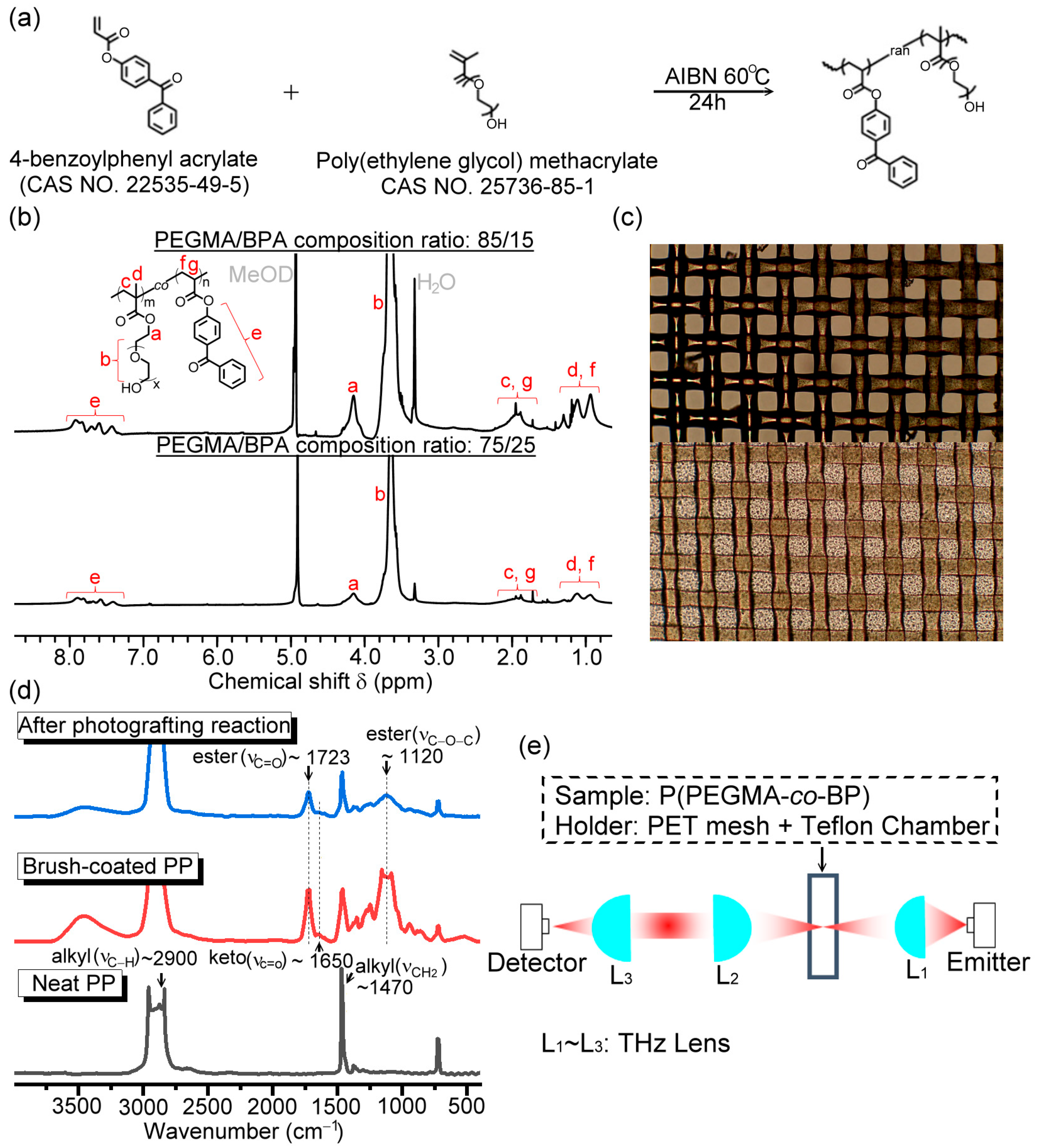
3.1. THz Characterization of the Copolymer Brushes on the PET Mesh Membrane
Figure 3a illustrates the measured THz wave transmittance of PET mesh membranes in 0.1–1 THz with and without surface modification of photografting polymer brush, P(PEGMA-
co-BPA), whose PEGMA/BPA composition ratio is 85/15 and grafting density is 0.76 mg/mm
3. The maximum UV illumination duration for photografting the polymer brush is 30 min. The timings to monitor the brush formation via THz wave transmittance are 0, 10, 20, and 30 min. Prior to photografting, only the blank PET mesh membrane was measured for the spectral transmittance. Compared with the surface-modified membranes, the THz transmittance of the blank PET mesh membrane is the highest because of the least absorption from the substrate materials. The spectral trend of blank PET mesh transmittance is evidently flattened due to the low loss porous structure, performing an average transmittance of 0.72. On the contrary, the transmittance of the surface-modified PET mesh membrane has an approximately negative correlation with THz frequency due to the overlayer of the P(PEGMA-
co-BPA) polymer brush. Thus, high-frequency THz waves have relatively low transmittance due to the intrinsic absorption of the crosslinked polymer network composed of P(PEGMA-
co-BPA) polymer brushes.
The interaction of the THz wave and P(PEGMA-
co-BPA) macromolecule is based on the THz electric-field (
)-induced molecular dipole moment (
) and
-driven molecular rotation through the torque between
and
[
10,
24,
25]. The time varying electric field from THz radiation can be significantly absorbed to drive the dipole oscillation and molecular rotation for the free macromolecules [
10,
24,
25]. Nevertheless, less THz power is depleted for the immobilized P(PEGMA-
co-BPA) macromolecule than the uncured copolymer macromolecule because the surface-anchored force of the former is resistive to the
-driven molecular rotation. This finding implies that the extent of intermolecular crosslinking among P(PEGMA-
co-BPA) macromolecules can be identified via intensity interrogation of THz-transmitted intensity from the surface-modified PET mesh-membrane during the polymerization process.
Above inference can be validated by the following experimental results as shown in
Figure 3. At the monitor timing of 0 min, the un-immobilized copolymer macromolecules of P(PEGMA-
co-BPA), without intermolecular crosslinking, strongly absorb THz waves, consequently performing the lowest transmittance compared with those under the longer durations of UV exposure (
Figure 3a). This condition indicates that the immobilization of P(PEGMA-
co-BPA) on the PET mesh membrane via the photografting process with a UV-illuminated duration within 10–30 min reduces the THz field energy absorption, which is caused by the THz-driving molecular dipole oscillations of P(PEGMA-
co-BPA) [
10,
21,
22]. That is, the anchoring force of the copolymer macromolecule resists the resonantly dipolar oscillation. The surface-modified PET mesh after UV-induced graft polymerization has a relatively higher THz wave transmittance than that prior to UV-induced grafting.
The spectral curves in
Figure 3a show the 0.20, 0.40, and 0.61 THz wave transmittances for the blank PET mesh membrane, and the different monitor timings of UV-induced graft polymerization are summarized in
Figure 3b. The finding clarifies that the THz wave transmittance of the surface-modified PET mesh membrane increases to the maximum values within 10 min. However, the THz wave transmittance slightly reduces at grafting time within 10–30 min because the temperature of the surface-modified PET mesh slightly rises when absorbing UV light from 10 to 30 min. The thermal motion of the copolymeric macromolecule slightly lose the surface anchoring and consequently enhances THz electric field absorption even when immobilized. The experiment result in
Figure 3 expresses that the duration of UV exposure time should not be less than 10 min for a complete graft polymerization process of P(PEGMA-
co-BPA) copolymer brush. In addition, to detect immobilization of P(PEGMA-
co-BPA), the sensing method based on intensity interrogation of the THz transmitted signals is better than the proposed phase interrogation method [
5,
9] because the measured phase variation of the transmitted THz pulse from the ultra-thin immobilized layer during the photograft polymerization is extremely small and ignored.
The P(PPEGMA-
co-BPA) overlayer is further used to surface-modify the PET mesh membrane with the 75/25 composition ratio of PEGMA/BPA and grafting density of 0.09 mg/mm
3. The experimental result of the broadband THz transmittance spectrum is shown in
Figure 4a measured by the same optical configuration (
Figure 1e). Within the available THz range of 0.1–1 THz, the measured THz wave transmittances of the copolymeric brush-coated PET mesh membranes (
Figure 4a) are indistinguishable among different UV exposure durations based on the system accuracy, but are considerably higher than those in
Figure 3a. The result in
Figure 4a reveals that the 0.09 mg/mm
3 grafting density of the copolymer brush with the 75/25 composite ratio of PEGMA/BPA monomer on the porous membrane is too small to be detected by the THz probe and is independent of the UV exposure duration. Experimental tests (i.e., Exp-1, -2 and -3) were conducted three times for the 85/15 and 75/25 composition ratios of PEGMA/BPA samples under 15 min UV exposure duration to ensure the completeness of graft polymerization. The grafting density of the copolymer brush for each sample can be obtained by comparing the sample weight with and without the graft-polymerized P(PEGMA-
co-BPA) brushes. The experimental results are summarized in
Figure 4b, showing that the amount of copolymer brushes formed by the 75/25 composition ratio of PEGMA/BPA monomer cannot sufficiently be grafted to PET mesh membranes to absorb THz wave power. The best composition ratio of PEGMA/BPA was experimentally validated at 85/15 to form a surface-modified layer with a sufficient amount of copolymer brushes that were graft-polymerized on the PET mesh membrane. It eventually provides a vast number of hydrophilic hydroxyl groups for water vapor adsorption and endows one sensor with an excellent humidity-responsive property.
3.2. RH-Sensing Analysis
Based on the experimental result of frequency-dependent THz absorption for a porous surface-modified membrane as shown in
Figure 3, the 0.4 THz wave was specifically applied to sense humidity in the Teflon chamber because of the relatively high transmittance decrement based on the response of the P(PEGMA-
co-BPA) molecule on the PET mesh membrane, which is compared with those of the 0.20 and 0.61 THz waves in
Figure 3. This finding indicates that the strong power absorption effect of P(PEGMA-
co-BPA) molecule occurs at 0.4 THz frequency and that the 0.4 THz wave is sensitive to surface variation for the water adsorption with P(PEGMA-
co-BPA) molecules.
Figure 5a shows the measured time-dependent 0.4 THz transmitted power of one layer of the blank PET mesh membrane, exposing to different RH percent levels (25–99%,
Table 1) inside the air-sealed chamber (
Figure 2). However, no power change is found among all of the RH levels during one period of 3500 s. This result indicates that various RH levels cannot be distinguished from the transmission power curves in
Figure 5a because of the approximate power voltage value. For example, for the 52% RH, the 0.4 THz wave sensing power is 0.0058 V on average, but other RHs are unidentified with detectable power difference. This finding reveals the consequence that the blank PET mesh membrane is hydrophobic and inactive to the environmental moisture [
18,
19]. Under the same THz wave-sensing scheme and RH conditions (
Figure 1c and
Figure 2, and
Table 1), this blank PET mesh membrane was further replaced with the surface-modified one for dynamic transmission power measurement of 0.4 THz wave, and the result is illustrated in
Figure 5b. In the experiment, the coated molecular density of the P(PEGMA-
co-BPA) copolymer brush is 1.57 mg/mm
3, contrary to the blank PET mesh membrane with 0 mg/mm
3 brush density. For measuring 52% RH of ambient laboratory humidity, no aqueous solution injection found, and the chamber was sealed. The responding voltage level of the 0.4 THz wave power in
Figure 5b is almost flattened with a constant voltage of approximately 0.0055 V on the average within the 3500 s recording duration. The 0.4 THz wave power from the 1.57 mg/mm
3 surface-modified mesh membrane is decreased to approximately 5.8% compared with that of the blank PET mesh membrane (
Figure 5a). The decreased THz transmittance resulted from the overlayer of P(PEGMA-
co-BPA) copolymer brush that was grafted on the PET mesh membrane. It eventually changes the hydrophobic surface property as hydrophilicity and increases the adsorption amount of water vapor, thereby depleting the 0.4 THz wave transmitted power.
When the RH of the chamber is lower than 52%, the 0.4 THz transmitted power is gradually raised within 0–500 s and approaches to saturation with an individual constant power voltage higher than that of 52% RH, as shown by the responding curves of 25%, 35%, and 44% RHs in
Figure 5b. The amounts of adsorbed water vapor at low humidity (i.e., 25%, 35%, and 44% RHs) are smaller than those at 52% RH, decreasing the absorption loss. In this condition, the water vapor was captured by the hydroxyl groups of the polymer chains or brushes grafted on the membrane substrate. Therefore, the relatively low RH results in relatively high THz transmittance. On the contrary, the 0.4 THz transmitted power gradually decreased within the initial 1000 s for the high RHs from 76% to 99%, and then the power trend became saturated nearly after 1500 s. For the microscopic version, the saturation of the power response curve expresses that adsorption and desorption of water vapor on the overlayer of the polymer brush approach equilibrium, and approximately 1000–1500 s is required to gradually achieve a steady power response (
Figure 5b). The high humidity accordingly leads to great THz power reduction because the large surface and volume densities of water vapor were physically adsorbed on the surface and infilled into holes of the hydrophilic mesh membrane (
Figure 5b). The result in
Figure 5b indicates that the measured power levels of different RHs (25–99%,
Table 1) in their saturation durations of response curves are significantly correlated to the adsorbed amounts of water vapor, which are proportional to the RH values. Additionally, the dynamically time-dependent curve of the THz transmission intensity within 3500 s exactly represents the interactive responses among the THz wave, surface-modified PET mesh membrane, and its environmental humidity, showing capability to quantitatively identify RH levels inside the air-sealed Teflon chamber.
The power difference of the saturated 0.4 THz waves, ΔV, between the test RH and the ambient RH could be used as the quantitative indicator for the hydrophilic adsorption of water vapor on the sensing mesh membrane. The negative (positive) power difference (ΔV) that represents the quantity of water vapor adsorbed on the hydrophilic mesh membrane at a specific RH is greater (less) than that at the ambient RH. The humidity-sensing experiment conducted for different polymer brush densities was separately operated at different days, and the relevant ambient laboratory RH is slightly deviated from 52% with an RH range of 44–52%, depending on the ambient temperature and pressure. Therefore, the saturated transmission power from a sensing membrane grafted with one polymer brush density at ambient laboratory RH should be measured as the reference power signal before each sensing experiment conducted on different days.
The power difference of the saturated 0.4 THz waves, ΔV, between the test RH and the ambient RH could be used as the quantitative indicator for the hydrophilic adsorption of water vapor on the sensing mesh membrane. The negative (positive) power difference (ΔV) that represents the quantity of water vapor adsorbed on the hydrophilic mesh membrane at a specific RH is greater (less) than that at the ambient RH. The humidity-sensing experiment conducted for different polymer brush densities was separately operated at different days, and the relevant ambient laboratory RH is slightly deviated from 52% with an RH range of 44–52%, depending on the ambient temperature and pressure. Therefore, the saturated transmission power from a sensing membrane grafted with one polymer brush density at ambient laboratory RH should be measured as the reference power signal before each sensing experiment conducted in different days.
In this humidity-sensing experiment, two surface modification approaches use the P(PEGMA-
co-BPA) copolymer brush: single-side surface modification and double-side surface modification. In the single-side surface modification, the solution of copolymer brushes was dip-coated on one side of the surface of a PET mesh membrane with a circular range of 22 mm diameter. Similarly, in the double-side surface modification, the solutions of the copolymer brushes were individually dip-coated on both (bottom and up) surfaces of one PET mesh membrane with the same circular area and location. The double-side surface modification was further treated once and twice with UV curing to immobilize copolymer brushes on the PET mesh membrane. The one-time UV curing with a 900 s duration is operated after dip-coating copolymer brush solution on the two sides of the PET mesh membrane, and the immobilized polymer brush density is 3.21 mg/mm
3 (
Figure 6). For longer curing duration, surfaces on both sides of the PET mesh membrane were individually dip-coated and cured, respectively, by copolymer brush solutions and UV illumination (900 s, 10 W), corresponding to the two-time UV-curing operation. The immobilized copolymer brush density of the two-time UV-curing operation is 3.23 mg/mm
3 (
Figure 6), which is slightly higher than that of the one-time UV operation.
However, their sensitivities, as shown in
Figure 6, are approximate and much lower than that of single-side surface modification. Although the polymer brush density of a single-side surface-modified PET mesh membrane is much lower than that of the double-side surface-modified one, the sensitivity of the single-side modification is much higher than that of the double-side modification. The results in
Figure 6 indicate that the quantities of active sites for effective adsorption of water vapors on the single-side surface-modified membrane are greater than those on the double-side surface-modified one. The low amounts of water vapor active sites that mainly resulted from the grafted and cured polymer brushes on the first-side surface are re-dissolved into the additional dip-coated THF solution on the other (second)-side surface of the porous PET membrane substrate (
Figure 1c). The hydrophilicity based on the process of double-side surface modification weakens to adsorb water vapor due to the decreased amount of hydrophilic polymer brushes. Moreover, the double-side surface-modified membrane decreases the hydrophilicity when the UV-exposing intensity of the UV-curing process on the unit surface area is insufficient for the sensing membrane with a brush density of 3.21 mg/mm
3, consequently reducing the quantity of crosslinked polymeric brushes that possess hydroxyl groups.
The humidity-sensing performance of the sensing membranes with different brush densities (
Figure 6) is summarized in
Table 2, including the sensitivity (
S) and linearity (
R2) (i.e., slopes and the coefficient of determination of a linear fitting curve),
inaccuracy (
δα) in measurement (i.e., an error bar scale), detectable RH range (
R), and measured resolution of RH (
C) (i.e., the limit of detection, LOD). In addition, the sensitivity can also be represented by the power difference ratio
, defined as
, where
and
are the THz transmission power through the RH sensor at 25 and 99 %RH, respectively. The results in
Table 2 show that the sequence of humidity sensitivity for different brush densities grafted via single- and double-side surface modifications, denoted as single and double in the parentheses, is 1.57 (single) > 1.69 (single) > 0.62 (single) > 3.21 (double)
3.23 (double) mg/mm
3. The best sensitivity occurs at the brush density of 1.57 mg/mm
3 grafted on the one surface of a PET mesh membrane, and the LOD of the RH value is approximately 1%, which was estimated from the slope (
S) and
inaccuracy (
δα) (
Table 2) within the detectable range of 25–99%. This result indicates that an overly high or an overly low brush density can reduce the hydrophilicity of the sensing membrane.
For the single-layered deposition of polymer chains on the mesh membrane, increasing the polymer brush density can raise the quantities of hydroxyl groups to adsorb more water vapor. As a result, humidity sensitivity, such as the sensitivity sequence of 1.57 (single) > 0.62 (single) mg/mm
3, increases. However, an overly high grafting brush density would increase the thickness of the hydrophilic surface modification layer and reduce the adsorption efficiency of water vapor. This condition is probably due to the hydrophilic active sites of the polymer chains that are buried in the deep and inner section of the surface-modified layer to hinder water vapor diffusion and binding [
26]. For example, the depressed sensitivities were observed in the following conditions: the double-side surface modification of polymer brush and the single-side surface modification of a high brush density (e.g., 1.69 mg/mm
3). The most effective hydrophilic binding always occurs at the outermost surface, but the adsorption efficiency of water vapor decreased with the increasing layer thickness [
26].
To validate the conjecture, the water contact angle measurement was further conducted for the mesh membrane without and with hydrophilic surface modification by four types of polymeric brush densities, as shown in
Figure 7a. The sequence of contact angle for the mesh membranes grafted with different brush densities is pristine > 3.23 > 3.21 > 0.62 > 1.57 mg/mm
3, which is opposite to the sequence of humidity sensitivity, as shown in
Figure 7b. The pristine PET mesh membrane is hydrophobic with the largest water contact angle of ~90.63°, and the membrane with PEGMA brush density of 1.57 mg/mm
3 has the smallest contact angle of ~65.47°. The smaller contact angle represents the more hydrophilicity of surface for water vapor adsorption and, thus, with a high humidity sensitivity.
Figure 8a shows the dynamic RH-sensing performance of the sensing membrane grafted with 1.57 mg/mm
3 brush density for three continuous cycles of humidity exposure and exhaustion. This membrane for sensing humidity was enclosed into the humidity-controlled Teflon chamber with different saturated salt solutions (
Figure 2,
Table 1) and measured for its dynamic power transmission of the 0.4 THz wave, where the 46% RH is the ambient laboratory humidity. The dynamic recording of the THz-transmitted intensity from the sensing membrane starts when the saturated salt solution was injected in the air-sealed chamber at 0 s. During the sensing process, the water vapors are diffused, and the chamber is filled up and adsorbed on the sensing membrane until equilibrium, indicating the steadiness of the THz transmission power, as shown in
Figure 8a. While the steady state of measured THz wave power is achieved, corresponding to the equilibrium of humidity exposure, the air pump is turned on to drain the adsorbed moisture of the mesh membrane, as indicated by the red arrows in
Figure 8a. When the THz transmitted power achieved stability once again, corresponding to the equilibrium of humidity exhaustion, the air pump was turned off, as indicated by the green arrows in
Figure 8a. Then, the second cycle of humidity exposure starts for water vapor adsorption on the sensing membrane. The three repeated measurements for each RH have almost identical THz transmission power at steady states. This finding reveals that the interaction between water vapors and the hydrophilic surface, grafted with polymeric brushes, belongs to physical adsorption, and the capability of repeatable usage performs without any degradation of the sensing performance.
When the RH level in the Teflon chamber was at 44%, approximating to the ambient humidity of 46%, there was no evident variation of the transmitted THz power from the surface-modified mesh membrane within the three periods of humidity exposure and exhaustion. The transmitted THz power increased/decreased at the low/high humidity exposure, i.e., below/above the 44% RH, whose power variation trend is similar to that of the humidity-sensing results in
Figure 5b. The response and recovery times of the sensing membrane at different RHs, except for the ambient humidity, are illustrated in
Figure 8b. The results were obtained by averaging the exponential fits of the THz transmitted power curves for three cycles of humidity exposure and exhaustion in
Figure 8a. At the low RH (
35%), the water vapors are fast bonded with the vacant hydroxyl groups of the polymer brushes grafted on the membrane surface; thus, the response time is short. On the contrary, for the high RH (>75%), more vaporized water molecules existed in the chamber space, and they not only fully occupied the hydroxyl groups on the outer surface of membrane, but they also further diffused into the deeper depth of the membrane for hydrophilic adsorption, increasing the response time with an inaccuracy of 50–100 s (
Figure 8b). The recovery times of the sensing membrane (
Figure 8b), ranging from 123 to 200 s, are independent of the relative humidity and only associated with the exhausted speed of the air pump (23
/min).
The slow response time can be further improved in two aspects, including the porous polymer substrate and the humidity-sensitive layer. For the porous polymer substrate, increasing the surface area or porosity of the surface-modified mesh membrane or replacing the hydrophobic substrate with a hydrophilic one, such as the polyamide (PA) porous membrane, can greatly increase the number of moisture-adsorption sites to shorten the response time. For the humidity-sensitive layer, decreasing the thickness of a brush-coated layer can lower the diffusion resistance of water vapor within this solid layer [
7,
26], which is validated in the experimental results of
Figure 6, and, thus, the speed of water vapor adsorption rises. Additionally, hybridizing inorganic moisture adsorbents in the humidity-sensitive layer could also increase the adsorption efficiency of water vapor.
Table 3 presents the sensing performance of the proposed sensor in this work, which is compared with the recently demonstrated polymer-based humidity sensors. This summary reveals that our proposed sensor is competitive with those RH sensors in sensitivity, detectable RH range, and RH resolution. A high linearity of 98% among RH-dependent THz signals has been verified within a wide RH range. Moreover, our proposed sensor also has advantages of simple configuration, compactness, flexibility, low cost, and an easy fabrication process.