Effect of Silicone Oil on Properties and Performance of Ceramizable Styrene-Butadiene Rubber-Based Composites
Abstract
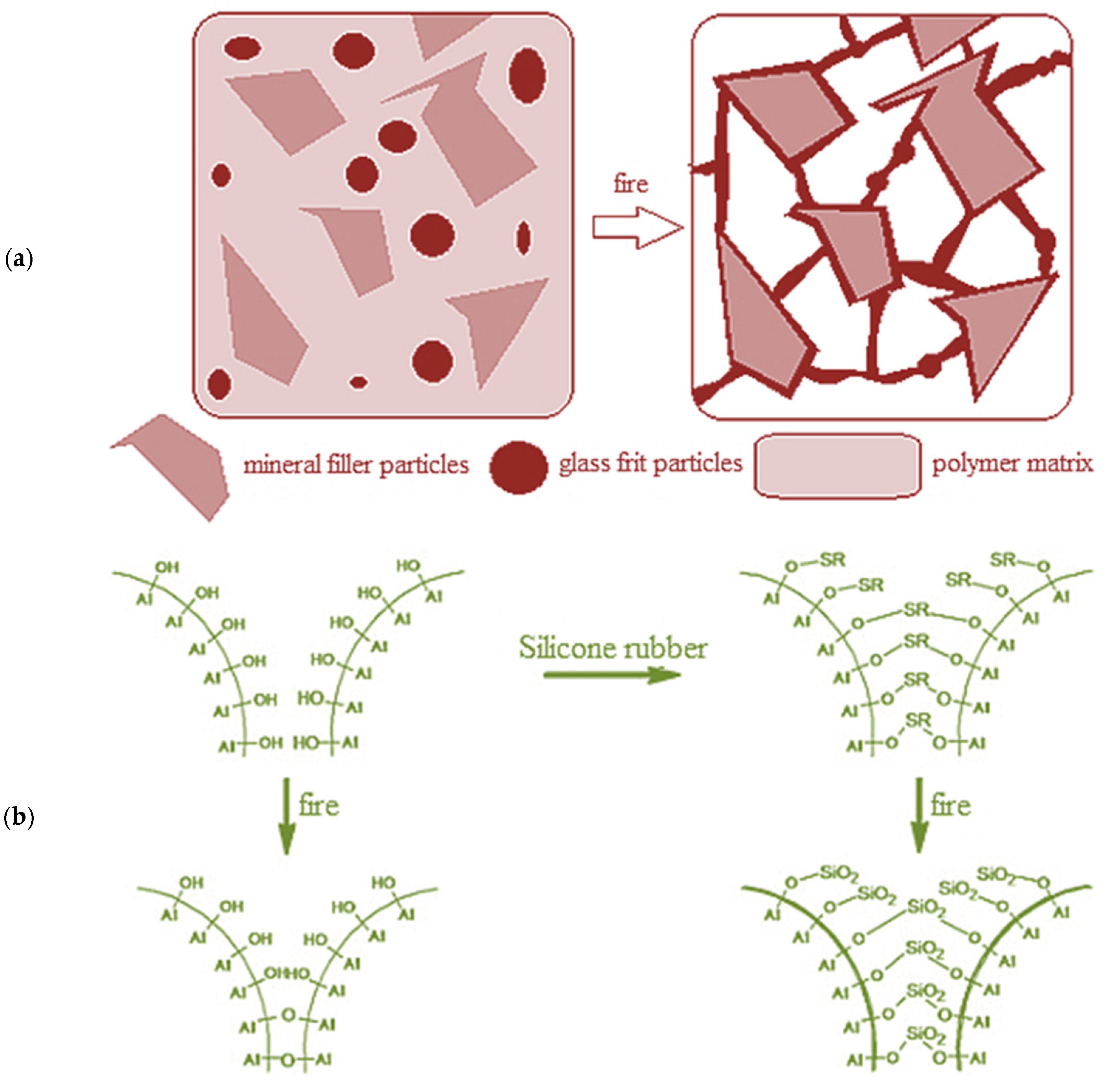
1. Introduction
2. Materials and Methods
2.1. Materials
- Elastomer base—synthesized by emulsion method with styrene-butadiene rubber (KER 1500, Synthos S.A., Oswiecim, Poland). The properties of this elastomer are as follows: Monney viscosity (ML 1 + 4; 100 °C)—45–55 ML, bonded styrene—22–25%, organic acids—5.0–7.5%, volatile matter—max. 0.7%, soap—max. 0.4%, total ash—max. 0.4%.
- Crosslinking agents—2,4-dichlorobenzoyl peroxide (50% paste) or sulfur. For sulfur vulcanization, we used N-cyclohexyl-2-benzothiazole sulfenamide (CBS) as an accelerator and stearic acid and zinc oxide as activators.
- Antioxidant—2,2,4-trimethyl-1,2-dihydroquinoline (TMQ).
- Mineral filler—mica phlogopite (PW30, LKAB Minerals GmbH, Lulea, Sweden) with a specific surface area of 2.8 m2/g.
- Fluxing agent—chemical composition of metal oxides (wt.%): 4 Li2O, 16 Na2O, 37 B2O3, 43 SiO2 (A 4015, Reimbold & Strick GmbH, Cologne, Germany), with softening point temperature of 540 °C.
- Plasticizer—silicone oil (Silikony Polskie Sp. z o. o., Nowa Sarzyna, Poland).
2.2. Preparation of Rubber Samples
2.3. Experimental Techniques
3. Results and Discussion
3.1. Viscoelastic Behavior of Composites
- (a)
- the complex shear modulus G* is equal to , where G′ is the storage shear modulus and G″ is the loss shear modulus;
- (b)
- the complex dynamic viscosity η* is equal to ; ω—frequency (Hz).
3.2. Mechanical Properties of Composites before Ceramization
3.3. Combustibility
3.4. Properties of Composites after Ceramization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Szumera, M. Influence of surface-modified montmorillonites on properties of silicone rubber-based ceramizable composites. J. Therm. Anal. Calorim. 2015, 119, 111–121. [Google Scholar] [CrossRef]
- Guo, J.; Chen, X.; Zhang, Y. Improving the Mechanical and Electrical Properties of Ceramizable Silicone Rubber/Halloysite Composites and Their Ceramic Residues by Incorporation of Different Borates. Polymers 2018, 10, 388. [Google Scholar] [CrossRef]
- Li, P.; Jin, H.; Liu, H. Effects of Bi2O3 on the microstructure, mechanical strength, and dielectric properties of CaZnSi2O6 glass ceramics with machinable precursors. Ceram. Int. 2022, 49, 13933–13939. [Google Scholar] [CrossRef]
- He, C.; Ji, Y.; Pei, D. Influence of platinum on ablation behavior of silicone rubber. Mater. Lett. 2022, 328, 133204. [Google Scholar] [CrossRef]
- Delebecq, E.; Hamdani-Devarennes, S.; Raeke, J.; Lopez Cuesta, J.-M.; Ganachaud, F. High residue contents indebted by platinum and silica synergistic action during the pyrolysis of silicone formulations. ACS Appl. Mater. Interfaces 2011, 3, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Bieliński, D.M.; Anyszka, R.; Pędzich, Z.; Dul, J. Ceramizable silicone rubber-based composites. J. Adv. Mater. Manuf. Charact. 2012, 1, 17–22. [Google Scholar]
- Chen, Y.; Liu, Z.; Huand, Y.; Liang, M.; Zou, H. Catalytic Graphitized Silicone Rubber Coatings with Highly Graphitized Ceramic Layer and Superior Ablation Resistance. Macromol. Mater. Eng. 2022, 307, 2200355. [Google Scholar] [CrossRef]
- Tang, K.; Yu, Y.; Xu, G.; Tang, X.; Zhang, A.; Ge, T.; Li, Y. Preparation of a Ceramifiable Phenolic Foam and Its Ceramization Behavior. Polymers 2022, 14, 1591. [Google Scholar] [CrossRef]
- Lou, F.; Yan, W.; Guo, W.; Wei, T.; Li, Q. Preparation and properties of ceramifiable flame-retarded silicone rubber composites. J. Therm. Anal. Calorim. 2017, 130, 813–821. [Google Scholar] [CrossRef]
- Lou, F.; Cheng, L.; Li, Q.; Wei, T.; Guan, X.; Guo, W. The combination of glass dust and glass fiber as fluxing agents for ceramifiable silicone rubber composites. RSC Adv. 2017, 7, 38805–38811. [Google Scholar] [CrossRef]
- Imiela, M.; Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Zarzecka-Napierała, M.; Szumera, M. Effect of carbon fibers on thermal properties and mechanical strength of ceramizable composites based on silicone rubber. J. Therm. Anal. Calorim. 2016, 124, 197–203. [Google Scholar] [CrossRef]
- Gong, X.; Wu, T.; Ma, J.; Zhao, D.; Shen, Y.; Wang, T. Improved self-supporting property of ceramifying silicone rubber composites by forming crystalline phase at high temperatures. J. Alloys Compd. 2017, 706, 322–329. [Google Scholar] [CrossRef]
- Imiela, M.; Anyszka, R.; Bieliński, D.M.; Lipińska, M.; Rybiński, R.; Syrek, B. Synergistic Effect of Mica, Glass Frit, and Melamine Cyanurate for Improving Fire Resistance of Styrene-Butadiene Rubber Composites Destined for Ceramizable Coatings. Coatings 2019, 9, 170. [Google Scholar] [CrossRef]
- Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Zarzecka-Napierała, M. Effect of mineral fillers type on properties of silicone rubber-based ceramizable composites. Part 1. Kinetics of vulcanization and mechanical properties of composites. Przem. Chem. 2014, 93, 1291–1295. [Google Scholar]
- Marosi, G.; Marton, A.; Anna, P.; Bertalan, G.; Marosfol, B.; Szep, A. Ceramic precursor in flame retardant systems. Polym. Degrad. Stab. 2002, 77, 259–265. [Google Scholar] [CrossRef]
- Imiela, M.; Anyszka, R.; Bieliński, D.M. Polimerowe Kompozyty Ceramizujące. Elastomery 2014, 18, 16–24. [Google Scholar]
- Morgan, A.B.; Chu, L.L.; Harris, J.D. A flammability performance comparison between synthetic and natural clays in polystyrene nanocomposites. Fire Mater. 2005, 29, 213–229. [Google Scholar] [CrossRef]
- PN-ISO 37:1998; Rubber and Thermoplastic Rubber—Determination of Tensile Strength Properties. Polish Committee for Standardization: Warsaw, Poland, 1998.
- Dick, J.S. Rubber Technology Compounding and Testing for Performance; Hanser Publishers: Munich, Germany, 2001. [Google Scholar]
- Gao, T.; Xie, R.; Zhang, L.; Gui, H.; Huang, M. Use of Rubber Process Analyzer for Characterizing the Molecular Weight Parameters of Natural Rubber. Int. J. Polym. Sci. 2015, 2015, 517260. [Google Scholar] [CrossRef]
- Dasgupta, S.; Agrawal, S.L.; Bandyopadhyay, S.; Mukhopadhyay, R. Improved polymer-filler interaction with an ecofriendly processing aid. Part 1. Prog. Rubber Plast. Recycl. Technol. 2009, 25, 141–164. [Google Scholar] [CrossRef]
- Perko, L.; Friesenbichler, W.; Obendrauf, W.; Bauchebner, V.; Chaloupka, G. Elongational viscosity of rubber compounds and improving corresponding models. Adv. Prod. Eng. Manag. 2013, 8, 126–133. [Google Scholar] [CrossRef]
- Lipińska, M.; Imiela, M. Viscoelastic properties and curing of polyhedral oligomeric silsesquioxanes POSS modified ethylene-propylene hydrogenated nitrile rubber EPM/HNBR composites. J. Multidiscip. Eng. Sci. Technol. 2017, 4, 8544–8555. [Google Scholar]
- Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Zarzecka-Napierała, M.; Imiela, M.; Rybiński, R. Processing and Properties of Fire Resistant EPDM Rubber-Based Ceramifiable Composites. High Temp. Mater. Process. 2017, 36, 963–969. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Bradło, D.; Żukowski, W.; Anyszka, R.; Imiela, M. Influence of cenospheric fillers on the thermal properties, ceramisation and flammability of nitrile rubber composites. J. Compos. Mater. 2018, 52, 2815–2827. [Google Scholar] [CrossRef]
- Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Parys, G.; Rybiński, P.; Zarzecka-Napierała, M.; Imiela, M.; Gozdek, T.; Siciński, M.; Okraska, M.; et al. Effect of mineral filler additives on flammability, processing and use of silicone-based ceramifiable composites. Polym. Bull. 2018, 75, 1731–1751. [Google Scholar] [CrossRef]






| A | Composition | |||
|---|---|---|---|---|
| Component | Reference | Peroxide | 10 phr | 15 phr |
| SBR | 100 | 100 | 100 | 100 |
| Mica | 200 | 200 | 200 | 200 |
| Glass frit | 100 | 100 | 100 | 100 |
| Curatives (sulfur-based) | 10 | - | 10 | 10 |
| Peroxide | - | 1.5 | - | - |
| Plasticizer | - | - | 10 | 15 |
| B | Vulcanization parameters | |||
| Scorch time (t05) | 2 min 30 s | 3 min 0 s | 1 min 45 s | 1 min 45 s |
| Torque t05 (dNm) | 4.93 | 5.19 | 3.48 | 2.30 |
| Optimum curing time (t90) | 19 min | 44 min | 5 min | 5 min |
| Torque t90 (dNm) | 28.35 | 23.07 | 16.02 | 12.74 |
| Name of the Composite | Frequency (rad/s) | Low Strain | High Strain | ||||
|---|---|---|---|---|---|---|---|
| G′ (kPa) | G″ (kPa) | η* (Pa·s) | G′ (kPa) | G″ (kPa) | η* (Pa·s) | ||
| Reference | 0.628 | 268.5 | 57.5 | 2746,188 | 62.9 | 60.9 | 875,638 |
| 5.024 | 305.3 | 65.5 | 390,361 | 112.3 | 95.9 | 184,605 | |
| 94.2 | 412.1 | 109.9 | 28,435 | 174.0 | 156.2 | 15,585 | |
| 314 | 471.1 | 122.9 | 9738 | 192.0 | 148.7 | 4857 | |
| Peroxide | 0.628 | 274.7 | 217.2 | 3,502,335 | 55.7 | 55.9 | 803,391 |
| 5.024 | 413.6 | 305.3 | 642,537 | 80.9 | 82.3 | 144,289 | |
| 94.2 | 854.4 | 545.0 | 67,560 | 140.8 | 145.3 | 13,490 | |
| 314 | 1040.0 | 602.0 | 24,033 | 152.8 | 144.6 | 4208 | |
| 10 phr | 0.628 | 253.8 | 193.8 | 3,193,494 | 54.2 | 55.5 | 775,826 |
| 5.024 | 455.4 | 291.9 | 676,193 | 84.8 | 80.8 | 146,383 | |
| 94.2 | 910.7 | 474.2 | 68,452 | 147.6 | 138.5 | 13,496 | |
| 314 | 1085.8 | 427.0 | 23,335 | 163.6 | 143.9 | 4358 | |
| 15 phr | 0.628 | 202.8 | 157.6 | 2,568,287 | 44.2 | 46.3 | 640,604 |
| 5.024 | 394.7 | 245.2 | 580,843 | 71.8 | 72.9 | 127,817 | |
| 94.2 | 816.7 | 421.9 | 61,281 | 133.3 | 126.9 | 12,269 | |
| 314 | 978.1 | 332.7 | 20,663 | 137.2 | 117.8 | 3617 | |
| Parameter | Reference | Peroxide | 10 phr | 15 phr |
|---|---|---|---|---|
| TES (N/mm) | 22 ± 2 | 12 ± 1 | 18 ± 1 | 16 ± 3 |
| SE100 (MPa) | 3.2 ± 0.1 | 4.5 ± 0.4 | 2.6 ± 0.1 | 2.1 ± 0.1 |
| SE200 (MPa) | 3.4 ± 0.1 | - | 2.8 ± 0.1 | 2.3 ± 0.1 |
| SE300 (MPa) | 3.7 ± 0.1 | - | 3.0 ± 0.3 | 2.5 ± 0.6 |
| TS (MPa) | 4.7 ± 0.1 | 4.7 ± 0.4 | 3.3 ± 0.3 | 3.1 ± 0.5 |
| Eb (%) | 449 ± 11 | 148 ± 11 | 330 ± 30 | 345 ± 82 |
| Hardness (°ShD) | 22 ± 1 | 21 ± 1 | 19 ± 1 | 16 ± 1 |
| Parameter | Reference | Peroxide | 10 phr | 15 phr |
|---|---|---|---|---|
| ti (s) | 133 | 77 | 92 | 87 |
| to (s) | 446 | 370 | 386 | 356 |
| HRRp (kW/m) | 112.9 | 183.9 | 192.1 | 162.7 |
| HRRm (kW/m) | 35.6 | 100.8 | 104.9 | 97.7 |
| tHRR (s) | 210 | 190 | 195 | 195 |
| HRRp/tHRR (kW/ms) | 0.54 | 0.97 | 0.99 | 0.83 |
| THR (MJ/m2) | 12.8 | 29.8 | 31.3 | 26.6 |
| EHCp (MJ/kg) | 74.5 | 72.9 | 78.7 | 77.1 |
| EHCm (MJ/kg) | 10.7 | 25.3 | 26.2 | 24.0 |
| MLRp (g/s) | 0.180 | 0.118 | 0.120 | 0.123 |
| MLRm (g/s) | 0.029 | 0.035 | 0.035 | 0.036 |
| MARHE (kW/ms) | 0.13 | 0.26 | 0.24 | 0.26 |
| ml (%) | 24.6 | 24.5 | 24.7 | 24.6 |
| Name of the Composite | Average Maximum Force (N) | ||
|---|---|---|---|
| 1100 °C | 950 °C | 550–1000 °C | |
| Reference | 222 ± 32 | 214 ± 40 | 223 ± 73 |
| Peroxide | 153 ± 47 | 258 ± 73 | 240 ± 49 |
| 10 phr | 123 ± 42 | 214 ± 40 | 226 ± 40 |
| 15 phr | 130 ± 32 | 223 ± 73 | 395 ± 114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imiela, M.; Bieliński, D.M.; Lipińska, M.; Rybiński, P. Effect of Silicone Oil on Properties and Performance of Ceramizable Styrene-Butadiene Rubber-Based Composites. Polymers 2023, 15, 3204. https://doi.org/10.3390/polym15153204
Imiela M, Bieliński DM, Lipińska M, Rybiński P. Effect of Silicone Oil on Properties and Performance of Ceramizable Styrene-Butadiene Rubber-Based Composites. Polymers. 2023; 15(15):3204. https://doi.org/10.3390/polym15153204
Chicago/Turabian StyleImiela, Mateusz, Dariusz M. Bieliński, Magdalena Lipińska, and Przemysław Rybiński. 2023. "Effect of Silicone Oil on Properties and Performance of Ceramizable Styrene-Butadiene Rubber-Based Composites" Polymers 15, no. 15: 3204. https://doi.org/10.3390/polym15153204
APA StyleImiela, M., Bieliński, D. M., Lipińska, M., & Rybiński, P. (2023). Effect of Silicone Oil on Properties and Performance of Ceramizable Styrene-Butadiene Rubber-Based Composites. Polymers, 15(15), 3204. https://doi.org/10.3390/polym15153204








