. Anal. 2010, 51, 284–287. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron Agents for Neutron Capture Therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Kiger, W.S.; Palmer, M.R.; Riley, K.J.; Zamenhof, R.G.; Busse, P.M. Pharamacokinetic Modeling for Boronophenylalanine-Fructose Mediated Neutron Capture Therapy: 10B Concentration Predictions and Dosimetric Consequences. J. Neurooncol. 2003, 62, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Wongthai, P.; Hagiwara, K.; Miyoshi, Y.; Wiriyasermkul, P.; Wei, L.; Ohgaki, R.; Kato, I.; Hamase, K.; Nagamori, S.; Kanai, Y. Boronophenylalanine, a Boron Delivery Agent for Boron Neutron Capture Therapy, Is Transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015, 106, 279–286. [Google Scholar] [CrossRef]
- Mi, P.; Yanagie, H.; Dewi, N.; Yen, H.-C.; Liu, X.; Suzuki, M.; Sakurai, Y.; Ono, K.; Takahashi, H.; Cabral, H.; et al. Block Copolymer-Boron Cluster Conjugate for Effective Boron Neutron Capture Therapy of Solid Tumors. J. Control. Release 2017, 254, 1–9. [Google Scholar] [CrossRef]
- Nomoto, T.; Inoue, Y.; Yao, Y.; Suzuki, M.; Kanamori, K.; Takemoto, H.; Matsui, M.; Tomoda, K.; Nishiyama, N. Poly(Vinyl Alcohol) Boosting Therapeutic Potential of p -Boronophenylalanine in Neutron Capture Therapy by Modulating Metabolism. Sci. Adv. 2020, 6, eaaz1722. [Google Scholar] [CrossRef]
- Chauhan, N.P.S.; Hosmane, N.S.; Mozafari, M. Boron-Based Polymers: Opportunities and Challenges. Mater. Today Chem. 2019, 14, 100184. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Janczura, M.; Luliński, P.; Sobiech, M. Imprinting Technology for Effective Sorbent Fabrication: Current State-of-Art and Future Prospects. Materials 2021, 14, 1850. [Google Scholar] [CrossRef] [PubMed]
- Shahhoseini, F.; Azizi, A.; Bottaro, C.S. A Critical Evaluation of Molecularly Imprinted Polymer (MIP) Coatings in Solid Phase Microextraction Devices. TrAC Trends Anal. Chem. 2022, 156, 116695. [Google Scholar] [CrossRef]
- Sobiech, M.; Luliński, P.; Wieczorek, P.P.; Marć, M. Quantum and Carbon Dots Conjugated Molecularly Imprinted Polymers as Advanced Nanomaterials for Selective Recognition of Analytes in Environmental, Food and Biomedical Applications. TrAC Trends Anal. Chem. 2021, 142, 116306. [Google Scholar] [CrossRef]
- Luliński, P. Molecularly Imprinted Polymers Based Drug Delivery Devices: A Way to Application in Modern Pharmacotherapy. A Review. Mater. Sci. Eng. C 2017, 76, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Tuwahatu, C.A.; Yeung, C.C.; Lam, Y.W.; Roy, V.A.L. The Molecularly Imprinted Polymer Essentials: Curation of Anticancer, Ophthalmic, and Projected Gene Therapy Drug Delivery Systems. J. Control. Release 2018, 287, 24–34. [Google Scholar] [CrossRef]
- Haupt, K.; Medina Rangel, P.X.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef]
- Hroboňová, K.; Vybohová, V.; Lomenova, A.; Špačková, A.; Svitková, V. Characterization of Kinetic, Thermodynamic, and Binding Properties of L-Phenylalanine Molecularly Imprinted Polymer. Monatsh. Chem. 2022, 153, 1037–1047. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Janczura, M.; Sobiech, M.; Luliński, P. Insight into the Morphology, Pore Structure and Sorption Properties of 4-hydroxy-3-nitrophenylacetic Acid Imprinted Poly(Acrylic Acid-Co-Ethylene Glycol Dimethacrylate) Sorbent. Polym. Test. 2021, 93, 106983. [Google Scholar] [CrossRef]
- Sobiech, M.; Giebułtowicz, J.; Luliński, P. Computational and Experimental Studies of Magnetic Molecularly Imprinted Sorbent with High Specificity towards Aceclofenac. Microchem. J. 2023, 186, 108272. [Google Scholar] [CrossRef]
- Frish, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Dassault Systèmes BIOVIA. Discovery Studio Modeling Environment, Release 2021; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Kvamme, B. Thermodynamic Properties and Dielectric Constants in Water/Methanol Mixtures by Integral Equation Theory and Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2002, 4, 942–948. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Karim, K.; Piletska, E.V.; Day, C.J.; Freebairn, K.W.; Legge, C.; Turner, A.P.F. Recognition of Ephedrine Enantiomers by Molecularly Imprinted Polymers Designed Using a Computational Approach. Analyst 2001, 126, 1826–1830. [Google Scholar] [CrossRef]
- Gul, S.; Shah, N.; Arain, M.B.; Rahman, N.; Rehan, T.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Fabrication of Magnetic Core Shell Particles Coated with Phenylalanine Imprinted Polymer. Polym. Test. 2019, 75, 262–269. [Google Scholar] [CrossRef]
- Mori, Y.; Suzuki, A.; Yoshino, K.; Kakihana, H. Complex Formation of p-Boronophenylalanine with Some Monosaccharides. Pigment. Cell Res. 1989, 2, 273–277. [Google Scholar] [CrossRef]
- Zhou, J.; Sheth, S.; Zhou, H.; Song, Q. Highly Selective Detection of L-Phenylalanine by Molecularly Imprinted Polymers Coated Au Nanoparticles via Surface-Enhanced Raman Scattering. Talanta 2020, 211, 120745. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Yao, S.; Huang, W.; Xin, Y.; Zhu, M.; Song, H. Highly Selective Recognition of L-phenylalanine with Molecularly Imprinted Polymers Based on Imidazolyl Amino Acid Chiral Ionic Liquid. Chirality 2019, 31, 824–834. [Google Scholar] [CrossRef]
- Widiyanti, P.; Amali, M.A. Aminatun Poly(Ethylene Glycol)Dimethacrylate—Nanofibrillated Cellulose Bionanocomposites as Injectable Hydrogel for Therapy of Herniated Nucleus Pulposus Patients. J. Mater. Res. Technol. 2020, 9, 12716–12722. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, X.; Li, P.; Huang, G.; Feng, S.; Shen, C.; Han, B.; Zhang, X.; Jin, F.; Xu, F.; et al. Bioinspired Engineering of Honeycomb Structure—Using Nature to Inspire Human Innovation. Prog. Mater. Sci. 2015, 74, 332–400. [Google Scholar] [CrossRef]
- Hanemann, T.; Honnef, K. Viscosity and Refractive Index Adjustment of Poly(Methyl Methacrylate-Co-Ethyleneglycol Dimethacrylate) for Application in Microoptics. Polym. Adv. Technol. 2015, 26, 294–299. [Google Scholar] [CrossRef]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Kennedy, J.E.; Higginbotham, C.L. Mechanical Properties and Thermal Behaviour of PEGDMA Hydrogels for Potential Bone Regeneration Application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, A.; Raffa, V.; Canfarotta, F.; Signore, G.; Piletsky, S.; MacDonald, M.P.; Cuschieri, A. In Vivo Recognition of Human Vascular Endothelial Growth Factor by Molecularly Imprinted Polymers. Nano Lett. 2017, 17, 2307–2312. [Google Scholar] [CrossRef]
- Farzaneh, S.; Asadi, E.; Abdouss, M.; Barghi-Lish, A.; Azodi-Deilami, S.; Khonakdar, H.A.; Gharghabi, M. Molecularly Imprinted Polymer Nanoparticles for Olanzapine Recognition: Application for Solid Phase Extraction and Sustained Release. RSC Adv. 2015, 5, 9154–9166. [Google Scholar] [CrossRef]





| Code of MIP | Cross-Linker (g, mmol) | SiO2@MPS (mg) |
|---|---|---|
| MIP1b | ethylene glycol dimethacrylate (1), 1.98, 10 | without |
| MIP1h | ethylene glycol dimethacrylate (1), 1.98, 10 | 1000 |
| MIP2h | poly(ethylene) glycol dimethacrylate (2), 5.50, 10 | 1000 |
| MIP3h | trimethylolpropane trimethacrylate (3), 3.38, 10 | 1000 |
| MIP4h | triethylene glycol dimethacrylate (4), 2.86, 10 | 1000 |
| MIP5h | divinylbenzene (5), 1.30, 10 | 1000 |
| Functional Monomer | Complexation Energy (∆Ec, kcal mol−1) |
|---|---|
| Methacrylic acid | −75.13 |
| Acrylic acid | −75.07 |
| 2-(Diethylamino)ethyl methacrylate | −72.00 |
| 2-Hydroxyethyl methacrylate | −68.26 |
| N-Allylurea | −61.37 |
| Acrylamide | −60.62 |
| 1,1,1,3,3,3-Hexafluoroisopropyl methacrylate | −59.98 |
| 4-Vinylpyridine | −57.41 |
| 2-Acrylamido-2-methylpropane sulfonic acid | −56.55 |
| N-Allylaniline | −49.63 |
| 2-(Trifluoromethyl)acrylic acid | −48.63 |
| 1-Allylpiperazine | −40.91 |
| Isopropenylbenzene | −33.62 |
| No. of Polymer | Binding Capacities ± S.D. (B, ng g−1) | Distribution Ratio (Kd, L g−1) | IF | ||
|---|---|---|---|---|---|
| MIP | NIP | MIP | NIP | ||
| 1b | 269 ± 20 | 79.42 ± 0.26 | 0.041 | 0.009 | 4.36 |
| 1h | 330.4 ± 4.6 | 197.4 ± 1.9 | 0.055 | 0.027 | 2.04 |
| 2h | 225 ± 28 | 175 ± 24 | 0.032 | 0.023 | 1.38 |
| 3h | 208 ± 11 | 299 ± 47 | 0.029 | 0.047 | 0.61 |
| 4h | 176.9 ± 1.8 | 249 ± 18 | 0.023 | 0.036 | 0.64 |
| 5h | 130.4 ± 3.2 | 254 ± 34 | 0.016 | 0.037 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcer, E.; Sobiech, M.; Giebułtowicz, J.; Sochacka, M.; Luliński, P. Molecularly Imprinted Polymers Specific towards 4-Borono-L-phenylalanine—Synthesis Optimization, Theoretical Analysis, Morphology Investigation, Cytotoxicity, and Release Studies. Polymers 2023, 15, 3149. https://doi.org/10.3390/polym15143149
Balcer E, Sobiech M, Giebułtowicz J, Sochacka M, Luliński P. Molecularly Imprinted Polymers Specific towards 4-Borono-L-phenylalanine—Synthesis Optimization, Theoretical Analysis, Morphology Investigation, Cytotoxicity, and Release Studies. Polymers. 2023; 15(14):3149. https://doi.org/10.3390/polym15143149
Chicago/Turabian StyleBalcer, Emilia, Monika Sobiech, Joanna Giebułtowicz, Małgorzata Sochacka, and Piotr Luliński. 2023. "Molecularly Imprinted Polymers Specific towards 4-Borono-L-phenylalanine—Synthesis Optimization, Theoretical Analysis, Morphology Investigation, Cytotoxicity, and Release Studies" Polymers 15, no. 14: 3149. https://doi.org/10.3390/polym15143149
APA StyleBalcer, E., Sobiech, M., Giebułtowicz, J., Sochacka, M., & Luliński, P. (2023). Molecularly Imprinted Polymers Specific towards 4-Borono-L-phenylalanine—Synthesis Optimization, Theoretical Analysis, Morphology Investigation, Cytotoxicity, and Release Studies. Polymers, 15(14), 3149. https://doi.org/10.3390/polym15143149






