Chemical Recycling of PET Using Catalysts from Layered Double Hydroxides: Effect of Synthesis Method and Mg-Fe Biocompatible Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Mg-Fe Catalysts
2.2. PET Glycolysis and Catalyst Reuse
2.3. Characterization of Mg-Fe Materials and BHET Products
2.4. PET Glycolysis Kinetics and Neural Network Modeling
3. Results
3.1. Characterization of the Catalyst
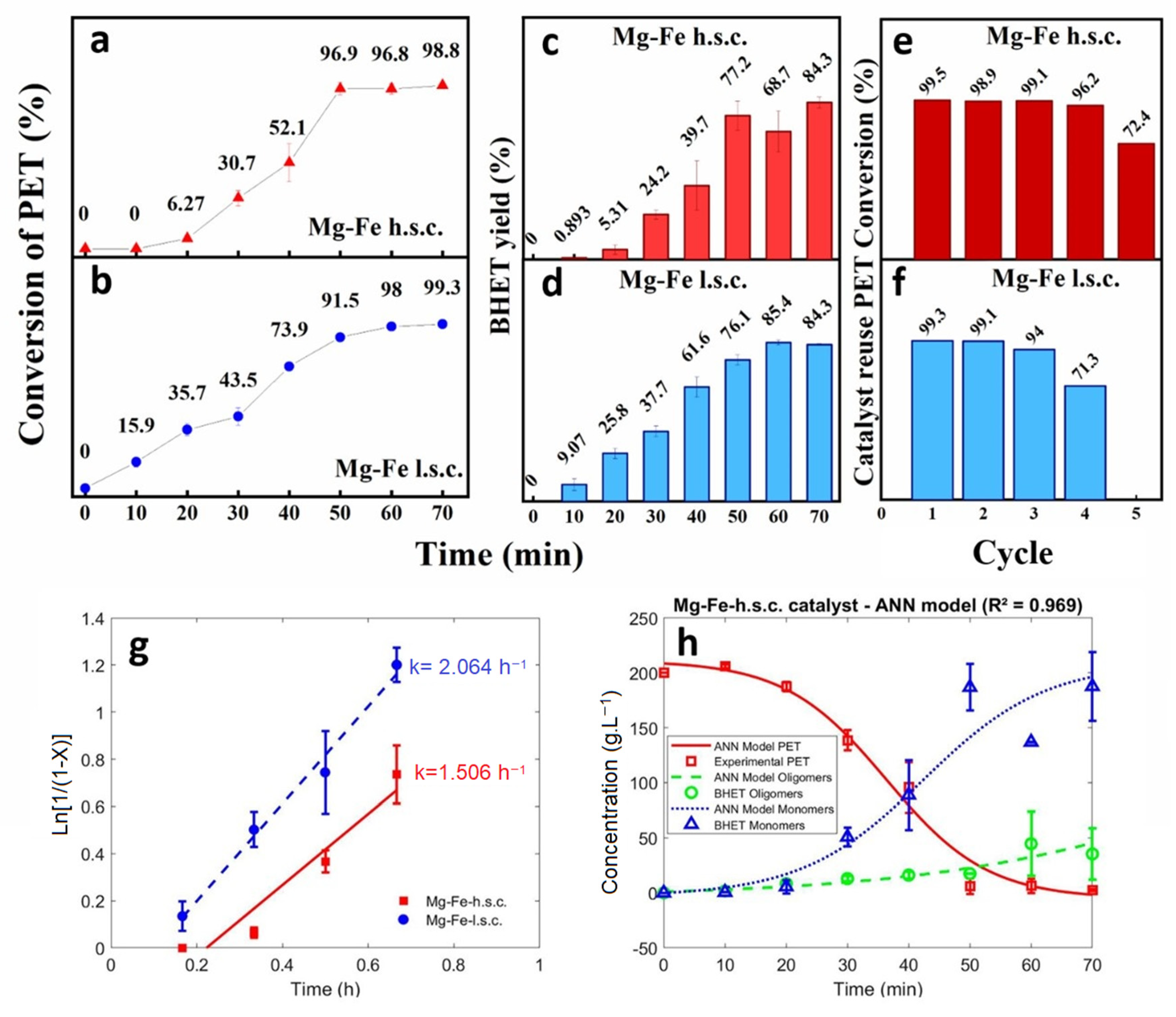
3.2. PET Glycolysis
3.3. Kinetics of PET Glycolysis over Mg-Fe Catalysts
3.4. Catalyst Reuse for PET Glycolysis
3.5. Characterization of the BHET Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the catalytic glycolysis of polyethylene terephthalate. J. Environ. Manag. 2021, 296, 113267. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Veksha, A.; Ahamed, A.; Wu, X.Y.; Liang, L.; Chan, W.P.; Giannis, A.; Lisak, G. Technical and environmental assessment of laboratory scale approach for sustainable management of marine plastic litter. J. Hazard. Mater. 2022, 421, 126717. [Google Scholar] [CrossRef]
- Mendiburu-Valor, E.; Mondragón, G.; González, N.; Kortaberria, G.; Martin, L.; Eceiza, A.; Pena-Rodriguez, C. Valorization of urban and marine PET waste by optimized chemical recycling. Resour. Conserv. Recycl. 2022, 184, 106413. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Hamid, M.K.A.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Imran, M.; Lee, K.G.; Imtiaz, Q.; Kim, B.; Han, M.; Cho, B.G.; Kim, D.H. Metal-Oxide-Doped Silica Nanoparticles for the Catalytic Glycolysis of Polyethylene Terephthalate. J. Nanosci. Nanotechnol. 2011, 11, 824–828. [Google Scholar] [CrossRef]
- Imran, M.; Kim, D.H.; Al-Masry, W.A.; Mahmood, A.; Hassan, A.; Haider, S.; Ramay, S.M. Manganese, cobalt-, and zinc-based mixed-oxide spinels as novel catalysts for the chemical recycling of poly (ethylene terephthalate) via glycolysis. Polym. Degrad. Stab. 2013, 98, 904–915. [Google Scholar] [CrossRef]
- Cano, I.; Martin, C.; Fernandes, J.A.; Lodge, R.W.; Dupont, J.; Casado-Carmona, F.A.; Lucena, R.; Cardenas, S.; Sans, V.; Pedro, I. Paramagnetic ionic liquid-coated SiO2@Fe3O4 nanoparticles—The next generation of magnetically recoverable nanocatalysts applied in the glycolysis of PET. Appl. Catal. B Environ. 2020, 260, 118110. [Google Scholar] [CrossRef]
- Wang, R.; Wang, T.; Yu, G.; Chen, X. A new class of catalysts for the glycolysis of PET: Deep eutectic solvent@ZIF-8 composite. Polym. Degrad. Stab. 2021, 183, 109463. [Google Scholar] [CrossRef]
- Guo, Z.; Adolfsson, E.; Tam, P.L. Nanostructured micro particles as a low-cost and sustainable catalyst in the recycling of PET fiber waste by the glycolysis method. Waste Manag. 2021, 126, 559–566. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, Y.; Yu, G.; Chen, X. Glycolysis of polyethylene terephthalate: Magnetic nanoparticle CoFe2O4 catalyst modified using ionic liquid as surfactant. Eur. Polym. J. 2021, 155, 110590. [Google Scholar] [CrossRef]
- Cot, S.; Leu, M.K.; Kalamiotis, A.; Dimitrakis, G.; Sans, V.; Pedro, I.; Cano, I. An Oxalate-Bridged Binuclear Iron (III) Ionic Liquid for the Highly Efficient Glycolysis of Polyethylene Terephthalate under Microwave Irradiation. ChemPlusChem 2019, 84, 786–793. [Google Scholar] [CrossRef]
- Bartolome, L.; Imran, M.; Lee, K.G.; Sangalang, A.; Ahn, J.K.; Kim, D.H. Superparamagnetic γ-Fe2O3 nanoparticles as an easily recoverable catalyst for the chemical recycling of PET. Green Chem. 2014, 16, 279–286. [Google Scholar] [CrossRef]
- Nabid, M.R.; Bide, Y.; Fereidouni, N.; Etemadi, B. Maghemite/nitrogen-doped graphene hybrid material as a reusable bifunctional catalyst for glycolysis of polyethylene terephthalate. Polym. Degrad. Stab. 2017, 144, 434–441. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Harding, D.R.K.; Eshaq, G.H.; Elmetwally, A.E. Fe3O4-boosted MWCNT as an efficient sustainable catalyst for PET glycolysis. Green Chem. 2016, 18, 3997–4003. [Google Scholar] [CrossRef]
- Krisbiantoro, P.A.; Chiao, Y.W.; Liao, W.; Sun, J.P.; Tsutsumi, D.; Yamamoto, H.; Kamiya, Y.; Wu, K.C.W. Catalytic glycolysis of polyethylene terephthalate (PET) by solvent-free mechanochemically synthesized MFe2O4 (M= Co, Ni, Cu and Zn) spinel. J. Chem. Eng. 2022, 450, 137926. [Google Scholar] [CrossRef]
- Cha, Y.; Park, Y.J.; Kim, D.H. Hydrodynamic synthesis of Fe2O3@ MoS20D/2D-nanocomposite material and its application as a catalyst in the glycolysis of polyethylene terephthalate. RSC Adv. 2021, 11, 16841–16848. [Google Scholar] [CrossRef]
- Jeong, J.M.; Jin, S.B.; Son, S.G.; Suh, H.; Moon, J.M.; Choi, B.G. Fast and facile synthesis of two-dimensional Fe III nanosheets based on fluid-shear exfoliation for highly catalytic glycolysis of poly (ethylene terephthalate). React. Chem. Eng. 2021, 6, 297–303. [Google Scholar] [CrossRef]
- Alzuhairi, M.A.A.; Khalil, B.I.; Hadi, R.S. Nano MgO catalyst for chemical depolymerization of polyethylene terephthalate (PET). Iraq. J. Phys. 2018, 16, 85–93. [Google Scholar] [CrossRef]
- Eshaq, G.H.; Elmetwally, A.E. (Mg–Zn)–Al layered double hydroxide as a regenerable catalyst for the catalytic glycolysis of polyethylene terephthalate. J. Mol. Liq. 2016, 214, 1–6. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Ivanyuk, G.Y.; Krivovichev, S.V.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Mikhailova, Y.A. Crystal Chemistry of Pyroaurite from the Kovdor Pluton, Kola Peninsula, Russia, and the Långban Fe–Mn deposit, Värmland, Sweden. Geol. Ore Depos. 2019, 59, 652–661. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, Q.; Liang, M.; Sun, W. Sb(III) and Sb(V) removal from water by a hydroxyl-intercalated, mechanochemically synthesized Mg-Fe-LDH. Appl. Clay Sci. 2020, 196, 105766. [Google Scholar] [CrossRef]
- Ji, L.; Li, S.; Xu, H.; Huang, W.; Qu, Z.; Yan, N. Morphology control enables [SnS4] 4− clusters and MgFe-LDHs dual active sites for the adsorption of mercury and arsenic ions. J. Chem. Eng. 2022, 433, 133761. [Google Scholar] [CrossRef]
- Arias, S.; Vasconcelos, D.P.; Libório, D.O.; Gonzalez, J.F.; Câmara, A.G.; Barbosa, C.M.B.M.; Frety, R.; Pacheco, J.G.A. Hydrogen-free deoxygenation of industrial vegetable oil waste using Ce, Zr-NiAl catalysts for second-generation biofuels production. Mol. Catal. 2022, 529, 112554. [Google Scholar] [CrossRef]
- Arias, S.; González, J.F.; Sousa, L.V.; Barbosa, C.B.M.; Silva, A.O.S.; Frety, R.; Pacheco, J.G.A. Influence of Ni/Al ratio on the fast pyrolysis of myristic acid when adsorbed on unsupported mixed oxides derived from layered double hydroxides. Catal. Today 2021, 381, 181–191. [Google Scholar] [CrossRef]
- Do Nascimento, L.A.; Peçanha, S.R.S.; Arias, S.; Santos, B.S.; Pacheco, J.G.A.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Barros, I.D.C.L. NiAlCe mixed oxides obtained from layered double hydroxides applied to anisole hydrodeoxygenation. Catal. Today 2022, 394, 282–294. [Google Scholar] [CrossRef]
- Arias, S.; Sousa, L.V.; Barbosa, C.B.M.; Silva, A.O.S.; Fréty, R.; Pacheco, J.G.A. Preparation of NiAlZr-terephthalate LDHs with high Al and Zr content and their mixed oxides for cyclohexane dehydrogenation. Appl. Clay Sci. 2018, 166, 137–145. [Google Scholar] [CrossRef]
- Del Olmo, N.S.; Carloni, R.; Ortega, P.; Garcia-Gallego, S.; De la Mata, F.J. Metallodendrimers as a promising tool in the biomedical field: An overview. Adv. Organomet. Chem. 2020, 74, 1–52. [Google Scholar] [CrossRef]
- Serrano, M.C.; Pagani, R.; Vallet-Regı, M.; Pena, J.; Ramila, A.; Izquierdo, I.; Portolés, M.T. In vitro biocompatibility assessment of poly (ε-caprolactone) films using L929 mouse fibroblasts. Biomaterials 2004, 25, 5603–5611. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, X.; Zhao, F.; Hu, Y.; Xiong, S.; Guo, Y.; Huang, P.; Yang, B. A study on the biocompatibility of MgO coating prepared by anodic oxidation method on magnesium metal. Bionic Eng. 2020, 17, 76–91. [Google Scholar] [CrossRef]
- Khalid, A.; Norello, R.N.; Abraham, A.N.; Tetienne, J.P.; Karle, T.J.; Lui, E.W.C.; Xia, K.; Tran, P.A.; O’connor, A.J.; Mann, B.C.; et al. Biocompatible and biodegradable magnesium oxide nanoparticles with in vitro photostable near-infrared emission: Short-term fluorescent markers. Nanomaterials 2019, 9, 1360. [Google Scholar] [CrossRef] [Green Version]
- Kansara, K.; Patel, P.; Shukla, R.K.; Pandya, A.; Shanker, R.; Kumar, A.; Dhawan, A. Synthesis of biocompatible iron oxide nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2018, 13, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, Y.; Cha, R.; Ran, B.; Mou, K.; Wang, H.; Xie, Q.; Sun, J.; Jiang, X. The biocompatibility evaluation of iron oxide nanoparticles synthesized by a one pot process for intravenous iron supply. RSC Adv. 2016, 6, 14329–14334. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Fridoni, M.; Abdollahifar, M.; Manjili, H.K.; Davaran, S.; Danafar, H. New insight about biocompatibility and biodegradability of iron oxide magnetic nanoparticles: Stereological and in vivo MRI monitor. Sci. Rep. 2019, 9, 7173. [Google Scholar] [CrossRef] [Green Version]
- Damayanti; Wu, H.S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.V.; Hassani, A.; Eghbali, P.; Nidheesh, P.V. Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100965. [Google Scholar] [CrossRef]
- Thao, N.T.; Trung, H.H. Selective oxidation of styrene over Mg–Co–Al hydrotalcite like-catalysts using air as oxidant. Catal. Commun. 2014, 45, 153–157. [Google Scholar] [CrossRef]
- Da Silva, W.O.; Lima, S.H.; Dias, M.L.; Sirelle, L.; Pacheco Filho, J.G.A.; De Andrade, J.N.; Do Nascimento Júnior, H.S.; Lopes, D.E.B.; Arcanjo, A.P.C.; De Castro, A.M. Catalisadores e Processo Para Obtenção de Poliéster Reciclado. INPI. BR Patent 102018068454-0, 19 April 2022. [Google Scholar]
- Lima, G.R.; Monteiro, W.F.; Ligabue, R.; Santana, R.M.C. Titanate Nanotubes as New Nanostrutured Catalyst for Depolymerization of PET by Glycolysis Reaction. Mater. Res. 2017, 20, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Siročik, A.P.; Fijačko, A.; Hrnjak-Murgič, Z. Chemical Recycling of Postconsumer Poly (ethylene-terephthalate) Bottles—Depolymerization Study. Chem. Biochem. Eng. Q. 2013, 27, 65–71. Available online: https://hrcak.srce.hr/99439 (accessed on 15 June 2023).
- Chen, F.; Zhou, Q.; Bu, R.; Yang, F.; Li, W. Kinetics of Poly (ethylene terephthalate) Fiber Glycolysis in Ethylene Glycol. Fibers Polym. 2015, 16, 1213–1219. [Google Scholar] [CrossRef]
- Souza, T.P.C.; Silva, R.J.M.C.L.; Melo, J.C.; Tschoeke, I.C.P.; Silva, J.P.; Pacheco, J.G.A.; Silva, J.M.F. Kinetic modeling of cottonseed oil transesterification with ethanol. React. Kinet. Mech. Catal. 2019, 128, 707–722. [Google Scholar] [CrossRef]
- Irineu, M.D.; De Aquino, R.V.S.; Barbosa, A.A.; Pacheco, J.G.A.; da Rocha, O.R.S. Degradation of textile dye mixture by heterogeneous photocatalysis employing neural network modeling. Desalin. Water Treat. 2022, 280, 128–138. [Google Scholar] [CrossRef]
- Bergstra, J.; Bengio, Y. Random search for hyper-parameter optimization. J. Mach. Learn. Res. 2012, 13, 281–305. Available online: http://jmlr.org/papers/v13/bergstra12a.html (accessed on 20 June 2023).
- Hadj-Abdelkader, N.H.; Beltrao-Nunes, A.; Belkhadem, F.; Benselka, N.; Roy, R.; Azzouz, A. New insights in MgAl and MgFe-LDH affinity towards carbon dioxide—Role of the hydrophilic character on CO2 retention strength. Appl. Clay Sci. 2020, 198, 105829. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Valim, J.B. Layered double hydroxides: Structure, synthesis, properties and applications. Quim. Nova 1998, 21, 300–311. [Google Scholar] [CrossRef]
- Coelho, A.; Perrone, O.M.; Gomes, E.; Silva, R.; Thomeo, J.C.; Boscolo, M. Mixed metal oxides from sucrose and cornstarch templated hydrotalcite-like LDHs as catalysts for ethyl biodiesel synthesis. Appl. Catal. A 2017, 532, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Giulietti, M.; Seckler, M.M.; Derenzo, S.; Ré, M.I.; Cekinski, E. Industrial crystallization and precipitation from solutions: State of the technique. Braz. J. Chem. Eng. 2001, 18, 423–440. [Google Scholar] [CrossRef]
- Kowalik, P.; Konkol, M.; Kondracka, M.; Próchniak, W.; Bicki, R.; Wiercioch, P. Memory effect of the CuZnAl-LDH derived catalyst precursor—In situ XRD studies. Appl. Catal. A 2013, 464–465, 339–347. [Google Scholar] [CrossRef]
- Elhalil, A.; Qourzal, S.; Mahjoubi, F.Z.; Elmoubarki, R.; Farnane, M.; Tounsadi, H.; Sadiq, M.; Abdennouri, M.; Barka, N. Defluoridation of groundwater by calcined Mg/Al layered double hydroxide. Emerg. Contam. 2016, 2, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Vozniuk, O.; Tabanelli, T.; Tanchoux, N.; Millet, J.M.; Albonetti, S.; Renzo, F.; Cavani, F. Mixed-oxide catalysts with spinel structure for the valorization of biomass: The chemical-loop reforming of bioethanol. Catalysts 2018, 8, 332. [Google Scholar] [CrossRef] [Green Version]
- Nabid, M.R.; Bide, Y.; Jafari, M. Boron nitride nanosheets decorated with Fe3O4 nanoparticles as a magnetic bifunctional catalyst for post-consumer PET wastes recycling. Polym. Degrad. Stab. 2019, 169, 108962. [Google Scholar] [CrossRef]
- El Mejjatti, A.; Harit, T.; Riahi, A.; Khiari, R.; Bouabdallah, I.; Malek, F. Chemical recycling of poly (ethylene terephthalate). Application to the synthesis of multiblock copolyesters. Express Polym. Lett. 2014, 8, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Veljković, V.B.; Stamenković, O.S.; Todorović, Z.B.; Lazić, M.L.; Skala, D.U. Kinetics of sunflower oil methanolysis catalyzed by calcium oxide. Fuel 2009, 88, 1554–1562. [Google Scholar] [CrossRef]
- Capeletti, M.R.; Passamonti, F.J. Optimization of reaction parameters in the conversion of PET to produce BHET. Polym. Eng. Sci. 2017, 58, 1500–1507. [Google Scholar] [CrossRef]
- Chen, C.H. Study of Glycolysis of Poly (ethylene terephthalate) Recycled from Postconsumer Soft-Drink Bottles. III. Further Investigation. J. Appl. Polym. Sci. 2003, 87, 2004–2010. [Google Scholar] [CrossRef]
- Nica, S.; Hanganu, A.; Tanase, A.; Duldner, M.; Iancu, S.; Draghici, C.; Filip, P.I.; Bartha, E. Glycolytic Depolymerization of Polyethylene Terephtalate (PET) Wastes Organic vs. metal-catalysis. Rev. Chim. Bucharest 2015, 66, 1105–1111. [Google Scholar]
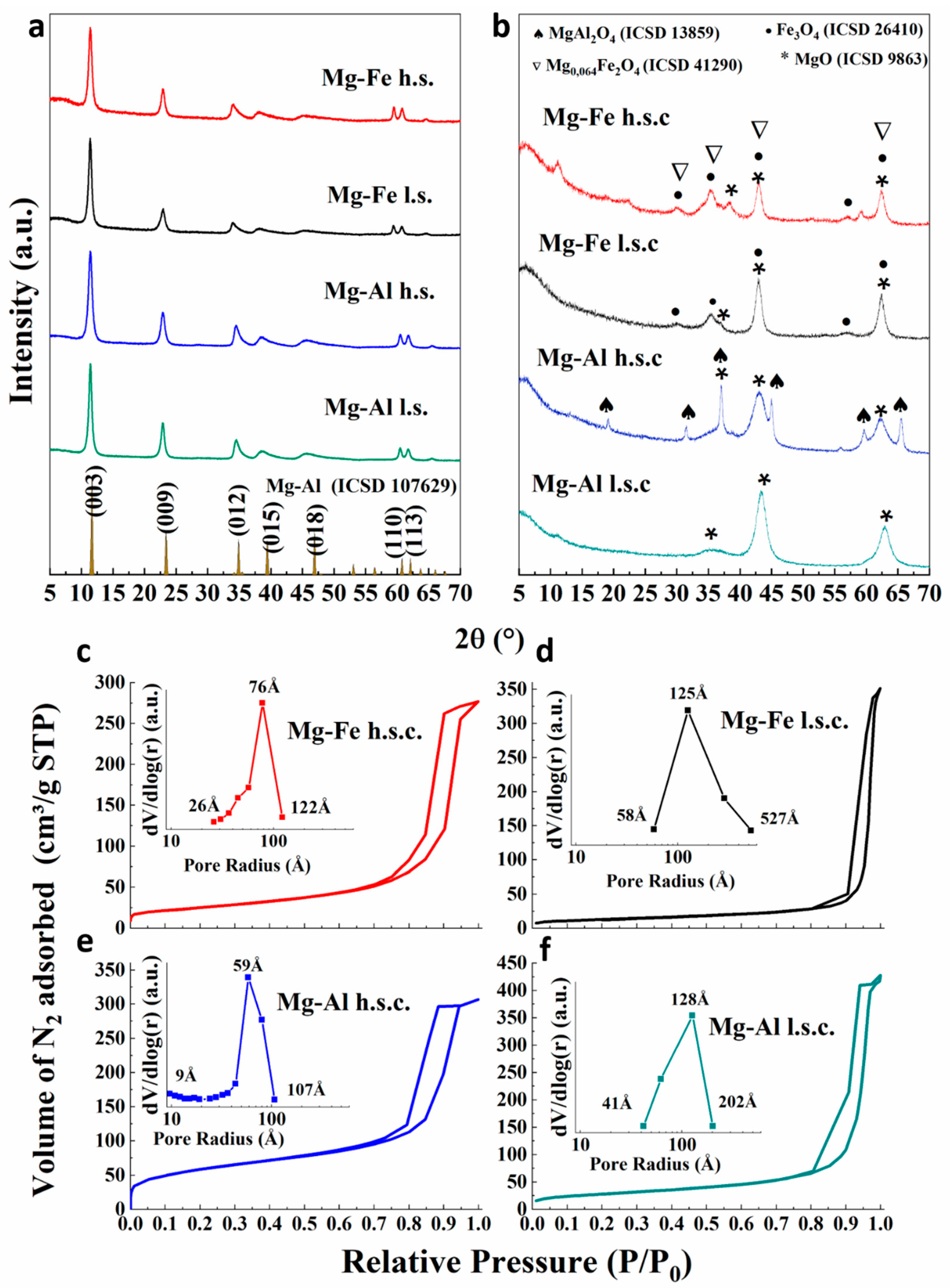


| Oxide Catalyst | Surface Area a (m2·g−1) | Pore Volume b (cm3·g−1) | Average Diameter b (Å) | Mg (%) | Fe (%) | Al (%) | X c |
|---|---|---|---|---|---|---|---|
| Mg-Fe-h.s.c. | 89 | 0.42 | 138 | 41.0 | 59.0 | 0.39 | |
| Mg-Fe-l.s.c. | 45 | 0.53 | 272 | 41.3 | 58.7 | 0.38 | |
| Mg-Al-h.s.c. | 208 | 0.44 | 104 | 57.2 | 42.8 | 0.40 | |
| Mg-Al-l.s.c. | 99 | 0.64 | 175 | 56.5 | 43.5 | 0.41 |
| Catalyst | PET Conversion (%) | BHET Molar Yield (%) | BHET Oligomer Selectivity (%) | BHET Monomer Selectivity (%) |
|---|---|---|---|---|
| Mg-Fe-h.s.c. | 96.8 ± 3.2 | 68.7 ± 11.0 | 23.6 ± 12.3 | 76.4 ± 12.3 |
| Mg-Fe-l.s.c. | 97.4 ± 2.6 | 85.4 ± 1.3 | 16.0 ± 5.5 | 84.0 ± 5.5 |
| Mg-Al-h.s.c. | 64.0 ± 10.7 | 52.1 ± 9.3 | 21.2 ± 6.0 | 78.8 ± 6.0 |
| Mg-Al-l.s.c. | 98.8 ± 0.1 | 84.0 ± 0.0 | 20.4 ± 4.9 | 79.6 ± 4.9 |
| Catalyst | Parameters | PET Conversion (%) | BHET Yield (%) | Reference |
|---|---|---|---|---|
| Mn3O4 with silica | Catalyst:PET = 1.0 wt% EG:PET = 11 300 °C, 1.3 h, 10.9 atm | - | >90% | [6] |
| ZnMn2O4 spinels | Catalyst:PET = 1.0 wt% EG:PET = 17.2 260 °C, 1.5 h, 5 atm | - | 92.2 mol% | [7] |
| Ionic liquids Fe3O4@SiO2@(mim) [FeCl4] | Catalyst:PET = 15 wt% EG:PET = 0.01 180 °C, 24 h, 1 atm | - | 100% | [8] |
| MOFs DES@ZIF-8 | Catalyst:PET = 0.4% EG:PET = 5 195 °C, 0.42 h, 1 atm | 100% | 83.2% | [9] |
| Mg-Al-O@Fe3O4 | Catalyst:PET = 0.5 wt% EG:PET = 5 240 °C, 1.5 h, 1 atm. | - | 80 mol% | [10] |
| Cobalt-ferrite CoFe2O4/C10-OAC | Catalyst:PET = 2 wt% EG:PET = 5 195 °C, 2.5 h, 1 atm | 100% | 95.4% | [11] |
| γ-Fe2O3 | Catalyst:PET = 0.05 wt% EG:PET = 3.3 300 °C, 1 h, 1 atm | - | 90% | [13] |
| Fe2O3/N-graphene | Catalyst:PET = 10 wt% EG:PET = 13.3 195 °C, 3 h, 1 atm | - | 100% | [14] |
| Fe3O4-carbon-nanotubes | Catalyst: PET = 5% ethanediol:PET = 10 190 °C, 2 h, 1 atm | - | 100% | [15] |
| Spinel ferrites of zinc or copper ZnFe2O4 | Catalyst:PET = 4% EG:PET = 5 190 °C, 6 h, 1 atm | 100% | 79.2% | [16] |
| Fe2O3@MoS2-2D nanocomposites | Catalyst:PET = 1.0 wt% EG:PET = 4 225 °C, 3 h, 1 atm | 97% | 90% | [17] |
| 2D-Fe(III) nanosheets | Catalyst:PET = 0.01 wt% EG:PET = 18.5 200 °C, 0.5 h, 1 atm | 100% | 100% | [18] |
| MgO | Catalyst:PET = 0.25% EG:PET = 4 190 °C, 5 h, 1 atm | - | 81.5% | [19] |
| Mg-Zn-Al (Mg–Zn)–Al | Catalyst:PET = 1.0 wt% EG:PET = 10 196 °C, 3 h, 1 atm | - | 75%. | [20] |
| Mg-Fe h.s.c. | Catalyst:PET = 0.5 wt% EG:PET = 5 200 °C, 1 h, 1 atm | 96.8% | 68.7% | This work |
| Mg-Fe l.s.c. | Catalyst:PET = 0.5 wt% EG:PET = 5 200 °C, 1 h, 1 atm | 97.4% | 85.4% | This work |
| Mg-Al h.s.c. | Catalyst:PET = 0.5 wt% EG:PET = 5 200 °C, 1 h, 1 atm | 64.0% | 52.1% | This work |
| Mg-Al l.s.c. | Catalyst:PET = 0.5 wt% EG:PET = 5 200 °C, 1 h, 1 atm | 98.8% | 84.0% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcanjo, A.P.; Liborio, D.O.; Arias, S.; Carvalho, F.R.; Silva, J.P.; Ribeiro, B.D.; Dias, M.L.; Castro, A.M.; Fréty, R.; Barbosa, C.M.B.M.; et al. Chemical Recycling of PET Using Catalysts from Layered Double Hydroxides: Effect of Synthesis Method and Mg-Fe Biocompatible Metals. Polymers 2023, 15, 3274. https://doi.org/10.3390/polym15153274
Arcanjo AP, Liborio DO, Arias S, Carvalho FR, Silva JP, Ribeiro BD, Dias ML, Castro AM, Fréty R, Barbosa CMBM, et al. Chemical Recycling of PET Using Catalysts from Layered Double Hydroxides: Effect of Synthesis Method and Mg-Fe Biocompatible Metals. Polymers. 2023; 15(15):3274. https://doi.org/10.3390/polym15153274
Chicago/Turabian StyleArcanjo, Ana P., Denisson O. Liborio, Santiago Arias, Florival R. Carvalho, Josivan P. Silva, Bernardo D. Ribeiro, Marcos L. Dias, Aline M. Castro, Roger Fréty, Celmy M. B. M. Barbosa, and et al. 2023. "Chemical Recycling of PET Using Catalysts from Layered Double Hydroxides: Effect of Synthesis Method and Mg-Fe Biocompatible Metals" Polymers 15, no. 15: 3274. https://doi.org/10.3390/polym15153274
APA StyleArcanjo, A. P., Liborio, D. O., Arias, S., Carvalho, F. R., Silva, J. P., Ribeiro, B. D., Dias, M. L., Castro, A. M., Fréty, R., Barbosa, C. M. B. M., & Pacheco, J. G. A. (2023). Chemical Recycling of PET Using Catalysts from Layered Double Hydroxides: Effect of Synthesis Method and Mg-Fe Biocompatible Metals. Polymers, 15(15), 3274. https://doi.org/10.3390/polym15153274








