Ladder Polyphenylsilsesquioxanes and Their Niobium–Siloxane Composite as Coating Materials: Spectroscopy and Atomic Oxygen Resistance Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.4. Atomic-Oxygen Exposition
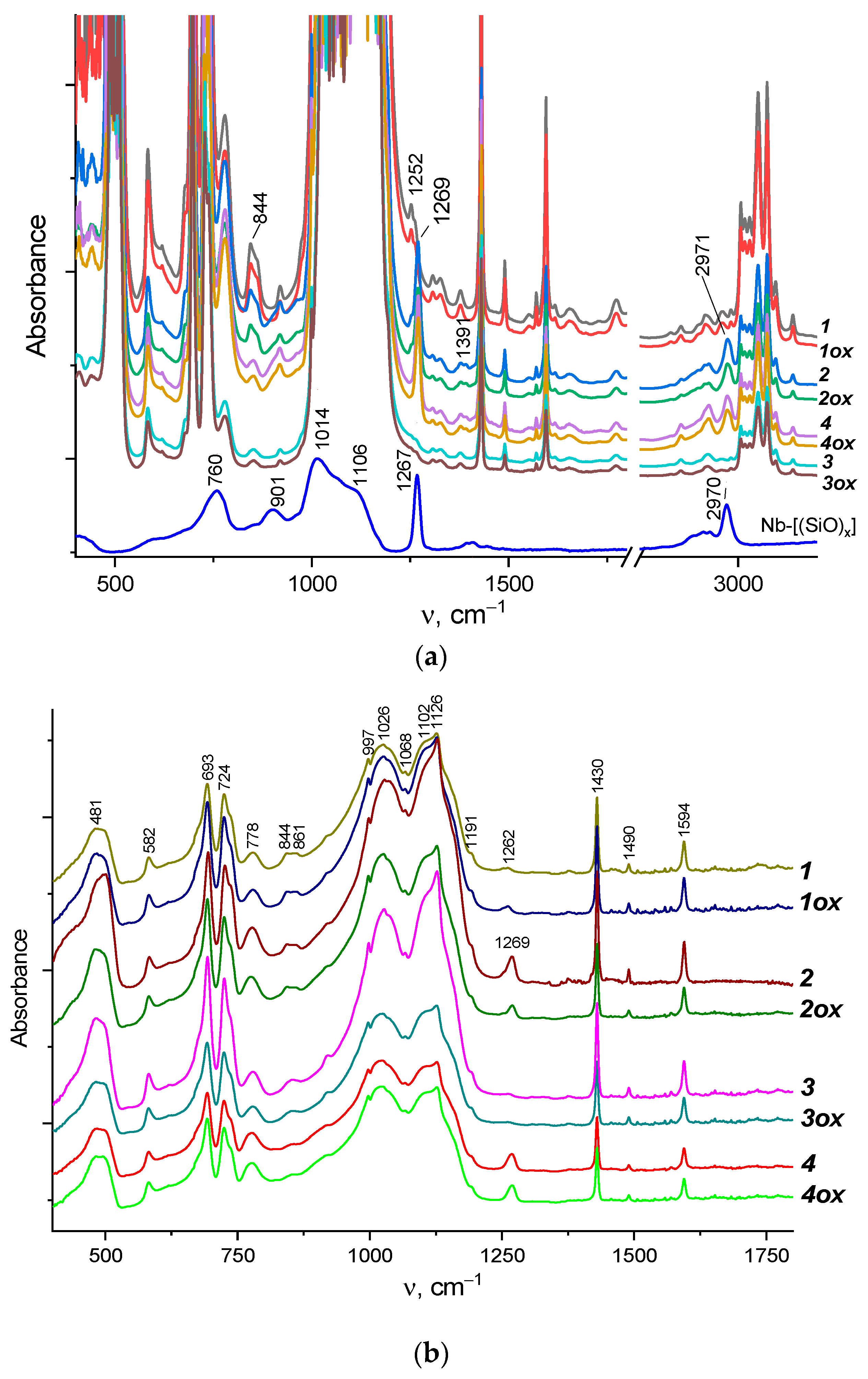
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Li, Y.; Qian, Y.; Qi, H.; Li, J.; Sun, J. Mechanically Robust Atomic Oxygen-Resistant Coatings Capable of Autonomously Healing Damage in Low Earth Orbit Space Environment. Adv. Mater. 2018, 30, 1803854. [Google Scholar] [CrossRef]
- Gordo, P.; Frederico, T.; Melício, R.; Duzellier, S.; Amorim, A. System for space materials evaluation in LEO environment. Adv. Space Res. 2020, 66, 307–320. [Google Scholar] [CrossRef]
- Verker, R.; Bolker, A.; Carmiel, Y.; Gouzman, I.; Grossman, E.; Minton, T.K.; Remaury, S. Ground testing of an on-orbit atomic oxygen flux and ionizing radiation dose sensor based on material degradation by the space environment. Acta Astronaut. 2020, 173, 333–343. [Google Scholar] [CrossRef]
- Bond, D.K.; Goddard, B.; Singleterry, R.C., Jr.; León, S.B. Evaluating the effectiveness of common aerospace materials at lowering the whole body effective dose equivalent in deep space. Acta Astronaut. 2019, 165, 68–95. [Google Scholar] [CrossRef]
- Smith, C.T.G.; Delkowki, M.; Anguita, J.V.; Cox, D.C.; Haas, C.; Silva, S.R.P. Complete Atomic Oxygen and UV Protection for Polymer and Composite Materials in a Low Earth Orbit. ACS Appl. Mater. Interfaces 2021, 13, 6670–6677. [Google Scholar] [CrossRef]
- Chen, J.; Ding, N.; Li, Z.; Wang, W. Organic polymer materials in the space environment. Prog. Aerosp. Sci. 2016, 83, 37–56. [Google Scholar] [CrossRef]
- Tennyson, R.C. Protection of polymeric materials from atomic oxygen. High Perform. Polym. 1999, 11, 157–165. [Google Scholar] [CrossRef]
- Banks, B.A.; De Groh, K.K.; Rutledge, S.K.; DiFilippo, F.J. Prediction of In-Space Durability of Protected Polymers Based on Ground Laboratory Thermal Energy Atomic Oxygen. In Protection of Materials and Structures from the Low Earth Orbit Space Environment: Proceedings of ICPMSE-3; Springer: Houten, The Netherlands, 1996; pp. 89–100. [Google Scholar] [CrossRef] [Green Version]
- Leger, L.J. Atomic Oxygen Reactions with Shuttle Materials at Orbital Altitudes. In NASA TM 58246 (NASA Technical Memorandum 58246); NASA: Washington, DC, USA, 1982. [Google Scholar]
- Gilman, J.W.; Schlitzer, D.S.; Lichtenhan, J.D. Low Earth orbit resistant siloxane copolymers. J. Appl. Polym. Sci. 1996, 60, 591–596. [Google Scholar] [CrossRef]
- Chen, D.Z.; Xu, C.; Gouzman, I.; Minton, T.K. Effect of Hyperthermal Atomic Oxygen on Space-Grade CV-11440 Silicone. ACS Appl. Polym. Mater. 2022, 4, 3627–3635. [Google Scholar] [CrossRef]
- Ouyang, M.; Yuan, C.; Muisener, R.J.; Boulares, A.; Koberstein, J.T. Conversion of Some Siloxane Polymers to Silicon Oxide by UV/Ozone Photochemical Processes. Chem. Mater. 2000, 12, 1591–1596. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Hillborg, H.; Ankner, J.F.; Gedde, U.W.; Smith, G.D.; Yasuda, H.K.; Wikström, K. Crosslinked polydimethylsiloxane exposed to oxygen plasma studied by neutron reflectometry and other surface specific techniques. Polymer 2000, 41, 6851–6863. [Google Scholar] [CrossRef]
- Dworak, D.P.; Banks, B.A.; Karniotis, C.A.; Soucek, M.D. Evaluation of Protective Silicone/Siloxane Coatings in Simulated Low-Earth-Orbit Environment. J. Spacecr. Rocket. 2006, 43, 393–401. [Google Scholar] [CrossRef]
- Wang, K.; Chen, M.; Lei, G.; Wang, X. Analysis of Physical−Chemical Properties and Space Environment Adaptability of Two-Component RTV Silicone Rubber. ACS Omega 2021, 6, 28477–28484. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T. Polymer-Composite Materials for Radiation Protection. ACS Appl. Mater. Interfaces 2012, 4, 5717–5726. [Google Scholar] [CrossRef]
- Xing, A.; Gao, Y.; Yin, J.; Ren, G.; Liu, H.; Ma, M. Preparation and atomic oxygen erosion resistance of silica film formed on silicon rubber by sol–gel method. Appl. Surf. Sci. 2010, 256, 6133–6138. [Google Scholar] [CrossRef]
- Kayhan, N.; Razavi, R.S.; Choopani, S. Evaluation of two new white silicone thermal control paints under atomic oxygen. Prog. Org. Coat. 2012, 74, 603–607. [Google Scholar] [CrossRef]
- Duo, S.; Li, M.; Zhu, M.; Zhou, Y. Polydimethylsiloxane/silica hybrid coatings protecting Kapton from atomic oxygen attack. Mater. Chem. Phys. 2008, 112, 1093–1098. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, Y.; Zhang, X.; Li, Z.; Zhao, L.; Wang, Z.; Gao, W. Highly Homogeneous Polysiloxane Flexible Coating for Low Earth Orbital Spacecraft with Ultraefficient Atomic Oxygen Resistance and Self-Healing Behavior. ACS Appl. Polym. Mater. 2019, 1, 3253–3260. [Google Scholar] [CrossRef]
- Duo, S.; Chang, Y.; Liu, T.; Zhang, H. Atomic oxygen erosion resistance of polysiloxane/POSS hybrid coatings on Kapton. Phys. Procedia 2013, 50, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Al-Ostaz, A.; Jaradat, M.; Rahmani, F.; Nouranian, S.; Rushing, G.; Manasrah, A.; Alkhateb, H.; Finckenor, M.; Lichtenhan, J. Substantially enhanced durability of polyhedral oligomeric silsequioxane-polyimide nanocomposites against atomic oxygen erosion. Eur. Polym. J. 2017, 92, 233–249. [Google Scholar] [CrossRef]
- Mu, H.; Wang, X.; Li, Z.; Xie, Y.; Gao, Y.; Liu, H. Preparation and atomic oxygen erosion resistance of SiOx coating formed on polyimide film by plasma polymer deposition. Vacuum 2019, 165, 7–11. [Google Scholar] [CrossRef]
- Wohl, C.J.; Belcher, M.A.; Ghose, S.; Connell, J.W. Modification of the surface properties of polyimide films using polyhedral oligomeric silsesquioxane deposition and oxygen plasma exposure. Appl. Surf. Sci. 2009, 255, 8135–8144. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Guo, Y.D.; Qi, H.R.; An, Y.C.; Jia, Y.J.; Tan, Y.Y.; Liu, J.G.; Wu, B.H. Preparation and Properties of Intrinsically Atomic-Oxygen Resistant Polyimide Films Containing Polyhedral Oligomeric Silsesquioxane (POSS) in the Side Chains. Polymers 2020, 12, 2865. [Google Scholar] [CrossRef]
- Lei, X.F.; Chen, Y.; Zhang, H.P.; Li, X.J.; Yao, P.; Zhang, Q.Y. Space survivable polyimides with excellent optical transparency and self-healing properties derived from hyperbranched polysiloxane. ACS Appl. Mater. Interfaces 2013, 5, 10207–10220. [Google Scholar] [CrossRef]
- Miyazaki, E.; Tagawa, M.; Yokota, K.; Yokota, R.; Kimoto, Y.; Ishizawa, J. Investigation into tolerance of polysiloxane-block-polyimide film against atomic oxygen. Acta Astronaut. 2010, 66, 922–928. [Google Scholar] [CrossRef]
- Qian, M.; Murray, V.J.; Wei, W.; Marshall, B.C.; Minton, T.K. Resistance of POSS polyimide blends to hyperthermal atomic oxygen attack. ACS Appl. Mater. Interfaces 2016, 8, 33982–33992. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Guo, Y.D.; Yang, Y.B.; Yu, Q.; Liu, J.G.; Lv, F.Z. Atomic Oxygen-Resistant Polyimide Composite Films Containing Nanocaged Polyhedral Oligomeric Silsesquioxane Components in Matrix and Fillers. Nanomater 2021, 11, 141. [Google Scholar] [CrossRef]
- Brook, M.A. Silicon in Organic, Organometallic, and Polymer Chemistry; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, A.S.; Hwang, S.S.; Baek, K.Y. Structural Control of Fully Condensed Polysilsesquioxanes: Ladderlike vs Cage Structured Polyphenylsilsesquioxanes. Macromolecules 2015, 48, 6063–6070. [Google Scholar] [CrossRef]
- Handke, M.; Handke, B.; Kowalewska, A.; Jastrzębski, W. New polysilsesquioxane materials of ladder-like structure. J. Mol. Struct. 2009, 924, 254–263. [Google Scholar] [CrossRef]
- Pohl, S.; Janka, O.; Fuglein, E.; Kickelbick, G. Thermoplastic Silsesquioxane Hybrid Polymers with a Local Ladder-Type Structure. Macromolecules 2021, 54, 3873–3885. [Google Scholar] [CrossRef]
- Ershova, T.O.; Anisimov, A.A.; Temnikov, M.N.; Novikov, M.A.; Buzin, M.I.; Nikiforova, G.G.; Muzafarov, A.M. A Versatile Equilibrium Method for the Synthesis of High-Strength, Ladder-like Polyphenylsilsesquioxanes with Finely Tunable Molecular Parameters. Polymers 2021, 13, 4452. [Google Scholar] [CrossRef]
- Ershova, T.; Anisimov, A.; Krylov, F.; Polshchikova, N.; Temnikov, M.; Shchegolikhina, O.; Muzafarov, A. A new highly efficient method for the preparation of phenyl-containing siloxanes by condensation of phenylsilanols in liquid ammonia. Chem. Eng. Sci. 2022, 247, 116916. [Google Scholar] [CrossRef]
- Andropova, U.; Serenko, O.; Tebeneva, N.; Tarasenkov, A.; Buzin, M.; Afanasyev, E.; Sapozhnikov, D.; Bukalov, S.; Leites, L.; Aysin, R.; et al. Atomic oxygen erosion resistance of polyimides filled hybrid nanoparticles. Polym. Test. 2020, 84, 106404. [Google Scholar] [CrossRef]
- Tebeneva, N.A.; Meshkov, I.B.; Tarasenkov, A.N.; Polshchikova, N.V.; Kalinina, A.A.; Buzin, M.I.; Serenko, O.A.; Zubavichus, Y.V.; Katsoulis, D.E.; Muzafarov, A.M. Polyfunctional branched metallosiloxane oligomers and composites based on them. J. Organomet. Chem. 2018, 868, 112–121. [Google Scholar] [CrossRef]
- Tarasenkov, A.N.; Parshina, M.S.; Tebeneva, N.A.; Borisov, K.M.; Goncharuk, G.P.; Shevchenko, V.G.; Ponomarenko, S.A.; Muzafarov, A.M. Metalloalkoxysiloxanes-cured polydimethylsiloxane compositions filled with silica component for special applications: Dielectric and mechanical properties. Express Polym. Lett. 2022, 16, 846–870. [Google Scholar] [CrossRef]
- Shchegolikhina, O.I.; Pozdnyakova, Y.A.; Molodtsova, Y.A.; Korkin, S.D.; Bukalov, S.S.; Leites, L.A.; Lyssenko, K.A.; Peregudov, A.S.; Auner, N.; Katsoulis, D.E. Synthesis and properties of stereoregular cyclic polysilanols: Cis-[PhSi(O)OH]4, cis-[PhSi(O)OH]6, and tris-cis-tris-trans-[PhSi(O)OH]12. Inorg. Chem. 2002, 41, 6892–6904. [Google Scholar] [CrossRef]
- Novikov, L.S.; Chernik, V.N.; Voronina, E.N.; Vernigorov, K.B.; Yablokova, M.Y. Atomic oxygen influence on polymer nanocomposites with different fillers. J. Spacecr. Rocket 2016, 53, 1012–1018. [Google Scholar] [CrossRef]
- ASTM E. 2089-00; Standard Practices for Ground Laboratory Atomic Oxygen Interaction Evaluation of Materials for Space Applications, Annual Book of ASTM Standards. ASTM Standards: West Conshohocken, PA, USA, 2000.
- Shapkin, N.P.; Razov, V.I.; Bazhenov, V.V.; Tutov, M.V.; Stavnisty, N.N.; Kuvchin, Y.N.; Voznesenskii, S.S.; Kuryavyi, V.G.; Slobodyuk, A.B. Polyvinyl- and Polyphenylsilsesquioxanes and their films: An investigation by X-ray difractometry, positron diagnostics, and 9SI NMR spectroscopy. Russ. Chem. Bull. 2011, 60, 1640–1646. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, T. Laddersiloxanes—silsesquioxanes with defined ladder structure. Russ. Chem. Rev. 2013, 82, 289–302. [Google Scholar] [CrossRef]

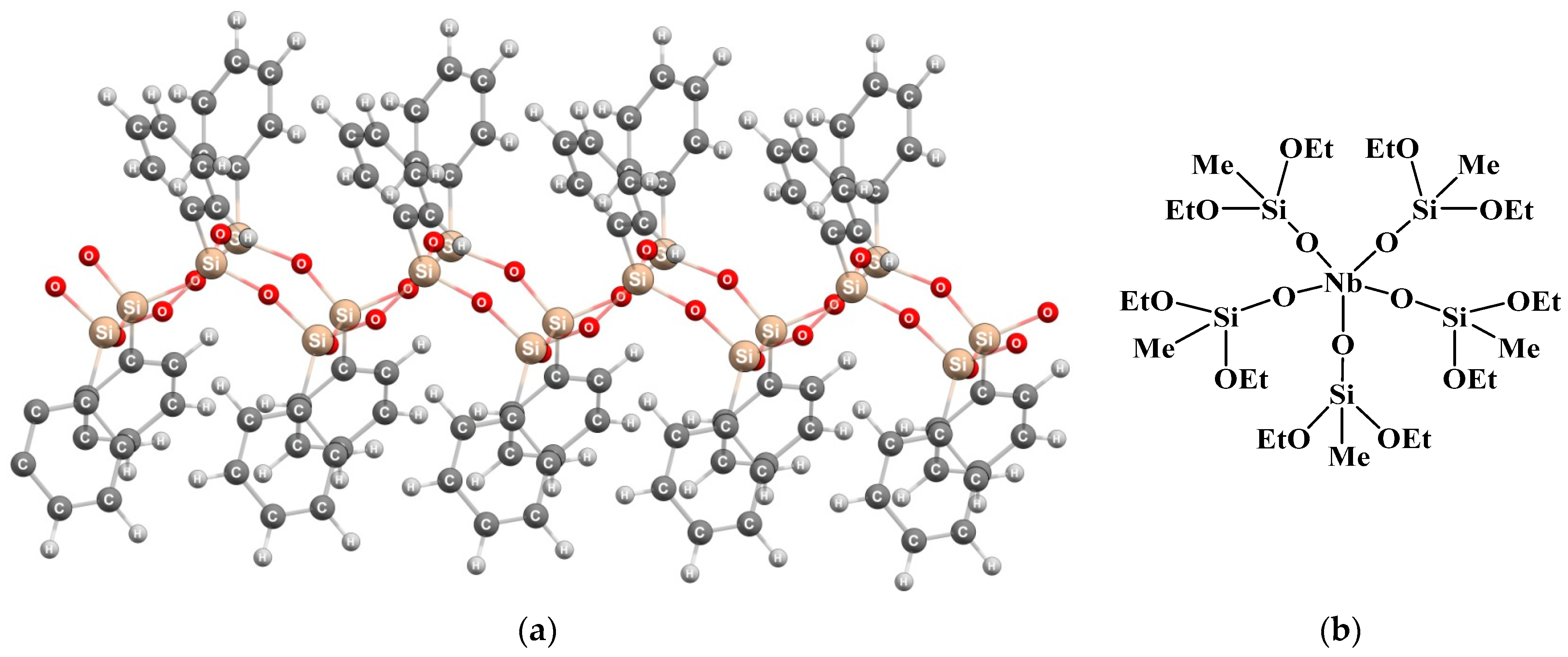

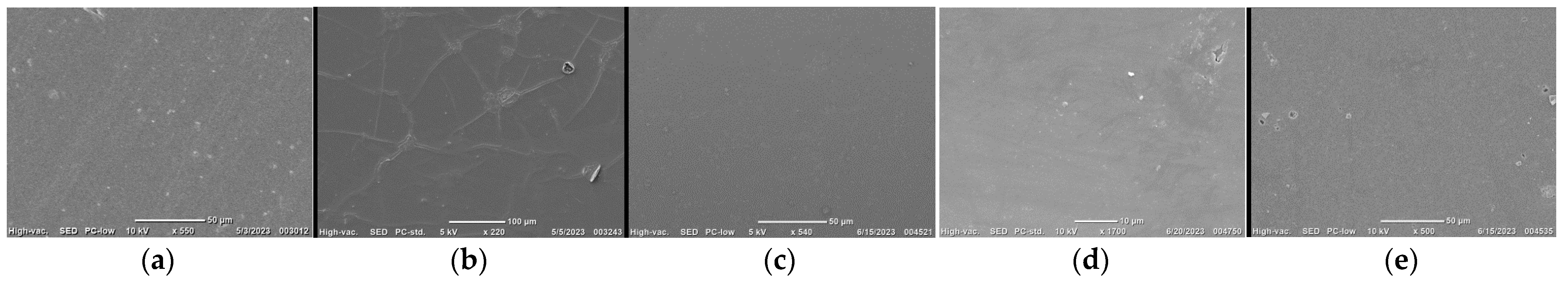
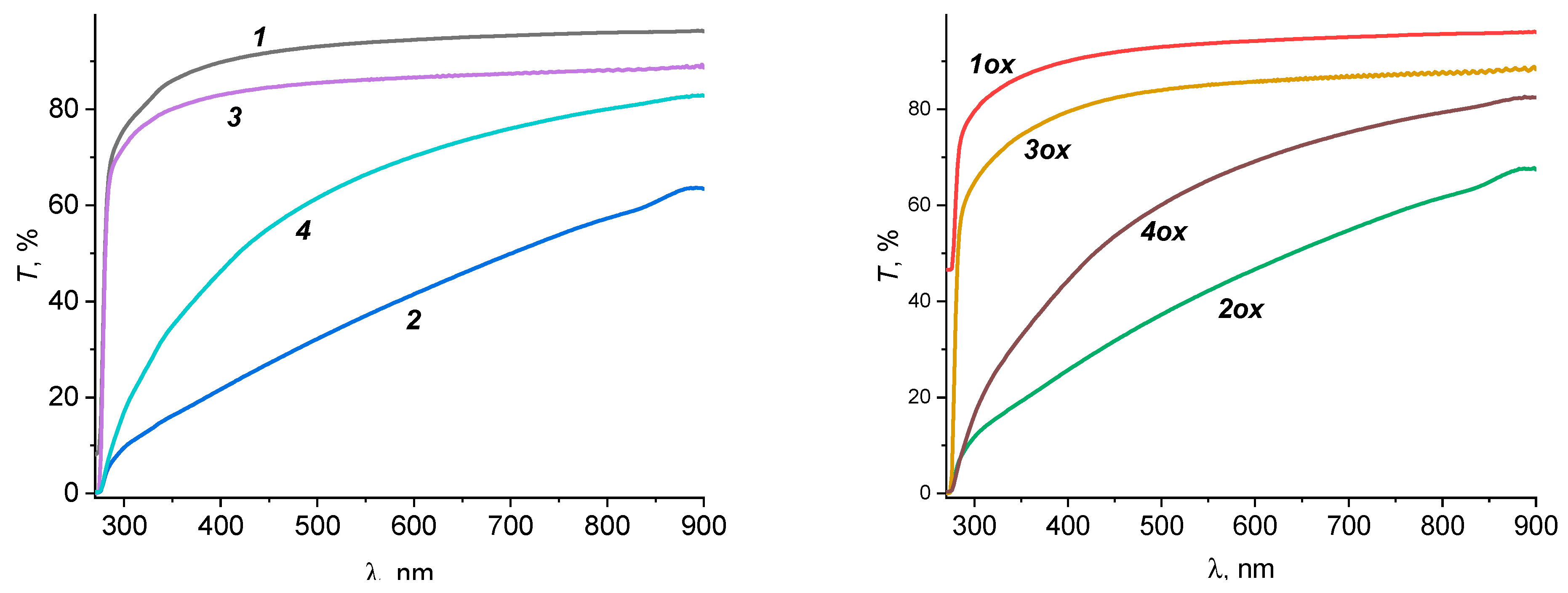
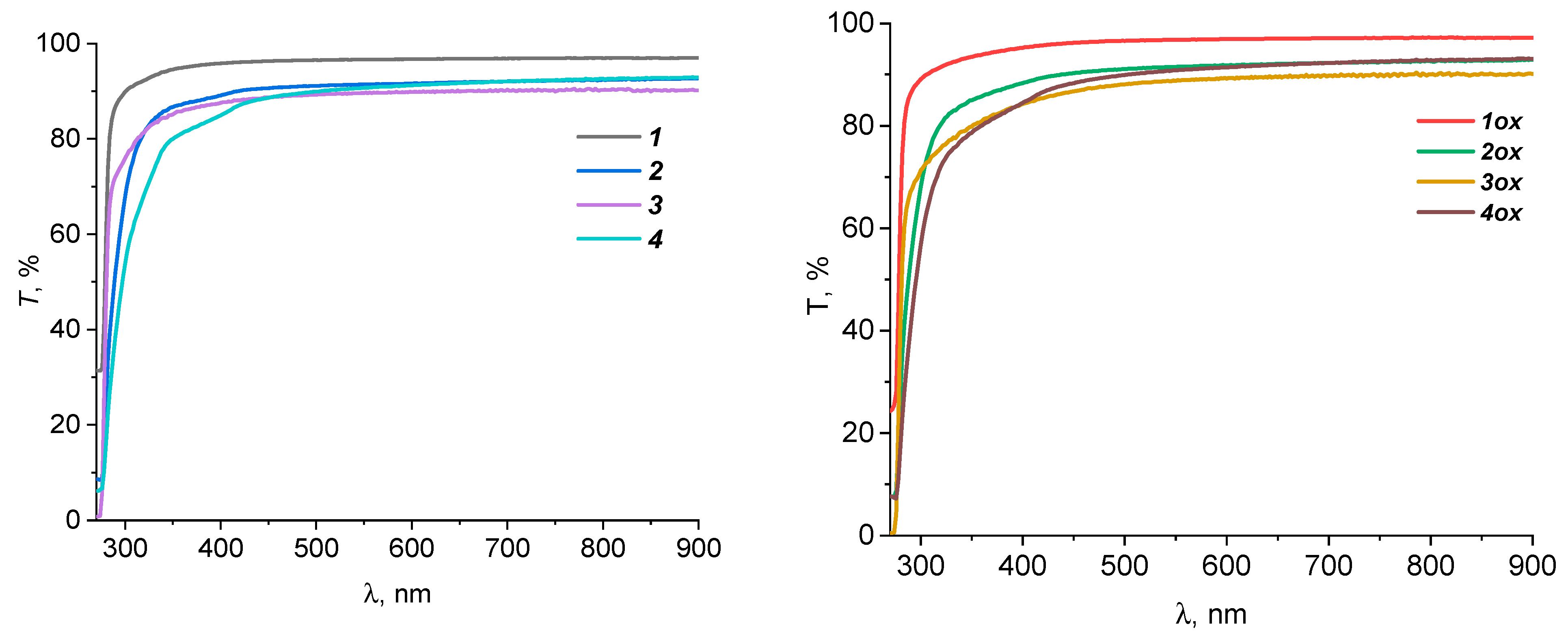

| Sample | σp, MPA | εp, % | E, MPA | Film Thickness, μm |
|---|---|---|---|---|
| L-PPSQ-322 | 26 ± 2 | 7.0 ± 1.0 | 930 ± 56 | 120 |
| L-PPSQ-322-Nb-[(SiO)x] | 18 ± 1 | 2.5 ± 0.2 | 900 ± 54 | 38 |
| L-PPSQ-1060 | 27 ± 2 | 6.0 ± 0.4 | 980 ± 59 | 50 |
| L-PPSQ-1060-Nb-[(SiO)x] | 40 ± 2 | 6.0 ± 0.4 | 1250 ± 75 | 27 |
| Sample | at F = 6.5 × 1020 Atom O/cm2 | at F = 10.0 × 1020 Atom O/cm2 |
|---|---|---|
| Kapton (reference) [7] | 3 × 10–24 | 3 × 10–24 |
| L-PPSQ-322 | 3.0 × 10–26 | 2.5 × 10–26 |
| L-PPSQ-322-Nb-[(SiO)x] | 3.0 × 10–26 | 2.4 × 10–26 |
| L-PPSQ-1060 | 5.5 × 10–26 | 4.8 × 10–26 |
| L-PPSQ-1060-Nb-[(SiO)x] | 4.6 × 10–26 | 4.0 × 10–26 |
| PDMS-Nb-[(SiO)x] | 1.7 × 10–25 | 1.3 × 10–25 |
| PI- Nb-[(SiO)x] [38] | 2.1 × 10–26 | 1.7 × 10–26 |
| PMDA−ODA/HBPSi [27] | 5.38 × 10–25 | |
| POSS PI [29] | 6.6 × 10–26 | |
| PI-30 [26] | 1.1 × 10–25 |
| Sample | τTS | τDT | Δτ | |||
|---|---|---|---|---|---|---|
| Before AO Exposure | After AO Exposure | Before AO Exposure | After AO Exposure | Before AO Exposure | After AO Exposure | |
| L-PPSQ-322 | 0.98 | 0.98 | 0.99 | 0.99 | 0.01 | 0.01 |
| L-PPSQ-322-Nb-[(SiO)x] | 0.59 | 0.63 | 0.95 | 0.94 | 0.36 | 0.31 |
| L-PPSQ-1060 | 0.86 | 0.84 | 0.89 | 0.87 | 0.03 | 0.03 |
| PPSQ-1060-Nb-[(SiO)x] | 0.79 | 0.79 | 0.94 | 0.94 | 0.15 | 0.15 |
| PDMS-Nb-[(SiO)x] | 0.92 | – | 0.90 | – | 0.02 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andropova, U.S.; Aysin, R.R.; Serenko, O.A.; Ershova, T.O.; Anisimov, A.A.; Chernik, V.N. Ladder Polyphenylsilsesquioxanes and Their Niobium–Siloxane Composite as Coating Materials: Spectroscopy and Atomic Oxygen Resistance Study. Polymers 2023, 15, 3299. https://doi.org/10.3390/polym15153299
Andropova US, Aysin RR, Serenko OA, Ershova TO, Anisimov AA, Chernik VN. Ladder Polyphenylsilsesquioxanes and Their Niobium–Siloxane Composite as Coating Materials: Spectroscopy and Atomic Oxygen Resistance Study. Polymers. 2023; 15(15):3299. https://doi.org/10.3390/polym15153299
Chicago/Turabian StyleAndropova, Ulyana S., Rinat R. Aysin, Olga A. Serenko, Tatyana O. Ershova, Anton A. Anisimov, and Vladimir N. Chernik. 2023. "Ladder Polyphenylsilsesquioxanes and Their Niobium–Siloxane Composite as Coating Materials: Spectroscopy and Atomic Oxygen Resistance Study" Polymers 15, no. 15: 3299. https://doi.org/10.3390/polym15153299





