Production of Graft Copolymers of Cod Collagen with Butyl Acrylate and Vinyl Butyl Ether in the Presence of Triethylborane—Prospects for Use in Regenerative Medicine
Abstract
:1. Introduction
- To adapt previously determined conditions for obtaining graft copolymers of collagen with methyl methacrylate (MMA) and acrylamide (AA) [18] using copolymer BA-VBE grafting;
- To characterize the target product using methods of physical and chemical analysis: gel permeation chromatography (GPC), scanning electron microscopy (SEM), elemental analysis (CHNS), and IR spectroscopy;
- To hydrolyze the CC-PBA-PVBE graft copolymer using collagenase, to isolate the grafted synthetic fragment, and to analyze it using GPC and Fourier-transform infrared spectroscopy (FTIR);
- To evaluate the cytotoxicity of the grafted copolymer using the MTT assay method.
2. Materials and Methods
2.1. Materials
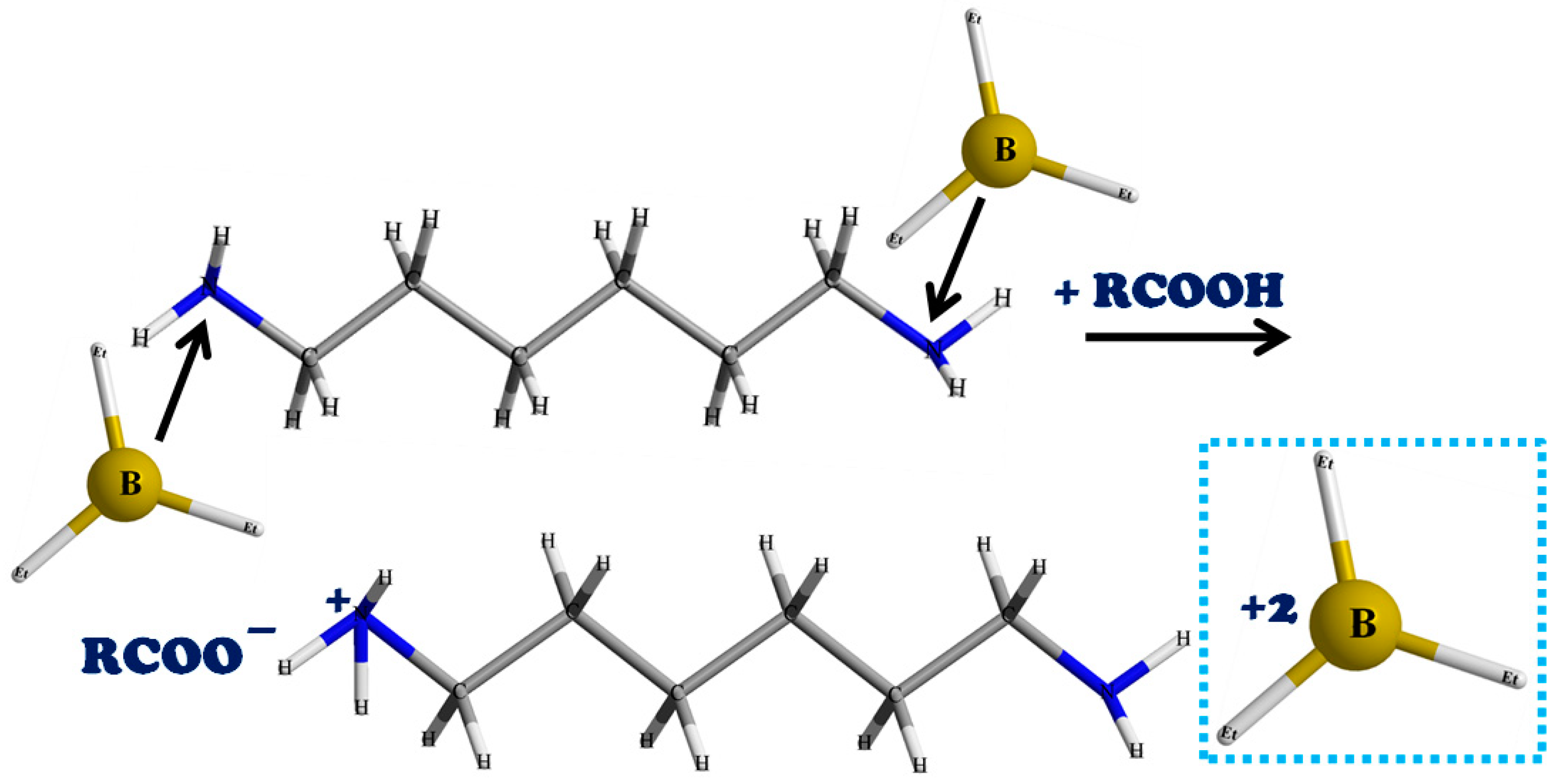
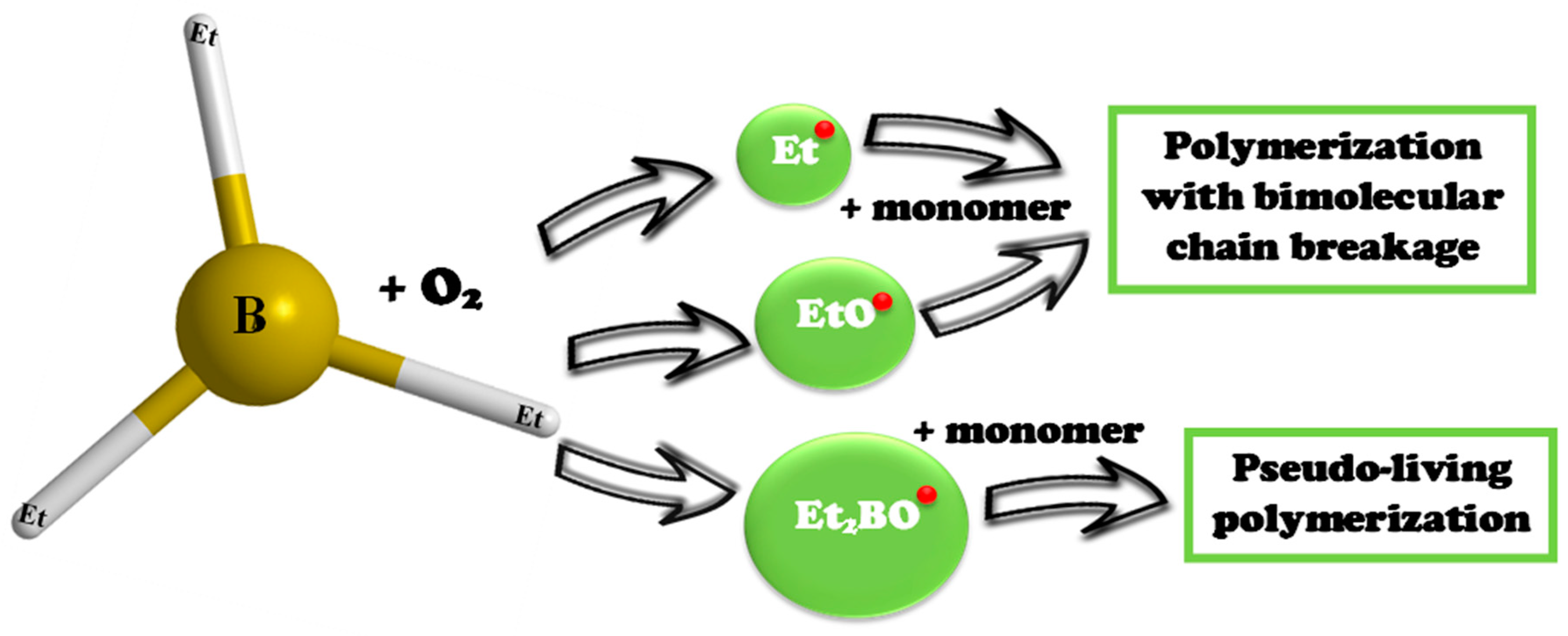
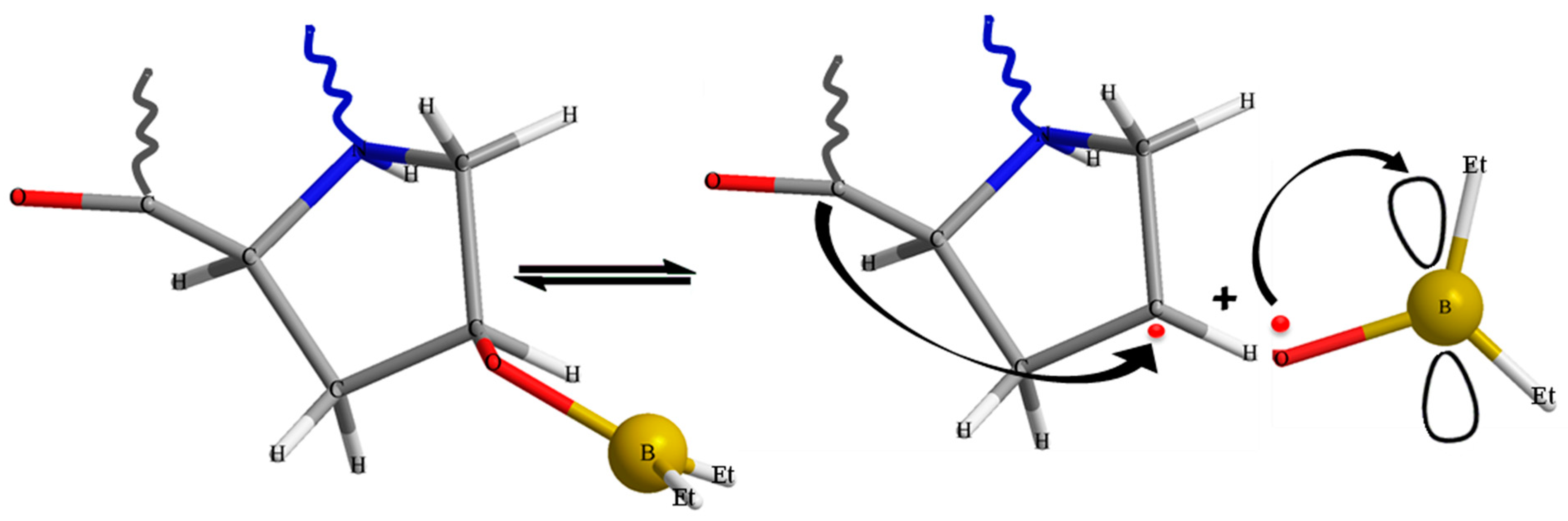
2.2. Synthesis of the Graft Copolymer CC-PBA-PVBE
2.3. Enzymatic Hydrolysis
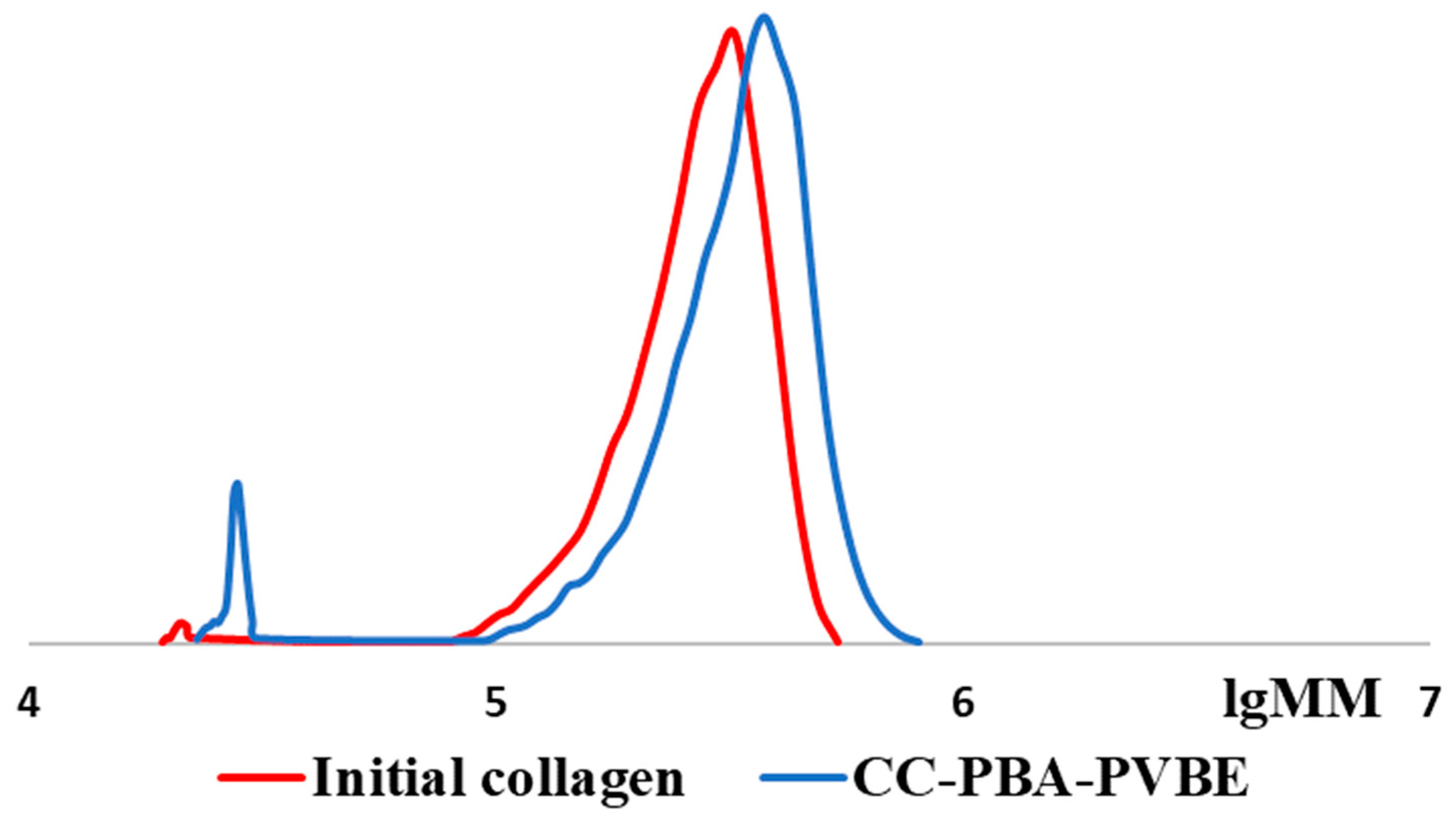
2.4. Gel-Penetrating Chromatography
2.5. FTIR Spectroscopy
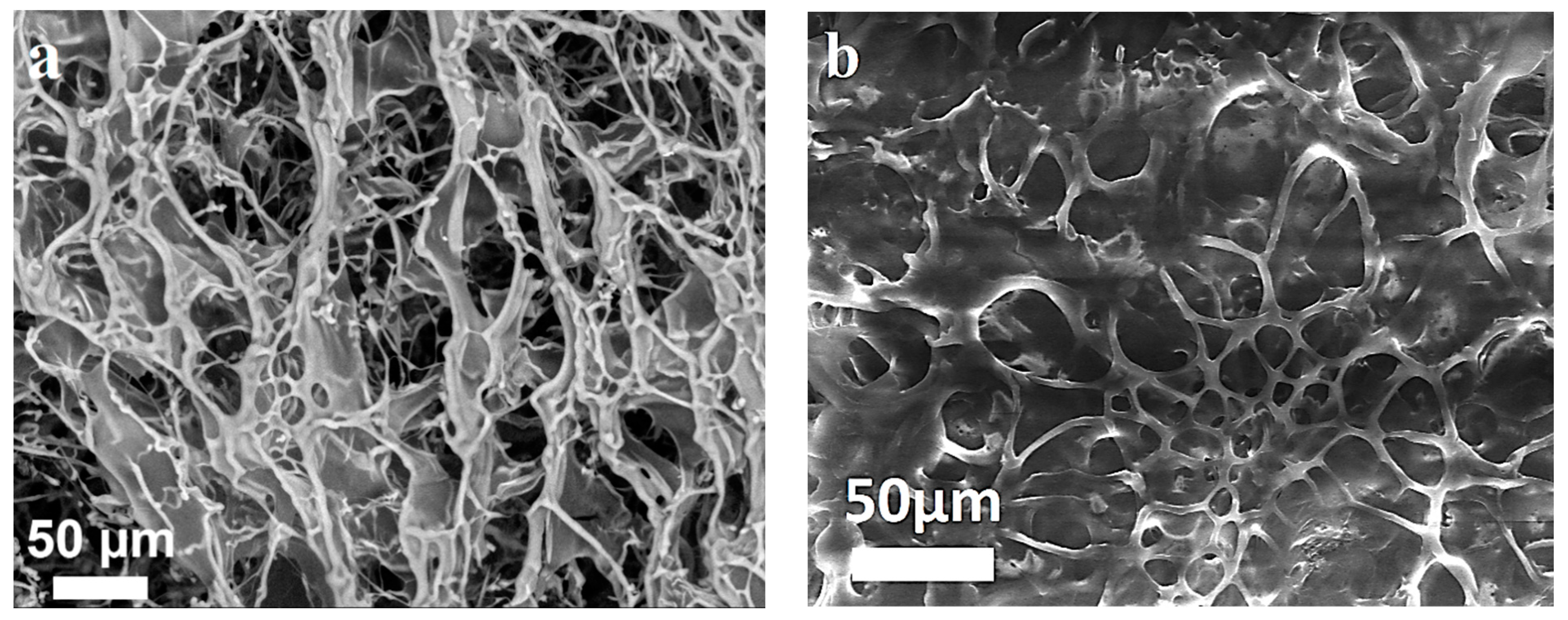
2.6. Scanning Electron Microscopy
2.7. Elemental Analysis
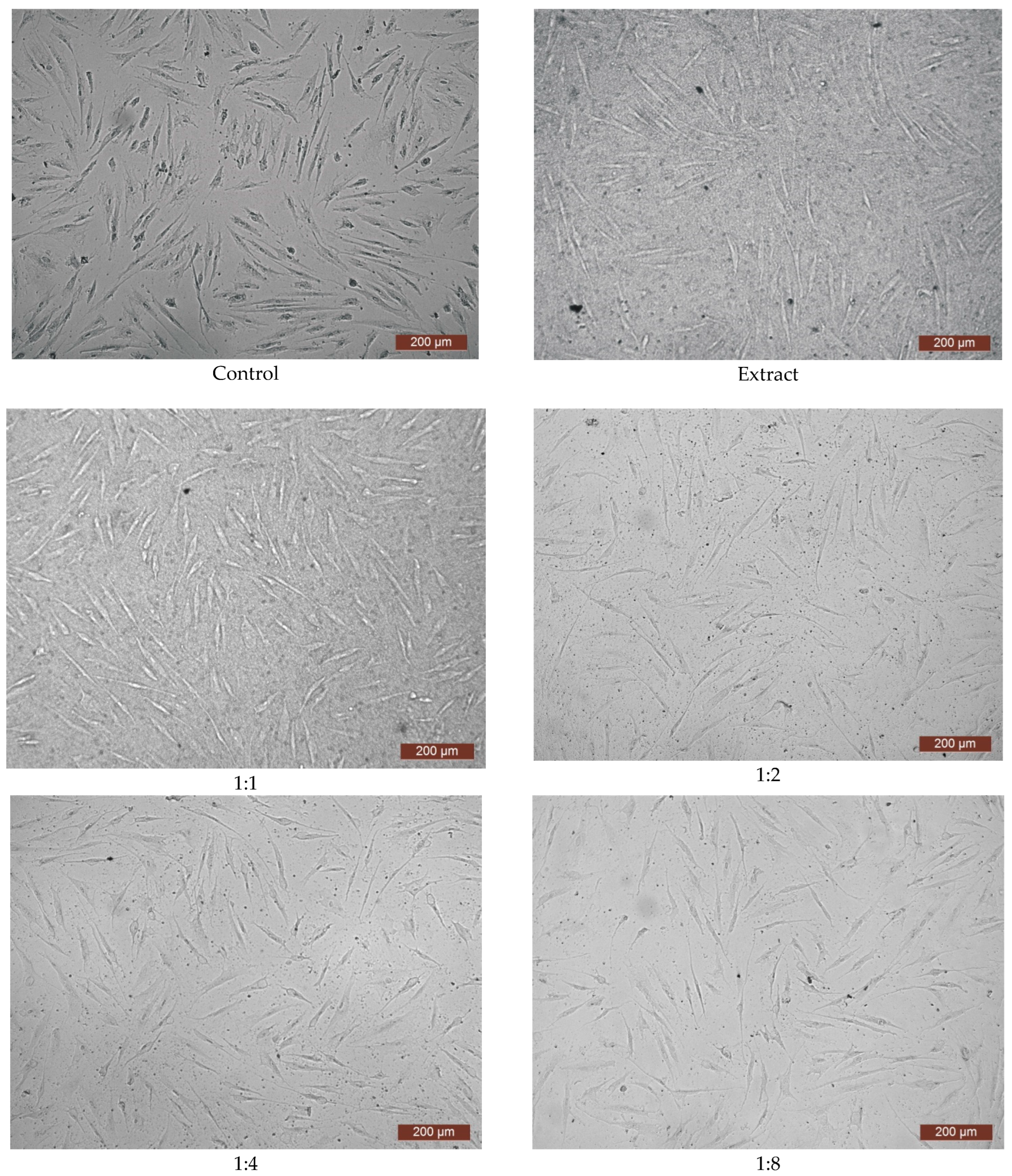
2.8. Cytotoxicity Examination via MTT Assay
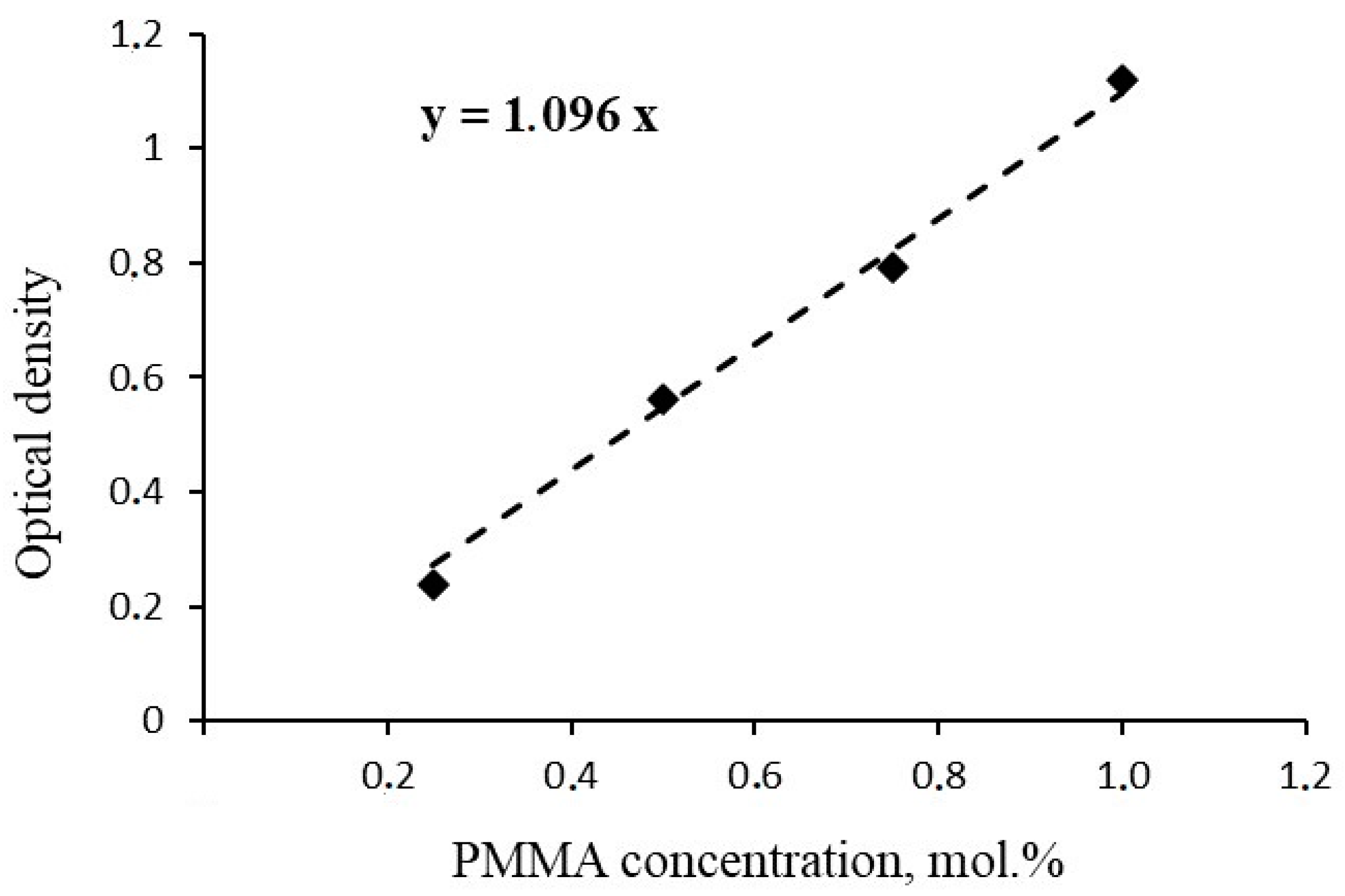
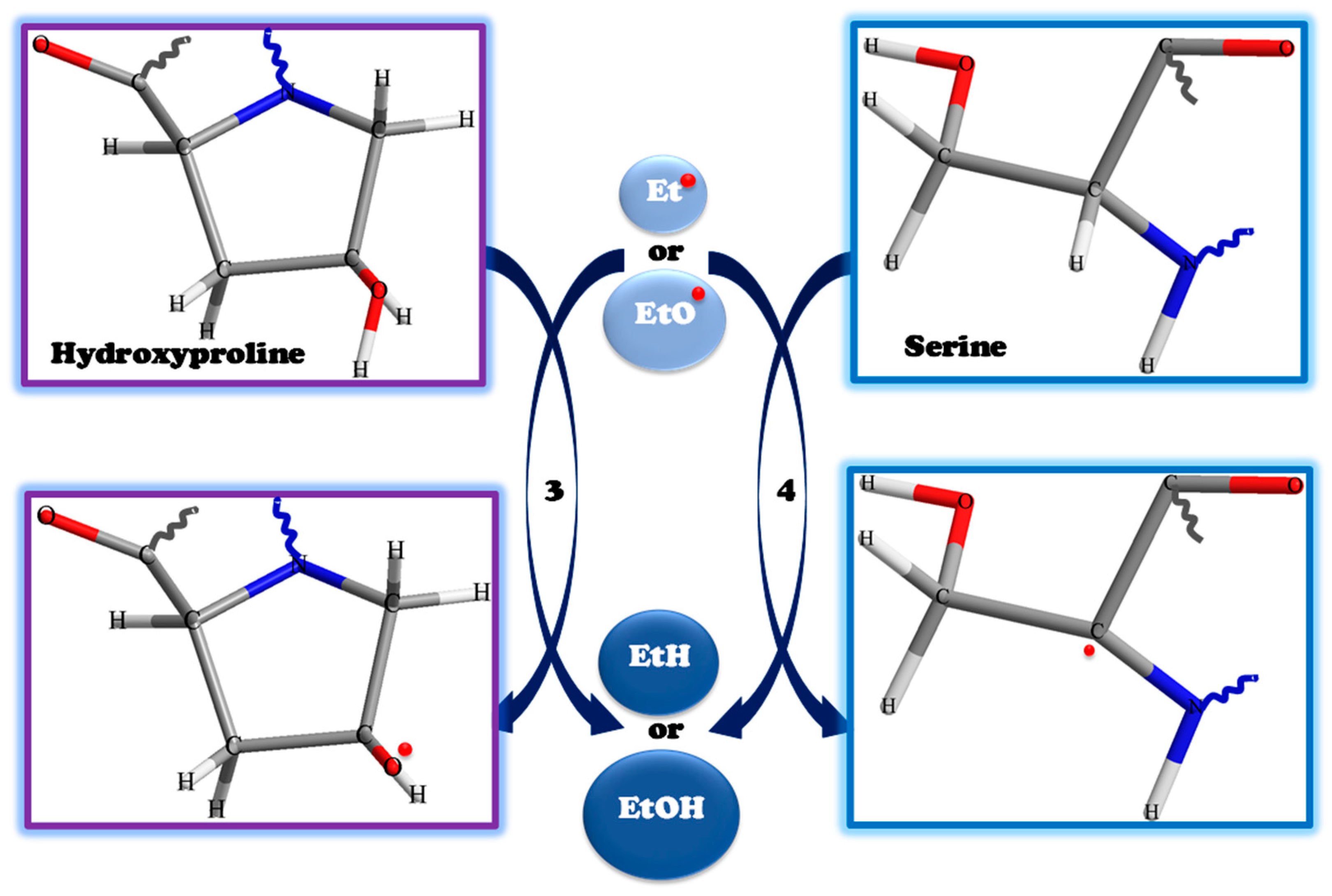
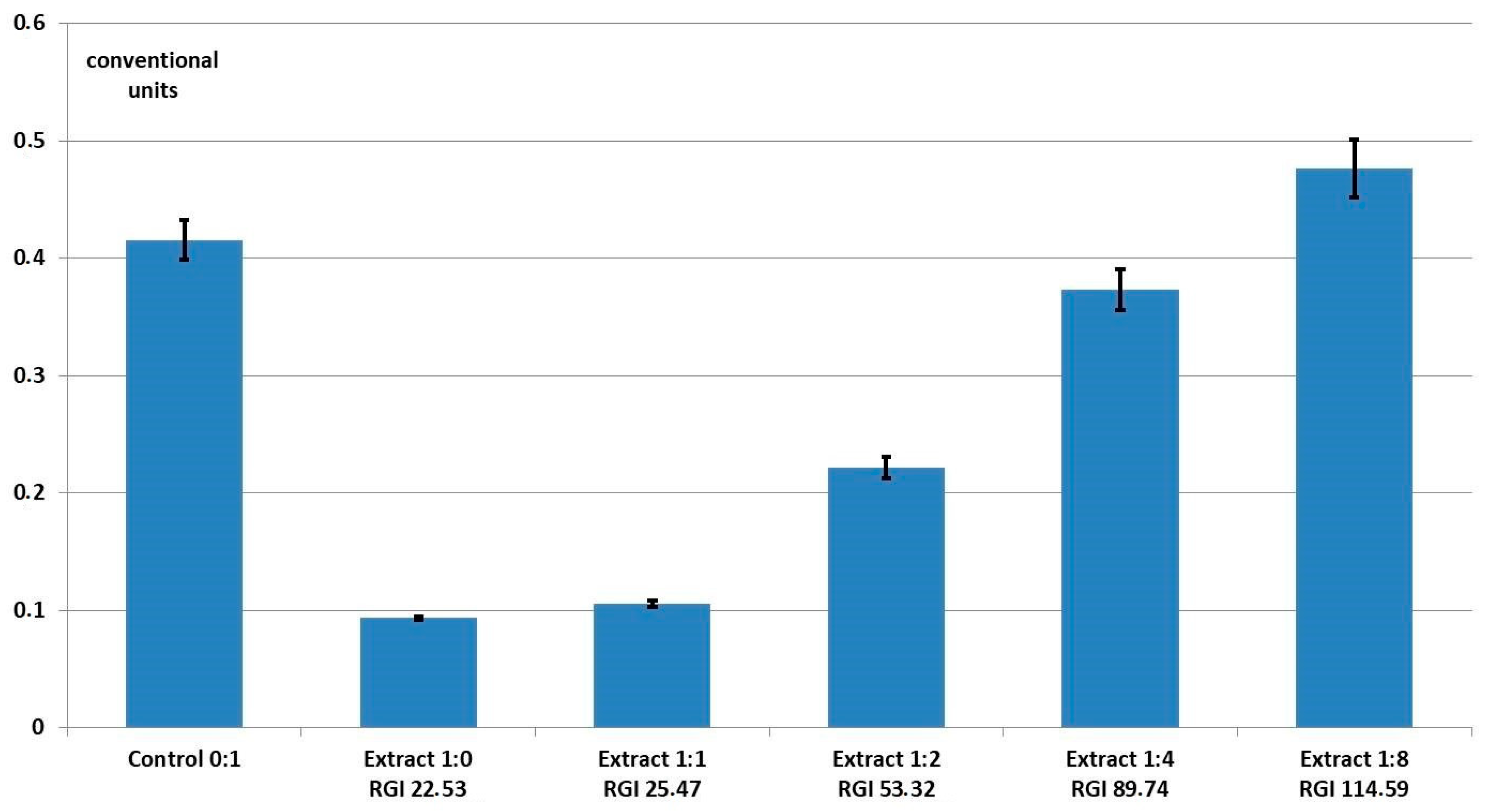
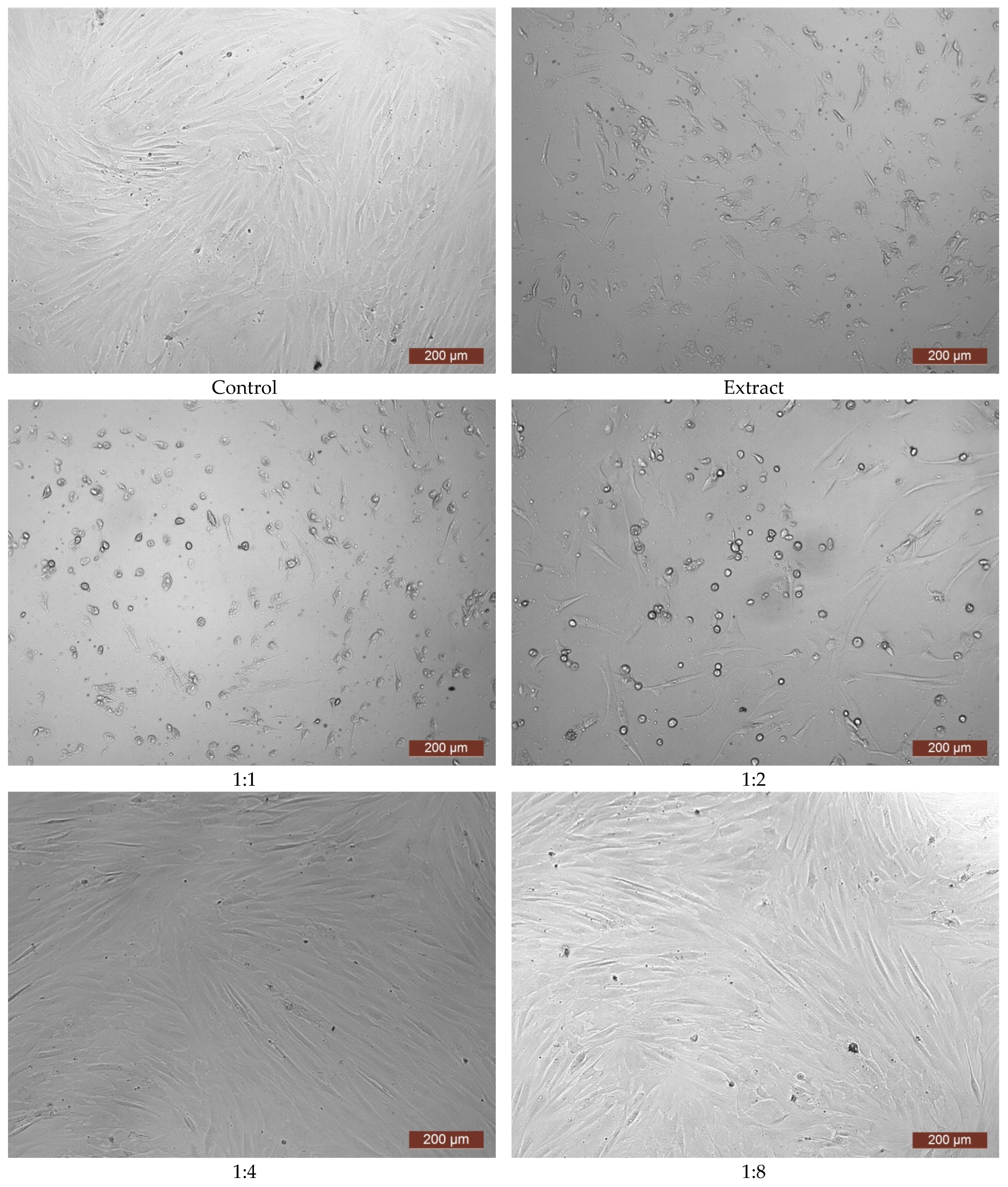
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manjari, M.S.; Aaron, K.P.; Muralidharan, C.; Rose, C. Highly biocompatible novel polyphenol cross-linked collagen scaffold for potential tissue engineering applications. React. Funct. Polym. 2020, 153, 104630. [Google Scholar] [CrossRef]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2021, 74, 92–103. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Marine bioactive peptides as potential antioxidants. Protein Pept. Sci. 2013, 14, 189–198. [Google Scholar] [CrossRef]
- Noohi, P.; Mahdavi, S.S.; Abdekhodaie, M.J.; Nekoofar, M.H.; Baradaran-Rafii, A. Photoreactive hydrogels based on type I collagen extracted from different sources as scaffolds for tissue engineering applications: A comparative study. Materialia 2023, 27, 101651. [Google Scholar] [CrossRef]
- Fassini, D.; Wilkie, I.C.; Pozzolini, M.; Ferrario, C.; Sugni, M.; Rocha, M.S.; Giovine, M.; Bonasoro, F.; Silva, T.H.; Reis, R.L. Diverse and productive source of biopolymer inspiration: Marine collagens. Biomacromolecules 2021, 22, 1815–1834. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Qiao, Y.; Tian, Y.; Liu, J.; Qin, S.; Wu, W. Extraction and characterization of type I collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process Biochem. 2018, 74, 156–163. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Furtado, M.; Chen, L.; Chen, Z.; Chen, A.; Cui, W. Development of fish collagen in tissue regeneration and drug delivery. Eng. Regen. 2022, 3, 217–231. [Google Scholar] [CrossRef]
- Ramanathan, G.; Jeyakumar, G.F.S.; Fardim, P. Biomimetic cellulose/collagen/silk fibroin as a highly interconnected 3D hybrid matrix for bone tissue engineering. Process Biochem. 2023, 129, 150–158. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Gelinsky, M.; Williams, D.S.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Marine collagen-chitosan-fucoidan/chondroitin sulfate cryo-biomaterials loaded with primary human cells envisaging cartilage tissue engineering. Int. J. Biol. Macromol. 2023, 241, 124510. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, X.; Li, S.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Preparation of aminated fish scale collagen and oxidized sodium alginate hybrid hydrogel for enhanced full-thickness wound healing. Int. J. Biol. Macromol. 2020, 164, 626–637. [Google Scholar] [CrossRef]
- Huang, W.-H.; Ding, S.-L.; Zhao, X.-Y.; Li, K.; Guo, H.-T.; Zhang, M.-Z.; Gu, Q. Collagen for neural tissue engineering: Materials, strategies, and challenges. Mater. Today Bio 2023, 20, 100639. [Google Scholar] [CrossRef]
- Xue, W.; Zhu, J.; Sun, P.; Yang, F.; Wu, H.; Li, W. Permeability of biodegradable film comprising biopolymers derived from marine origin for food packaging application: A review. Trends Food Sci. Technol. 2023, 136, 295–307. [Google Scholar] [CrossRef]
- Kalidas, S.; Sumathi, S. Mechanical, biocompatibility and antibacterial studies of gelatin/polyvinyl alcohol/silkfibre polymeric scaffold for bone tissue engineering. Heliyon 2023, 9, e16886. [Google Scholar] [CrossRef]
- Babić Radić, M.M.; Vukomanović, M.; Nikodinović-Runić, J.; Tomić, S.L. Development of nanographene oxide/2-hydroxyethyl methacrylate/gelatin/alginate and nanotitanium dioxide/2-hydroxyethyl methacrylate/gelatin/alginate polymeric systems for biomedical applications. In Advanced Nanoformulations; Hasnain, M.S., Nayak, A.K., Aminabhavi, T.M., Eds.; Academic Press: London, UK, 2023; Volume 3, pp. 771–810. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Said, N.S.; Yosri, N.; Hawash, H.B.; El-Sherif, D.M.; Abouzid, M.; Abdel-Daim, M.M.; Yaseen, M.; Omar, H.; Shou, Q.; et al. Gelatin nanofibers: Recent insights in synthesis, bio-medical applications and limitations. Heliyon 2023, 9, e16228. [Google Scholar] [CrossRef]
- Imani, F.; Karimi-Soflou, R.; Shabani, I.; Karkhaneh, A. PLA electrospun nanofibers modified with polypyrrole-grafted gelatin as bioactive electroconductive scaffold. Polymer 2021, 218, 123487. [Google Scholar] [CrossRef]
- Kuznetsova, Y.L.; Sustaeva, K.S.; Mitin, A.V.; Zakharychev, E.A.; Egorikhina, M.N.; Chasova, V.O.; Farafontova, E.A.; Kobyakova, I.I.; Semenycheva, L.L. Graft polymerization of acrylamide in an aqueous dispersion of collagen in the presence of tributylborane. Polymers 2022, 14, 4900. [Google Scholar] [CrossRef]
- Tanwar, A.; Kalode, P.; Roshni, V.; Prema, B.K.; Doshi, P.; Ottoor, D. Influence of nanofillers (Ag NPs and C. dots) on the controlled drug release profile of gelatin-grafted-polyacrylamide hydrogel: An in vitro study. Mater. Today Commun. 2023, 35, 105922. [Google Scholar] [CrossRef]
- Semenycheva, L.; Chasova, V.; Fukina, D.; Koryagin, A.; Valetova, N.; Suleymanov, E. Synthesis of polymethyl-methacrylate–collagen-graft copolymer using a complex oxide RbTe1.5W0.5O6 photocatalyst. Polym. Sci. Ser. D 2022, 5, 110–117. [Google Scholar] [CrossRef]
- Chasova, V.; Semenycheva, L.; Egorikhina, M.; Charykova, I.; Linkova, D.; Rubtsova, Y.; Fukina, D.; Koryagin, A.; Valetova, N.; Suleymanov, E. Cod gelatin as an alternative to cod collagen in hybrid materials for regenerative medicine. Macromol. Res. 2022, 30, 212–221. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Egorikhina, M.N.; Chasova, V.O.; Valetova, N.B.; Fukina, D.G.; Sukhareva, A.A.; Ostrosablin, A.N.; Kobyakova, I.I.; Farafontova, E.A.; Rubtsova, Y.P. An environmentally friendly solution related to the use of fish production waste to manufacture new materials for biomedicine. Examines Mar. Biol. Oceanogr. 2022, 5, 000606. [Google Scholar]
- Kuznetsova, Y.; Gushchina, K.; Sustaeva, K.; Mitin, A.; Egorikhina, M.; Chasova, V.; Semenycheva, L. Grafting of methyl methacrylate onto gelatin initiated by tri-butylborane—2,5-di-tert-butyl-p-benzoquinone system. Polymers 2022, 14, 3290. [Google Scholar] [CrossRef]
- Kuznetsova, Y.L.; Gushchina, K.S.; Lobanova, K.S.; Chasova, V.O.; Egorikhina, M.N.; Grigoreva, A.O.; Malysheva, Y.B.; Kuzmina, D.A.; Farafontova, E.A.; Linkova, D.D.; et al. Scaffold chemical model based on collagen—Methyl methacrylate graft copolymers. Polymers 2023, 15, 2618. [Google Scholar] [CrossRef]
- Semenycheva, L.; Chasova, V.; Fukina, D.; Koryagin, A.; Belousov, A.; Valetova, N.; Suleimanov, E. Photocatalytic synthesis of materials for regenerative medicine using complex oxides with β-pyrochlore structure. Life 2023, 13, 352. [Google Scholar] [CrossRef]
- Semenycheva, L.; Chasova, V.; Sukhareva, A.; Fukina, D.; Koryagin, A.; Valetova, N.; Smirnova, O.; Suleimanov, E. New composite materials with cross-linked structures based on grafted copolymers of acrylates on cod collagen. Appl. Sci. 2023, 13, 5455. [Google Scholar] [CrossRef]
- Kojima, K.; Iguchi, S.; Kajima, Y.; Yoshikuni, M. Grafting of methyl methacrylate onto collagen initiated by tributylborane. J. Appl. Polym. Sci. 1983, 28, 87–95. [Google Scholar] [CrossRef]
- Kuznetsova, Y.L.; Morozova, E.A.; Sustaeva, K.S.; Markin, A.V.; Mitin, A.V.; Batenykin, M.A.; Salomatina, E.V.; Shurygina, M.P.; Gushchina, K.S.; Pryazhnikova, M.I.; et al. Tributylborane in the synthesis of graft copolymers of collagen and polymethyl methacrylate. Russ. Chem. Bull. 2022, 71, 389–398. [Google Scholar] [CrossRef]
- Uromicheva, M.A.; Kuznetsova, Y.L.; Valetova, N.B.; Mitin, A.V.; Semenycheva, L.L.; Smirnova, O.N. Synthesis of grafted polybutyl acrylate copolymer on fish collagen. Proc. Univ. Appl. Chem. Biotechnol. 2021, 11, 16–25. [Google Scholar] [CrossRef]
- Ludin, D.V.; Zaitsev, S.D.; Kuznetsova, Y.L.; Markin, A.V.; Mochalova, A.E.; Salomatina, E.V. Starch-graft-poly(methyl acrylate) copolymer: The new approach to synthesis and copolymer characterization. J. Polym. Res. 2017, 24, 117. [Google Scholar] [CrossRef]
- Zhubanov, B.A.; Shaikhutdinov, E.M.; Osadchay, E.F. Simple Vinyl Ethers in Radical Polymerization; Nauka: Alma-Ata, Kazakhstan, SSSR, 1985; p. 158. [Google Scholar]
- Semenycheva, L.L.; Matkivskaya, Y.O.; Pegeeva, Y.O.; Pegeev, N.L.; Valetova, N.B.; Liogon’kaya, T.I.; Kurskii, Y.A. Microstructure of the chain of alkyl (meth)acrylate copolymers with vinyl butyl ether synthesized by the compensation method. Russ. Chem. Bull. 2021, 70, 1798–1803. [Google Scholar] [CrossRef]
- Gimaletdinova, I.A.; Absalyamova, L.R.; Spiridonov, A.V.; Amirov, N.B. Evaluation of the efficacy of altay (balsam tincture) in the treatment of ulcers associated H. PYLORI (Russian). Bull. Contemp. Clin. Med. 2015, 8, 36–40. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Kuznetsova, J.L.; Valetova, N.B.; Geras’kina, E.V.; Tarankova, O.A. Method for Production of Acetic Dispersion of High Molecular Fish Collagen. Patent RF, No. 2567171 C1, 6 October 2014. [Google Scholar]
- Dumaldar, M.N. A theoretical comparison between the simplex method and the basic line search algorithm. Optimization 2014, 65, 1–7. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gopal, B. Antimicrobial and cytotoxicity evaluation of aliovalent substituted hydroxyapatite. Appl. Surf. Sci. 2014, 303, 277–281. [Google Scholar] [CrossRef]
- Sun, B.; Li, C.; Mao, Y.; Qiao, Z.; Jia, R.; Huang, T.; Xu, D.; Yang, W. Distinctive characteristics of collagen and gelatin extracted from Dosidicus gigas skin. Int. J. Food Sci. Technol. 2021, 56, 3443–3454. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Matkivskaia, Y.O.; Valetova, N.B.; Chasova, Y.O.; Pegeeva, N.L.; Eloyan, A.L.; Kursky, Y.A.; Moikin, A.A. Specific features of “compensating” copolymerization of butyl acrylatewith vinyl butyl ether in the presence of triethylboron. Russ. Chem. Bull. 2017, 66, 1660–1664. [Google Scholar] [CrossRef]
- Nakanisi, K. Infrared Spectra and the Structure of Organic Compounds; Maltseva, A.A., Ed.; Mir: Moscow, Russia, 1965; p. 216. [Google Scholar]
- Murphy, S.; Ellis-Hutchings, R.; Finch, L.; Welz, S.; Wiench, K. In vitro genotoxicity studies: N-Butyl acrylate L5178Y mouse lymphoma (TK+/− locus assay), 2-Ethylhexyl acrylate gene mutation assay in Chinese hamster V79 cells, and 2-Ethylhexyl acrylate micronucleus test in human lymphocytes. Data Brief 2018, 20, 316–325. [Google Scholar] [CrossRef]
- Garay-Jimenez, J.C.; Gergeres, D.; Young, A.; Lim, D.V.; Turos, E. Physical properties and biological activity of poly(butyl acrylate-styrene) nanoparticle emulsions prepared with conventional and polymerizable surfactants. Nanomedicine 2009, 5, 443–451. [Google Scholar] [CrossRef] [Green Version]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Butyl-vinyl-ether (accessed on 14 July 2023).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenycheva, L.; Chasova, V.O.; Pegeev, N.L.; Uromicheva, M.A.; Mitin, A.V.; Kuznetsova, Y.L.; Farafontova, E.A.; Rubtsova, Y.P.; Linkova, D.D.; Egorikhina, M.N. Production of Graft Copolymers of Cod Collagen with Butyl Acrylate and Vinyl Butyl Ether in the Presence of Triethylborane—Prospects for Use in Regenerative Medicine. Polymers 2023, 15, 3159. https://doi.org/10.3390/polym15153159
Semenycheva L, Chasova VO, Pegeev NL, Uromicheva MA, Mitin AV, Kuznetsova YL, Farafontova EA, Rubtsova YP, Linkova DD, Egorikhina MN. Production of Graft Copolymers of Cod Collagen with Butyl Acrylate and Vinyl Butyl Ether in the Presence of Triethylborane—Prospects for Use in Regenerative Medicine. Polymers. 2023; 15(15):3159. https://doi.org/10.3390/polym15153159
Chicago/Turabian StyleSemenycheva, Lyudmila, Victoria O. Chasova, Nikita L. Pegeev, Marina A. Uromicheva, Alexander V. Mitin, Yulia L. Kuznetsova, Ekaterina A. Farafontova, Yulia P. Rubtsova, Daria D. Linkova, and Marfa N. Egorikhina. 2023. "Production of Graft Copolymers of Cod Collagen with Butyl Acrylate and Vinyl Butyl Ether in the Presence of Triethylborane—Prospects for Use in Regenerative Medicine" Polymers 15, no. 15: 3159. https://doi.org/10.3390/polym15153159






