The Microplastics Iceberg: Filling Gaps in Our Understanding
Abstract
:1. Introduction
2. Emerging Contamination: From Plastic to Microplastics
3. The Ubiquity of Microplastics in the Environment: Input Pathways and Transport
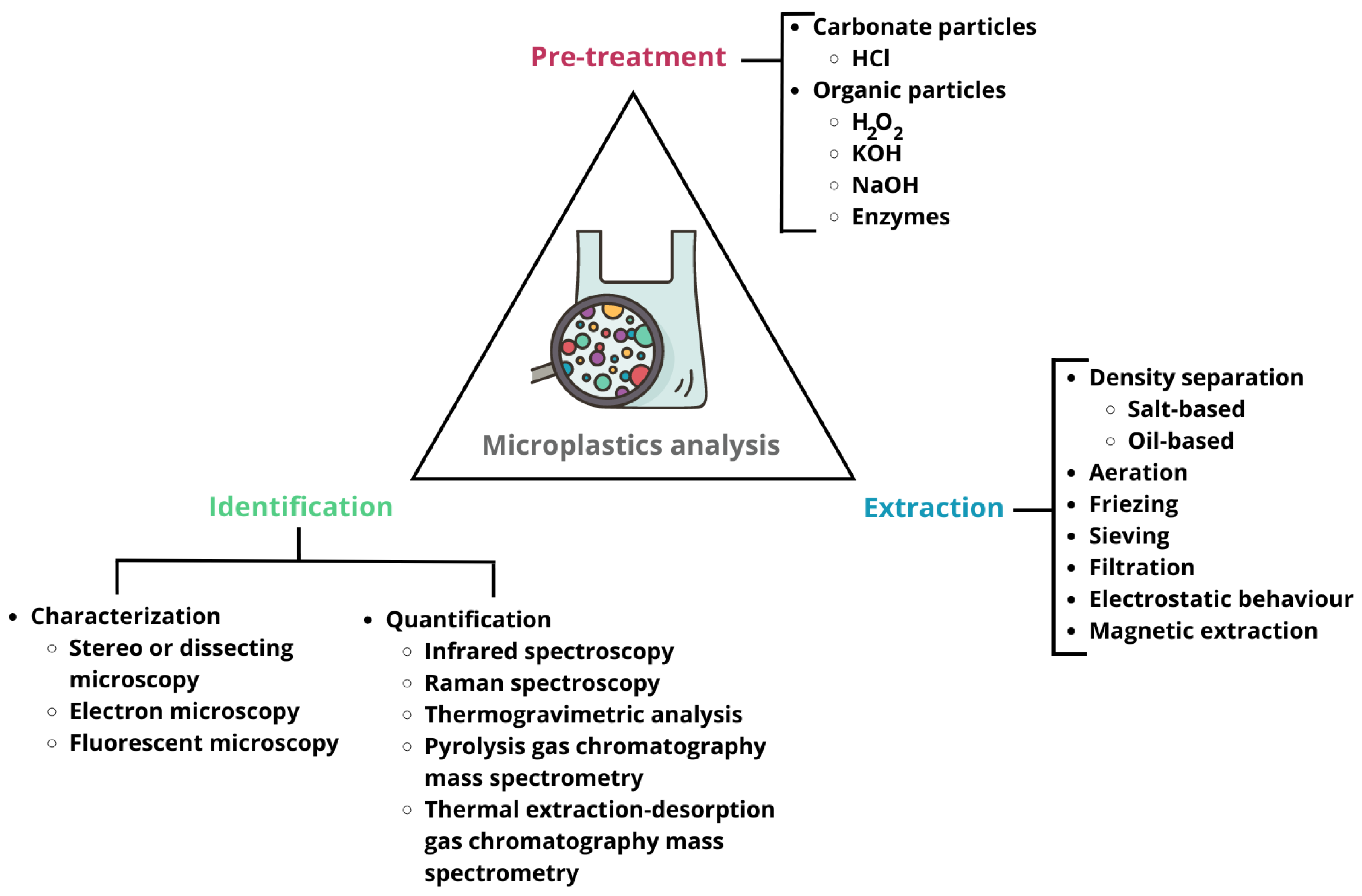
4. Unveiling Microplastics in the Environment: Advances and Challenges in Analytics
| Sample Type | Location | Study Findings | Pretreatment | Extraction and Identification Techniques | Ref. |
|---|---|---|---|---|---|
| Beach sand | The Netherlands | Intertidal zone: 29.10 ± 17.75 MPs per 50 g dw High tidal line: 25.20 ± 12.37 MPS per 50 g dw Supralittoral zone (30 m from the dunes): 21.30 ± 3.62 MPs per 50 g dw Supralittoral zone (10 m from the dunes): 25.90 ± 10.91 MPS per 50 g dw | Samples were dried (48 h, 75 °C), and stored at room temperature for extraction. | Samples were floated in NaCl solution (358.9 g L−1). After mixing for 2 min at 600 rpm, the mixture settled for 6 h, then the supernatant was filtered. MPs were counted on filter papers using a stereomicroscope. NaCl flotation was used. | [83] |
| Quartz sand Freshwater sediment Beach sand | Germany | 99% of the original sample mass was removed without loss of MPs. 150 g quartz sand reduced to 2.34 ± 0.17 g. 150 g freshwater sediment reduced to 2.33 ± 0.13 g. 150 g beach sand reduced to 2.00 ± 0.04 g. 150 g particulate matter reduced to 2.51 ± 0.23 g. | Sample was sieved through 20, 63, 200, 630, and 2000 mm sieves. 20 g of freeze-dried material, 100 mL of bidistilled water, and agate balls were added to each sieve, that was sonicated for 1 min, and sieved manually. The procedure was repeated 10 times and the remaining fractions were centrifuged and dried at 105 °C. | 150 g of sample material was spiked with 10 particles of each type of MP. Separation using the KWS electrostatic method, and each fraction was separated 3 times. Samples were digested, subjected to density separation, and characterized by Py-GC-MS. | [102] |
| Sand River sediment | Mass of sand was reduced by 98% after electrostatic separation. Mass reduction of sediment samples reduced by 70–78% after electrostatic separation, and above 99% after density separation. Recovery of MPs was polymer-specific: PCL: 50 ± 8%–74 ± 9%; LD-PE: 93 ± 9%–114 ± 9%; and PET 82 ± 11%–120 ± 18%. | Samples dried at 60 °C. Particles with diameters >2 mm were removed by sieving. Samples spiked with PCL, LD-PE, and PET (63–200 µm). | Electrostatic separation combined with density separation, identification, and quantification by DSC. | [104] | |
| Estuarine sediments | Japan | Fenton’s reagent affected the size of PE and PET. Recovery rates for density separation using an overflow column with top inflow (OC-T): 90.7% (±7.7% SD) for large MPs (>0.5 mm) and 95.0% (±12.5% SD) for small MPs (<0.5–0.160 mm). 91.7% of stained particles were confirmed as MPs. | 2 protocols were tested for organic matter removal: (1) 20 mL of a 30% H2O2 added to 60 mL of sample; (2) 20 mL of a 0.05 M Fe(II) solution (2.5 g of FeSO4.7H2O, 165 mL water and 1 mL of concentrated H2SO4) added to 60 mL of sample. Samples were left to react for 24 h at room temperature and then freeze-dried at 80 °C under vacuum. | Density separation was tested using 4 columns, filled with ZnCl2 solution (1.5 g cm−3): an SMI unit; a simple decanting column; an OC-T; and an overflow column with mid-level inflow (OC-M). MPs were concentrated on vacuum glass fiber filters and drops of a Nile red solution (1 mg Nile red dye in 1 mL 99.5% acetone, diluted with 100 mL distilled water) were spread. Stained filters were inspected with a binocular microscope. Automated epifluorescence microscopic image analysis of Nile red-stained filters with FT-IR validation for polymer identification. | [111] |
| Beach sediments | Foamed polystyrene (FPS) (0.3 to <5 mm): 80–17,500 particles m−2; 5–1206 pieces kg−1 dw PE and PP (0.3 to <5 mm): 0–1640 pieces m−2; 0–200 particles kg−1 dw | - | Samples floated in saline solution (200 mL:100 mL sediment) for 30 s, settled for 2 min, and then sieved through a 355 μm mesh. The residue was rinsed, vacuum-filtered, and MPs were collected via visual sorting and stereomicroscopy. Identification was done with FT-IR, and structural analysis utilized digital microscopy, FE-SEM, and X-ray CT. | [69] | |
| Bottom sediments | FPS (0.3 to <5 mm): 552–9128 particles m−2; 13–221 particles kg−1 dw PE and PP (0.3 to <5 mm): 210–1210 particles m−2; 5–29 particles kg−1 dw | Samples sieved through 5.6 mm and 355 μm mesh. | |||
| Bay surface water | FPS (0.3–<5 mm): 0.004–0.06 particles m−2 PE and PP (0.3–<5 mm): 0.03–0.17 particles m−2 | The residues on the 355 μm mesh were rinsed with distilled water and vacuum-filtered. MPs were collected from samples by visual sorting and stereomicroscopy. Identification was performed using FT-IR and the structure was analyzed by digital microscopy, FE-SEM, and X-ray CT. | |||
| Snow | Arctic and Alps | MPs: 0.02 × 103–154 × 103 N L−1 (80% ≤ 25 µm; 98% < 100 µm) Microfibers: 0.043 × 103–10.2 × 103 N L−1 (maximum length: 97% < 5 mm; 31% < 500 µm) European fibers were significantly longer than those from Arctic snow. | - | Samples were filtered using aluminum oxide (Al2O3) filters and dried in a desiccator for 2 days. Particles retained on filters were analyzed by FT-IR imaging. | [30] |
| Aqueous solution | Linearity (R2) > 0.994 Precision: 99.4–99.9%. Limit of detection (LOD): 19–21 μg mL−1 Limit of the quantification (LOQ): 74–85 mg mL−1 | MPs (PE granules < 300 μm, PET fibers 500 μm, and PS beads 0.5–1 mm) dissolved in a deuterated solvent at 50 °C | Samples were analyzed by NMR spectroscopy. | [113] | |
| Freshwater (inlet WTP) | Czech Republic | 1473 ± 34–3605 ± 497 particles L−1 Common sizes: 1–10 μm Common types: fibers, spherical, and fragments Common polymers: PET and PP | Oxidize each sample with 20 mL of 0.05 M Fe (II) and 20 mL of 30% H2O2, and stir for 30 min at 75 °C in 250 mL of sample. Allow samples to digest for 24 h. | Samples vacuum-filtered in two-step descending-mesh-size, dry PTFE filters (30 °C, 30 min). Particles retained on filters were analyzed by SEM; particles > 10 μm were analyzed by FT-IR; particles 1–10 μm were analyzed by μ-Raman spectroscopy. Elemental analysis was performed on selected particles using SEM-EDX. | [78] |
| Drinking water (outlet WTP) | 338 ± 76–628 ± 28 particles L−1 Common sizes: 1–10 μm Common types: fibers, spherical, and fragments Common polymers: PET PP and polyacrylamide | ||||
| Wastewater (effluent) | Germany | MP > 500 µm: 0 × 101–4 × 101 particles m−3 MP < 500 µm: 8 × 101–9 × 103 particles m−3 Common polymers: PE (59%) and PP (16%) | Samples were incubated at 70 °C for 24 h, then treated with enzymes (protease at 50 °C for 48 h, lipase at 40 °C for 96 h, and cellulase at 50 °C for 6 days). Finally, each sample was filtered through a 500 μm PA net. | <500 μm fractions were filtered with 10 μm stainless-steel screens, and then treated with H2O2 (35%) at 37 °C for 48 h, followed by incubation at 50 °C for 24 h. MPs were identified using ATR-FT-IR and μ-FT-IR spectroscopy techniques. | [56] |
| Sewage sludge | MP > 500 µm: not detected MP < 500 µm: 1 × 103–2.4 × 104 particles kg−1 dw | 125 g of each sample was diluted in 825 deionized water and then mixed with 400 g solid NaOH (24 h at 60 °C). Samples were neutralized with HCl. | Flotation with NaCl (1.14 g cm−3). After 96 h, supernatants were rinsed over a 500 μm PA net. Residues were visually inspected using an optical microscope and identification was carried out using ATR-FT-IR. Aliquots of fraction <500 μm filtered using 0.2 µm Al2O3 filters and analyzed by μ-FT-IR. | ||
| Lake water | Canada | 53,000–748,000 particles km−2 Common types: fibers (90%), films, and foam | Samples were filtered through a 250 μm mesh sieve and rinsed with deionized water. Each 250 mL sample was oxidized with 20 mL of Fe (II) (0.05 M) and 20 mL of 30% H2O2 while stirring for 30 min at 75 °C. Samples digested for 24 h. | Each sample was filtered through a 250 μm sieve. MPs were visually identified using a dissecting microscope and SEM-EDX. | [3] |
| Lake water | Finland | Total MP: 0.3 ± 0.1 particles L−1 Microfibers: 0.2 ± 0.1 particles L−1 | Samples were sieved and dried (40 h, 75 °C). Dried samples were oxidized with 20 mL of Fe (II) (0.05 M) and 60 mL of H2O2 (30%). Samples settled for 5 min, then were shaken and heated up to 75 °C. | Samples were vacuum-filtrated, and the glass filters were left to dry for 24 h at room temperature. Identification was performed by optical microscopy, FT-IR, and Raman spectroscopy. | [73] |
| Wastewater (influent) | Total MP: 56.7 ± 12.4 particles L−1 Microfibers: 52.6 ± 11.3 particles L−1 | Samples were sieved and dried (40 h, 75 °C). Dried samples were oxidized with 20 mL of Fe (II) (0.05 M) and 20 mL of H2O2 (30%). Samples settled for 5 min, then were agitated and heated up to 75 °C. | |||
| Sewage sludge | Activated sludge: 23 ± 4.2 particles g−1 dw microfibers: 21.7 ± 4.6 particles g−1 Digested sludge: 170.9 ± 28.7 particles g−1 dw microfibers: 161.0 ± 25.5 particles g−1 | Samples were stirred and subsamples (0.1 g dw) were dried at 45 °C for1 8 h. | Identification was performed by optical microscopy, FT-IR and Raman spectroscopy. | ||
| Wastewater (influent) | Spain | Primary effluent (451 ± 106 particles L−1) Fragment length: 53–2100 µm Fiber length: 104–4000 µm Secondary effluent (26 ± 14 particles L−1) Fragment length: 41–2890 µm Fiber length: 144–1824 µm | Filtration through 3 stainless steel meshes (375, 104 and 25 μm). Filters were exposed to H2O2 (33%) at 50 °C. After 20–24 h, the filters were rinsed with deionized water. | NaCl flotation was conducted, and samples were stirred for 24 h followed by settling for 24 h. Both supernatant and sediment were then filtered. Identification was performed using stereomicroscopy and µ-FT-IR. | [8] |
| Sewage sludge | Wet sludge (314 ± 145 particles L−1) fragment length: 36–377 µm fibers length: 213–4716 µm Dry sludge (302 ± 83 particles L−1) fragment length: 29–533 µm fibers length: 71–2224 µm | 30 mL of H2O2 (33%) was added to 1 g of sludge (at 50 °C). | |||
| Sewage sludge | China | 5553–13,460 particles kg−1 Common polymers: PET, PE, and polyacrylonitrile | Samples were dried, sieved, and predigested with 30% H2O2 at 70 °C. | Three flotation solutions (NaI, ZnCl2, and NaCl) with respective densities of 1.8, 1.5, and 1.2 g cm−3 were compared. Four membrane types (quartz, glass fiber, PTFE, and nylon) were tested for vacuum filtration of the supernatant. MPs on the membranes were digested with 30% H2O2 and H2SO4 at 70 °C for identification using stereomicroscopy and µ-FT-IR. | [81] |
| Soil | 420–1290 particles kg−1 Common polymers: PP, PE | ||||
| Alluvial soil | Slovenia | Recovery rate: Oil-based extraction: >97% for all vessels in alluvial soil; 80% for modified syringes in compost. Salt-based extraction: >93% and >98% for alluvial soil and compost, respectively The ZnCl2 solution could be reused up to 20 times without losing the desired density, reducing cost and environmental impact. Protocol was chosen to extract PE, PP, PS, PVC, and PET at various concentrations (10, 25, and 50 MPs) with cumulative recoveries >93% for all spiked MPs. | - | Oil-based extraction: 30 mL of H2O and 3 mL of olive oil were added to the sample. The vessel was capped and shaken. After 2 h of standing, the samples were frozen overnight at −18 °C. The frozen oil layer was removed, melted, and filtered through a GF/C filter (47 mm). The MPs were rinsed with n-hexane and H2O. Salt-based extraction: ZnCl2 solution (1.6 g cm−3; pH 3) was filtered with GF/C filter. The sample was transferred to 50 mL centrifuge tube and a ZnCl2 solution was added to the 50 mL mark. After shaking for 30 s, it was centrifuged at 9000 rpm for 15 min. The supernatant was filtered through a GF/C filter. For compost, an oxidation step was performed by submerging the filter in 60 mL of Fenton’s reagent for 2 h under constant stirring. The oxidized sample was filtered through a GF/C filter. The optimal extraction method was tested on samples spiked with 10, 25, and 50 MPs. Identification of MPs via HS-SPME-GC-MS. | [87] |
| Biowaste compost | 10 g of compost with MPs (10 and 20 MPs of LDPE or PET) was added to 60 mL of Fenton’s reagent and left for 2 h under constant stirring. The sample was filtered through the GF/C filter, and the sample remaining on the filter was oxidized using 50 mL of NaOH (1M) under constant stirring overnight at 50 °C. The sample was filtered through a 100 µm stainless-steel mesh. | ||||
| Lagooning sludge | Morocco | Fresh sludge: 40.5 ± 11.9 × 103 kg−1 (dw) Dewatered sludge: 36 ± 9.7 × 103 kg−1 (dw). Sludge dewatering resulted in a loss of MPs <500 μm. The quantity of MPs in compost varied with the proportion of sewage sludge. | 20 g of dry sample treated with Fenton’s reagent (H2O2 30% (v/v) + FeSO4 solution 20 mg mL−1). | For Py-GC/MS analysis, 1 mg of the freeze-dried sample was used. For Nile red staining, 1 g of digested sample was mixed with 50 μL Nile red (1 mg mL−1) and incubated on a shaker at 100 rpm for 60 min. MPs were separated using a ZnCl2 solution (1.38 g cm−1) and centrifuged. The supernatant was filtered through 0.45 μm cellulose nitrate filters with a graduated grid (100 μm). The filter paper was observed using a fluorescence microscope under blue light. Identification of suspected MPs using Raman spectroscopy | [112] |
| Soil Sediment Compost Sewage sludge Suspended particles of WWTP Indoor dust | Germany | Recoveries rates for PET: 94.5–107.1% Limit of determination 0.031 mg PET Limit of quantification 0.121 mg PET PET mass contents in environmental samples: <LOQ in agriculture soil up to 57,000 mg kg−1 in dust samples. | 20 g of sample was mixed with 20 mL of 1-butanol and 1 g of potassium hydroxide pellets. The mixture was heated to 115 °C in an oil bath under constant stirring for 1 h. Then, 50 mL of ultrapure water was added, and the system was mixed for 1 h at 300 rpm. The extract was vacuum-filtrated using a glass-fiber filter. Next, 10 mL of the aqueous phase was diluted (1:10) with ultrapure water, and the pH was adjusted to 2.5, using HCl (10%). Determination of the monomers, terephthalic acid, using LC-UV. Mass content verification using TED-GC-MS. | [110] | |
| Biosolids | Ireland | 4196–15,385 particles kg−1 dw. Common polymers: HDPE, PE, PET, PP, and PA. Common types: fibers (75.8%), fragments, films, and spheres | Biosolids treated by composting or thermal drying (TD): samples were soaked in water for 1 week, then transferred to a water bath (30 °C) for 24 h and shacked for 12 h. The samples were sieved through a 250 μm mesh. Biosolids treated by lime stabilization (LS) and biosolids treated by anaerobic digestion (AD): samples were soaked in tap water and washed through 250, 212, 63, and 45 μm sieves. | TD and AD Extraction: each sample (40 g) was elutriated. The extracted mixture was filtered through a 250 μm mesh, rinsed with ZnCl2 (1 M), and brought to a volume of 300 mL. The mixture was shaken (1 min) and settled (20 min). The settled material was drained, and the remaining sample was filtered (glass-fiber filter).LS Extraction: 10 g from each sample was filtered. Identification: Stereomicroscopy, ATR-FT-IR, and SEM. | [57] |
| Compost | Finland | Mean recovery rate: 90.5% for PE, PU, polycarbonate (PC), PET, PVC, and PS; 30% for tire wear particles (TWP). Sewage sludge compost: 2 acrylonitrile (butadiene styrene) (ABS) fragments, and 1 PE fiber. Biowaste compost: 1 PE fragment, 1 PET fiber, 1 ABS fragment and 1 blend ABS/PET fiber. | 10 mL H2O2 (30%), 1 mL of FeSO4.7H2O (2 mmol L−1), 1 mL of protocatechuic acid (2 mmol L−1), and 5 mL of H2O were added to 1 g of soil. Samples were kept in the hood for 1 h and then in the oven (40 °C) overnight. | Oxidized samples were transferred to custom-made PTFE tubes with 3 mL of olive oil, left to stand for 2 h, and frozen at −40 °C. Samples were filtered using glass microfiber filters. Filters were rinsed with water and n-hexane, air dried, and the MPs were collected with tweezers for identification with ATR-FT-IR. Each sample was extracted three times. | [84] |
| Agriculture soil | Mean recovery rate: 96.5% for PE, PU, PC, PET, PVC, and PS; 43% for TWP. 1 ABS fiber and PS fragment detected in soil from Mäkelä. | ||||
| Amended agricultural soil | Chile | 18–41 particles g−1 Common types: microfibers | Samples were milled (porcelain mortar), sieved (<1 mm), and dried. | 5 g of each sample was mixed with 20 mL of deionized water, centrifuged, and filtered with filter paper. 20 mL of NaCl was added to the sample, which was centrifuged and filtered with filter paper. 20 mL of ZnCl2 was added, centrifuged, and filtered with filter paper. MPs identified by stereomicroscopy. | [70] |
| Agricultural soil | Chai river valley, China | 0–5 cm layer: Fibers: 11,130–24,850 particles kg−1 Strings: 10–60 particles kg−1 Films: 460–1110 particles kg−1 fragments: 230–1360 particles kg−1 5–10 cm layer: Fibers: 9780–26,940 particles kg−1 Strings: 40–70 particles kg−1 Films: 410–1290 particles kg−1 Fragments: 170–15,700 particles kg−1 | 10 mL of H2O2 (35%) was added incrementally to 30.0 g of soil sample. FeSO4 (10%, 1 mL) was added, and sample was heated on sand bath (50 °C). After organic matter destruction, FeSO4 (10%, 1 mL) and NaOH (0.5 M, 30 mL) were added. Volumes were adjusted to 150 mL with distilled water, sonicated, and centrifuged. | Supernatant was collected and 150 mL of a saturated NaI solution (1.8 g cm−3) was added to the soil, followed by centrifugation. The procedure was repeated. The collected supernatant was filtered (1, 0.25, and 0.05 mm mesh). H2O2 (2 mL) was added for the digestion of labile organic. Solids were washed with distilled water, transferred to clean glass containers and oven-dried at 80 °C. MPs were optically sorted by a dissecting microscope. | [28] |
| Agricultural soil | Middle Franconia, Germany | 0–1.25 particles kg−1 dw 16 particles per 50 kg dw (5–1 mm): PE (62.50%, 10 particles) PP (25.00%, 4 particles) | Soil aggregates dissolved by adding 20 mL of H2O2 to 500 mL of sample. Size fractioning using sieves with mesh size of 5 and 1 mm. The procedure was repeated until all aggregates were dissolved. | MPs optically sorted under a magnifying lamp and by stereomicroscopy. Identification and quantification carried out by ATR-FT-IR. | [58] |
| Soil | United Kingdom | Average recoveries using high-gradient magnetic separation (HGMS) system: 96% for fibers and 92% for MPs in loam; 91% for fibers and 87% for MPs in high-carbon loamy sand; 96% for fibers and 89% for MPs in sandy loam; 97% for fibers; and 94% for MPs in high-clay sandy loam. Agricultural soil: HGMS extracted an average of 14 ± 4 fibers and 3 ± 1 MPs per 8 g sample extracted using HGMS; 8 ± 3 fibers and 1 ± 1 MPs per 8 g sample extracted by density separation. | 20 mL of ethanol was added to 4 g of soil spiked with 30 MPs (<1% (w/w)) which were prepared in the laboratory. Magnetic soil particle removal for each soil sample using HGMS system. MPs magnetized with modified iron nanoparticles. | HGMS: the sample was introduced in the system and the retained fraction containing MPs was filtered onto 1.2 μm Whatman GF/C glass-microfiber membrane filters. Density separation: 50 mL of a saturated zinc bromide solution (density ≈ 2.4 g mL−3) was added to the soil. The sample was shaken at 300 rpm for 5 min and settled overnight. The recovered material was treated with 10 mL 30% H2O2 for 1 h at 60 °C and filtered. The MPs were counted directly on the filter using a stereo microscope. ATR-FT-IR was used to confirm the compositions of the MPs. | [98] |
| Loamy sand (standard) | Germany | LOD: 0.07 wt % PET; LOQ: 1.72 wt % PET. MS signal intensities linearly responding to MPs concentrations. | Samples (with 1.61 ± 0.15 wt % organic matter) spiked with 0.23–4.59 wt % PET recycled MPs. | Calibration series was prepared with a standard loamy sand with 1.61 ± 0.15% organic content. DL-cysteine was used as the internal standard. PET quantified by TGA-MS. | [114] |
| Soil (standard) | Validation parameters for plastic contents of 250 μg g−1: R2 > 0.996, LOD: 1–86 ng Precision: 3.2–7.2% Recoveries: 70–128% The addition of nontarget polymers (PET, PVC and TWP) did not interfere with the quantification of the analytes. | Each reference soil (4 g) was spiked with 0.2 and 1.0 mg of PE, PP, and PS. Three clean-up protocols were tested: (1) adding 8 mL of methanol to the sample and agitating it for 60 min at 150 rpm, followed by centrifugation and evaporation of the supernatant; (2) performing Fenton digestion by adding 10 mL of FeSO4.7H2O solution (20 g L−1, pH 2) and 10 mL of H2O2 (30%) to the spiked soil, followed by 60 min in an ice bath and heating to 60 °C; (3) adding 4 mL of KAl(SO4)2.12H2O solution (500 mgL−1) to the soil, shaking for 60 min at 150 rpm and evaporation. Nonspiked soil and soil spiked with 0.2 mg of nontargeted plastics were also included for testing | Polymers extracted using 1,2,4-trichlorobenzene (TCB) and liquid sample aliquots analyzed by Py-GC-MS. | [115] | |
| Road Dust | Korea | Tire and road-wear microparticles (TRWMPs): 6,400–39,738 μg/g. Average concentration of TRWMPs in the industrial area of 22,581 μg/g and in the residential area of 9054 μg/g. | Moisture content was removed at 120 °C. | TGA to compare the thermal profile of road dust and tire tread particles; EGA-MS analysis to compare the organic composition of road dust and tire tread powder; identification and quantification of TRWMPs using Py-GC-MS. | [77] |
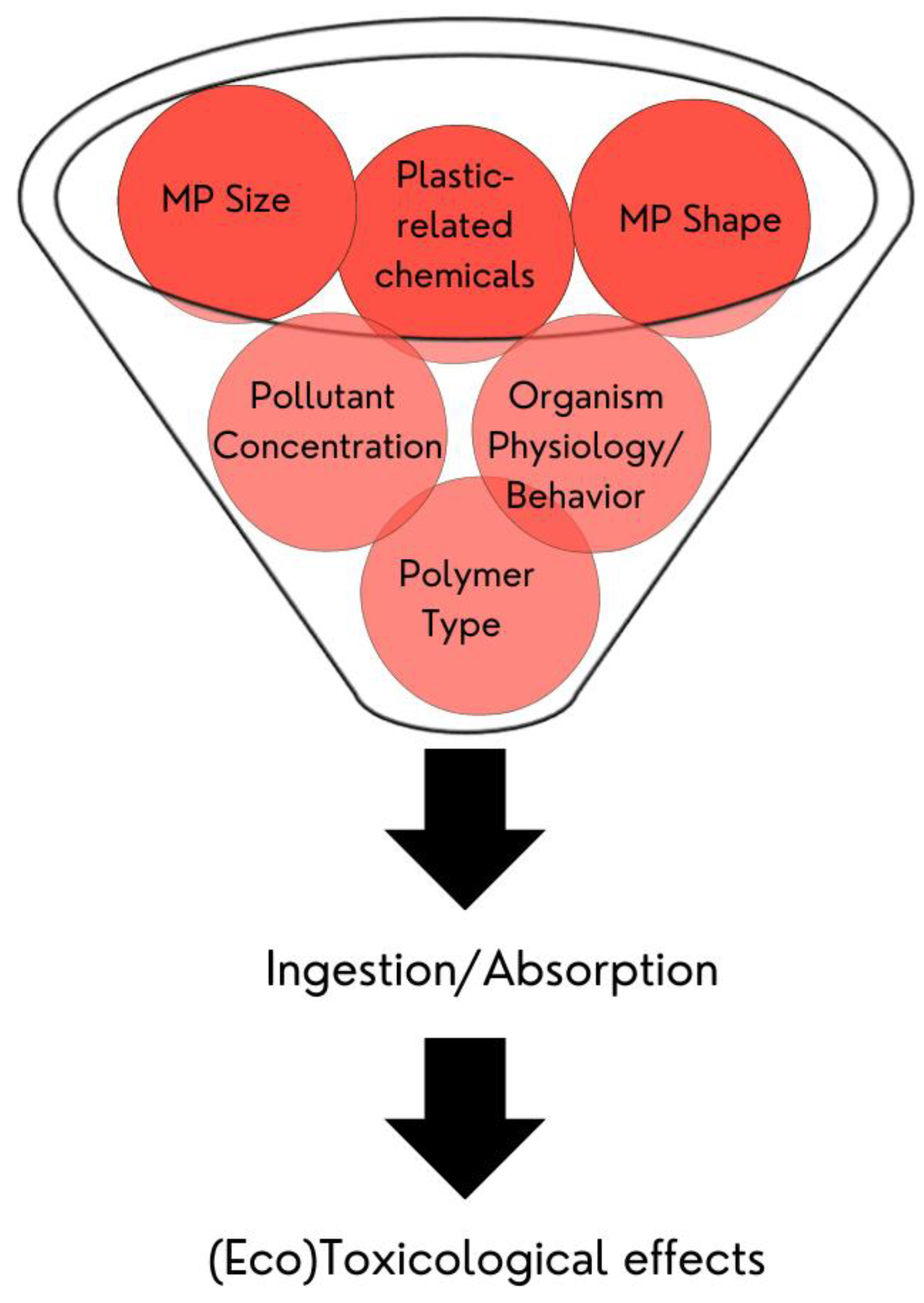
5. (Eco)Toxicological Effects of Microplastics
| Species | Exposure Characteristics | Toxicological Effects/Findings | Ref. |
|---|---|---|---|
| Artemia salina | PS (spherical; 5 μm); 1–100 mg L−1; 48 h and 14-day exposure tests | Deformation of epithelial cells in the midgut region after both acute exposures at 100 mg/L and chronic exposure at 1 mg L−1 | [117] |
| Carassius auratus | EVA (fiber; 0.7–5.0 mm), PS (fragments; 2.5–3.0 mm), PA (pellet; 4.9–5.0 mm); 6 weeks of exposure | Fibers detected in gills, gastrointestinal tract and feces; severe alterations in the livers of fish exposed to fibers; severe breakage of the dermal layer with hemorrhages in the lower jaws of fish exposed to fragments; hypertrophy of mucous cells in the lower jaw in fish exposed to pellet. | [11] |
| Cyprinodon variegatus | PE (spherical, 150–180 µm; irregular, 6–350 µm); 50 and 250 mg L−1; 4-day exposure test | Intestinal distention provoked by the accumulation of MPs in the digestive system; decrease in swimming activities; oxidative stress induced by irregularly shaped MPs. | [10] |
| Danio rerio | PS (fibers, fragments, beads); 50–500 μg L−1; 21-day exposure test | Shape-dependent accumulation in the gut: fibers > fragments > beads; mucosal damage and increased permeability; inflammation and metabolism disruption; gut microbiota dysbiosis and bacteria alterations. | [13] |
| PS (spherical; 5 μm); 50–500 μg L−1; 21-day exposure test | Accumulation of MPs in the gut; inflammation and oxidative stress in the gut tissues; gut microbiome perturbations: Proteobacteria decreased and Fusobacteria increased. | [14] | |
| PS (spherical; 50–500 nm); 0.1–10 mg L−1; 14-day exposure test | Tissue of the amputated plane was penetrable by MPs; inhibition of fin regeneration, both morphologically and functionally, of amputated larvae. | [15] | |
| PA, PE, PP, PVC (~70 μm); PS (0.1–5.0 μm); 0.001–10.0 mg L−1; 10-day exposure test | PA, PE, PP, and PVC caused intestinal damage including cracking of villi and splitting of enterocytes. | [16] | |
| MPs: PS (700 nm) | Signs of accumulation of particles around the heart region and within the blood stream; systemic immune responses; lipid metabolism and toxicity pathway significantly enriched. | [17] | |
| Fertilized eggs exposed to MPs (2 mg/L, red fluorescent spherical polymer particles), copper (Cu), and Cu + MPs, 96 h exposure | MPs did not significantly impact the early life stages of zebrafish; increased mortality, inhibition hatching rate, oxidative stress, AChE inhibition, and behavioral changes in zebrafish in the presence of Cu; antagonistic response of MPs to Cu. | [144] | |
| Dicentrachus labrax | Mixture of environmental MPs (35.29% PEVA; 5.88% HDPE; 17.65% PE; 11.76% LDPE; 23.53% PA; 5.88% PP); fragments and fibers (5–1 mm and 1 mm–300 µm) | Imbalance in the enzymatic defense mechanisms after a short-term exposure to MPs ranging from 1 mm to 300 µm. | [47] |
| Mytilus edulis | PLA (<250 μm); 10 μg/L and 100 μg L−1 8-day exposure test | No significant signs of oxidative stress or neurotoxicity; slight increase in CAT and glutathione-S-Transferase (GST) biomarker activities was observed. | [145] |
| Sparus aurata L. | PVC (40–150 μm); 100 and 500 mg kg−1; 30-day exposure test | Ingestion did not produce any significant alteration in humoral and cellular immunity; evidence of cellular and oxidative stress and damage in the liver and kidney. | [12] |
| Daphnia magna | PVC, PUR and PLA (<59 µm); 10–500 mg L−1 | PVC toxic to reproduction; PLA reduced survival. | [38] |
| PS (6 µm); 5–300 mg L−1; 120 h and 80-day exposure tests | EC50 of 34.3 (19.8–59.3) mg L−1 for juveniles and 52 (17.7–152.3) mg L−1; growth rate of mother animals and the body size of newborn declined with increasing dose of MPs. | [116] | |
| Caenorhabditis elegans | PA, PE, PP, PVC (~70 μm); PS (0.1–5.0 μm); 0.5–10.0 mg m−2; 2-day exposure test | 5.0 mg m−2 significantly inhibited survival rates, body length, and reproduction; oxidative stress and changes in intestinal calcium levels. | [16]] |
| Coturnix japonica | Naturally aged PS (fragments); 9-day oral exposure tests; three experimental groups (0, 11, and 22 MPs per day) | Lower body mass; increased ROS levels in muscle and liver, and higher production of MDA in various organs; reduction in SOD activity in the liver and intestine; MPs’ size and shape altered as they moved through the gastrointestinal tract. | [146] |
| Eisenia andrei | Tire particles (<600 µm) and PS (<500 µm, containing 1% hexabromocyclododecane (HBCD)); 28-day exposure test | No mortality, morphological, or avoidance behavior changes observed; AChE activity was not significantly affected after 14 and 28 days. | [147] |
| Eisenia fetida | PS (fragments, 100–1300 nm); 100–1000 μg kg−1; 14-day exposure test | Intestinal cells were damaged; changes in GSH level and SOD activity; induction of damages in DNA. | [119] |
| Enchytraeus crypticus | PA and PVC (13–18 μm; 90–150 μm); 20–120 g kg−1; 21-day exposure test | EC50 of 108 ± 8.5 g kg−1 for PA 13–18 μm size range; 25% reduction in reproduction for PA 90–50 μm. | [120] |
| Folsomia Candida | PVC (80–250 μm); 1 g Kg−1 dw; 56-day exposure test | Alterations of microbial community of the gut; significant reduction in body weight and reproduction; carbon and nitrogen contents increased in tissues. | [118] |
| Aporrectodea rosea | PLA, HDPE and fibers | The biomass of A. rosea exposed to HDPE was significantly reduced. | [25] |
| Allium fistulosum | PE (fibers); PA (spherical); PE, PS, Polyester, and PET (fragments) | Changes in biomass, tissue elemental composition, root traits, and soil microbial activities. | [121] |
| Cucumis sativus L. | PS-NPs (100, 300, 500, and 700 nm); 10 mg mL−1, 65-day exposure test | Decreased biomass induced by 300 nm PS-NPs; decreased chlorophyll content and soluble sugar induced by 300 nm PS-NPs; increase in the contents of MDA and proline in leaves induced by 700 nm PS-NPs. | [132] |
| Lepidium sativum | PP, PE, PVC, PE + PVC (0.125 mm); 184 ± 4 mg kg−1 | Effect on growth; induction of oxidative stress. | [122] |
| Lycopersicon esculentum Mill | One-month-old plants transplanted outdoors into 3.3 L terracotta pots with five different soil compositions: control, manure control, and soil + sewage sludge 109-days exposure test | Lower crop in plants grown under soil mixed with sewage sludge. | [131] |
| Lolium perenne | PLA, HDPE and fibers | Germination decreased with PE and fibers; reduction in shoot height with PLA; increase in chlorophyll a/chlorophyll b ratio suggesting a stronger inhibition of chlorophyll b synthesis in response to MPs. | [25] |
| Oryza sativa | PS and PTFE (~10 mm) combined with arsenic (As) III in Hoagland nutrient solution; 0.04 g L−1 MPs + 1.6 mg L−1 As(III); 0.1 g L−1 MPs + 3.2 mg L −1 As (III); and 0.2 g L−1 MPs + 4.0 mg L −1 As (III) | PS and PTFE hinder root growth and transpiration; As (III) damages chloroplast structure, reducing photosynthetic capacity and biomass, and impairing antioxidant enzyme structure, leading to membrane lipid peroxidation and membrane structure destruction; the presence of MPs restricts the uptake of As(III) in rice seedlings, reducing its content in tissues. | [148] |
| Triticum aestivum | LDPE, PET, PBT and starch-based biodegradable MP | Biodegradable MPs inhibited wheat growth and decreased the fruits and the shoot biomass. | [149] |
| Vicia faba | PS (5 μm and 100 nm); 10–100 mg L−1; 48 h exposure test | Inhibition of growth; induction of oxidative damage; accumulation of 100 nm sized MPs in root. | [150] |
6. What Does the Future Hold?
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef] [Green Version]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2019, 703, 134722. [Google Scholar] [CrossRef]
- Li, Y.; Chang, Q.; Duan, H.; Liu, Y.; Zhang, J.; Li, J. Occurrence, levels and profiles of brominated flame retardants in daily-use consumer products on the Chinese market. Environ. Sci. Process. Impacts 2019, 21, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, D.; Nowack, B. Polymer-Specific Modeling of the Environmental Emissions of Seven Commodity Plastics As Macro- and Microplastics. Environ. Sci. Technol. 2019, 53, 9664–9676. [Google Scholar] [CrossRef] [Green Version]
- Gago, J.; Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J.; Vazquez, A.; Carlos, J.; Zeferino, A.; et al. Standardised Protocol for Monitoring Microplastics in Seawater; JPI-Oceans BASE-MAN project; JPI Oceans AISBL: Brussels, Belgium, 2018. [Google Scholar]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef]
- Bellasi, A.; Binda, G.; Pozzi, A.; Galafassi, S.; Volta, P.; Bettinetti, R. Microplastic Contamination in Freshwater Environments: A Review, Focusing on Interactions with Sediments and Benthic Organisms. Environments 2020, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Jung, Y.-J.; Hong, N.-H.; Hong, S.H.; Park, J.-W. Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 2018, 129, 231–240. [Google Scholar] [CrossRef]
- Jabeen, K.; Li, B.; Chen, Q.; Su, L.; Wu, C.; Hollert, H.; Shi, H. Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 2018, 213, 323–332. [Google Scholar] [CrossRef]
- Espinosa, C.; Cuesta, A.; Esteban, M.Á. Effects of dietary polyvinylchloride microparticles on general health, immune status and expression of several genes related to stress in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 68, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Tian, L.; Gao, G.; Peng, S.; Zhang, J.; Wu, D.; Huang, J.; Hua, Q.; Lu, T.; Zhong, L.; et al. Inhibitory effects of polystyrene microplastics on caudal fin regeneration in zebrafish larvae. Environ. Pollut. 2020, 266, 114664. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Veneman, W.J.; Spaink, H.P.; Brun, N.R.; Bosker, T.; Vijver, M.G. Pathway analysis of systemic transcriptome responses to injected polystyrene particles in zebrafish larvae. Aquat. Toxicol. 2017, 190, 112–120. [Google Scholar] [CrossRef]
- Plastics–The Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Brussels, Belgium, 2019. [Google Scholar]
- Namazi, H. Polymers in our daily life. Bioimpacts 2017, 7, 73–74. [Google Scholar] [CrossRef]
- Payne, J.; McKeown, P.; Jones, M.D. A circular economy approach to plastic waste. Polym. Degrad. Stab. 2019, 165, 170–181. [Google Scholar] [CrossRef]
- Independent Group of Scientists Appointed by the Secretary-General. Global Sustainable Development Report 2019: The Future is Now–Science for Achieving Sustainable Development; United Nations: New York, NY, USA, 2019. [Google Scholar]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [Green Version]
- UNEP. Frontiers 2018/2019 Emerging Issues of Environmental Concern; United Nations Environment Programme: Nairobi, Kenya, 2019; ISBN 978-92-807-3553-6. [Google Scholar]
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of chemical contaminants with microplastics: Principles and perspectives. Sci. Total Environ. 2020, 706, 135978. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Plastics–The Facts 2022. Plastics Europe, Brussels, Belgium. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 15 May 2023).
- Chae, Y.; An, Y.-J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [Green Version]
- Ross, P.S.; Chastain, S.; Vassilenko, E.; Etemadifar, A.; Zimmermann, S.; Quesnel, S.-A.; Eert, J.; Solomon, E.; Patankar, S.; Posacka, A.M.; et al. Pervasive distribution of polyester fibres in the Arctic Ocean is driven by Atlantic inputs. Nat. Commun. 2021, 12, 106. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Machado, A.A.d.S.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, P.L.; Jazvac, K. The consequence that is plastiglomerate. Nat. Rev. Earth Environ. 2020, 1, 6–7. [Google Scholar] [CrossRef] [Green Version]
- De-la-Torre, G.E.; Pizarro-Ortega, C.I.; Dioses-Salinas, D.C.; Rakib, R.M.J.; Ramos, W.; Pretell, V.; Ribeiro, V.V.; Castro, Í.B.; Dobaradaran, S. First record of plastiglomerates, pyroplastics, and plasticrusts in South America. Sci. Total Environ. 2022, 833, 155179. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Pozo, K.; Urbina, W.; Gómez, V.; Torres, M.; Nuñez, D.; Přibylová, P.; Audy, O.; Clarke, B.; Arias, A.; Tombesi, N.; et al. Persistent organic pollutants sorbed in plastic resin pellet—“Nurdles” from coastal areas of Central Chile. Mar. Pollut. Bull. 2020, 151, 110786. [Google Scholar] [CrossRef]
- Zimmermann, L.; Göttlich, S.; Oehlmann, J.; Wagner, M.; Völker, C. What are the drivers of microplastic toxicity? Comparing the toxicity of plastic chemicals and particles to Daphnia magna. Environ. Pollut. 2020, 267, 115392. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- ter Halle, A.; Ghiglione, J.F. Nanoplastics: A Complex, Polluting Terra Incognita. Environ. Sci. Technol. 2021, 55, 14466–14469. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Henderson, L.; Green, C. Making sense of microplastics? Public understandings of plastic pollution. Mar. Pollut. Bull. 2020, 152, 110908. [Google Scholar] [CrossRef]
- Zitouni, N.; Bousserrhine, N.; Missawi, O.; Boughattas, I.; Chèvre, N.; Santos, R.; Belbekhouche, S.; Alphonse, V.; Tisserand, F.; Balmassiere, L.; et al. Uptake, tissue distribution and toxicological effects of environmental microplastics in early juvenile fish Dicentrarchus labrax. J. Hazard. Mater. 2020, 403, 124055. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef]
- Besseling, E.; Quik, J.T.K.; Sun, M.; Koelmans, A.A. Fate of nano- and microplastic in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: EU Policy Framework on Biobased, Biodegradable and Compostable Plastics; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- European Commission. Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Synthetic Polymer Microparticles-Draft; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Kawecki, D.; Wu, Q.; Gonçalves, J.S.; Nowack, B. Polymer-specific dynamic probabilistic material flow analysis of seven polymers in Europe from 1950 to 2016. Resour. Conserv. Recycl. 2021, 173, 105733. [Google Scholar] [CrossRef]
- da Costa, J.P.; Paço, A.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. Microplastics in soils: Assessment, analytics and risks. Environ. Chem. 2019, 16, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [Green Version]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’connell, B.; Healy, M.G.; O’connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef] [Green Version]
- Rillig, M.C.; Ingraffia, R.; de Souza Machado, A.A. Microplastic Incorporation into Soil in Agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef] [Green Version]
- van Weert, S.; Redondo-Hasselerharm, P.E.; Diepens, N.J.; Koelmans, A.A. Effects of Nanoplastics and Micro-plastics on the Growth of Sediment-Rooted Macrophytes. Sci. Total Environ. 2019, 654, 1040–1047. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; Li, Y.; Xiang, Y.; He, D.; Pan, X. Aging of microplastics affects their surface properties, thermal decomposition, additives leaching and interactions in simulated fluids. Sci. Total Environ. 2020, 714, 136862. [Google Scholar] [CrossRef]
- Fernández-González, V.; Andrade-Garda, J.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Impact of weathering on the chemical identification of microplastics from usual packaging polymers in the marine environment. Anal. Chim. Acta 2021, 1142, 179–188. [Google Scholar] [CrossRef]
- Valentine, K.; Cross, R.; Cox, R.; Woodmancy, G.; Boxall, A.B.A. Caddisfly Larvae are a Driver of Plastic Litter Breakdown and Microplastic Formation in Freshwater Environments. Environ. Toxicol. Chem. 2022, 41, 3058–3069. [Google Scholar] [CrossRef]
- Kwak, J.I.; An, Y.-J. Length- and polymer-dependent ecotoxicities of microfibers to the earthworm Eisenia andrei. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109354. [Google Scholar] [CrossRef]
- Selonen, S.; Dolar, A.; Kokalj, A.J.; Sackey, L.N.A.; Skalar, T.; Fernandes, V.C.; Rede, D.; Delerue-Matos, C.; Hurley, R.; Nizzetto, L.; et al. Exploring the impacts of microplastics and associated chemicals in the terrestrial environment–Exposure of soil invertebrates to tire particles. Environ. Res. 2021, 201, 111495. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Kissener, H.L.; Faltin, E.; Ryo, M.; Rillig, M.C. Tire abrasion particles negatively affect plant growth even at low concentrations and alter soil biogeochemical cycling. Soil Ecol. Lett. 2022, 4, 409–415. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef] [Green Version]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef]
- Sagawa, N.; Kawaai, K.; Hinata, H. Abundance and size of microplastics in a coastal sea: Comparison among bottom sediment, beach sediment, and surface water. Mar. Pollut. Bull. 2018, 133, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Bergami, E.; Singh, N.; Corsi, I. Plastic occurrence, sources, and impacts in Antarctic environment and biota. Water Biol. Secur. 2022, 1, 100034. [Google Scholar] [CrossRef]
- Seghers, J.; Stefaniak, E.A.; La Spina, R.; Cella, C.; Mehn, D.; Gilliland, D.; Held, A.; Jacobsson, U.; Emteborg, H. Preparation of a reference material for microplastics in water—Evaluation of homogeneity. Anal. Bioanal. Chem. 2021, 414, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- Andrade, J.; Fernández-González, V.; López-Mahía, P.; Muniategui, S. A low-cost system to simulate environmental microplastic weathering. Mar. Pollut. Bull. 2019, 149, 110663. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef]
- Youn, J.-S.; Kim, Y.-M.; Siddiqui, M.Z.; Watanabe, A.; Han, S.; Jeong, S.; Jung, Y.-W.; Jeon, K.-J. Quantification of tire wear particles in road dust from industrial and residential areas in Seoul, Korea. Sci. Total Environ. 2021, 784, 147177. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- da Costa, J.P.; Reis, V.; Paço, A.; Costa, M.; Duarte, A.C.; Rocha-Santos, T. Micro(nano)plastics–Analytical challenges towards risk evaluation. TrAC Trends Anal. Chem. 2019, 111, 173–184. [Google Scholar] [CrossRef]
- Miller, E.; Sedlak, M.; Lin, D.; Box, C.; Holleman, C.; Rochman, C.M.; Sutton, R. Recommended best practices for collecting, analyzing, and reporting microplastics in environmental media: Lessons learned from comprehensive monitoring of San Francisco Bay. J. Hazard. Mater. 2021, 409, 124770. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, A.; Jiang, X.; Gu, X. Are microplastics correlated to phthalates in facility agriculture soil? J. Hazard. Mater. 2021, 412, 125164. [Google Scholar] [CrossRef] [PubMed]
- Besley, A.; Vijver, M.G.; Behrens, P.; Bosker, T. A standardized method for sampling and extraction methods for quantifying microplastics in beach sand. Mar. Pollut. Bull. 2017, 114, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Scopetani, C.; Chelazzi, D.; Mikola, J.; Leiniö, V.; Heikkinen, R.; Cincinelli, A.; Pellinen, J. Olive oil-based method for the extraction, quantification and identification of microplastics in soil and compost samples. Sci. Total Environ. 2020, 733, 139338. [Google Scholar] [CrossRef]
- Song, X.; Wu, X.; Song, X.; Zhang, Z. Oil extraction following digestion to separate microplastics from mussels. Chemosphere 2021, 289, 133187. [Google Scholar] [CrossRef]
- Kononov, A.; Hishida, M.; Suzuki, K.; Harada, N. Microplastic Extraction from Agricultural Soils Using Canola Oil and Unsaturated Sodium Chloride Solution and Evaluation by Incineration Method. Soil Syst. 2022, 6, 54. [Google Scholar] [CrossRef]
- Prosenc, F.; Leban, P.; Šunta, U.; Kralj, M.B. Extraction and Identification of a Wide Range of Microplastic Polymers in Soil and Compost. Polymers 2021, 13, 4069. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, X.; Vogt, R.D. An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ. Pollut. 2019, 254, 113009. [Google Scholar] [CrossRef] [PubMed]
- Constant, M.; Billon, G.; Breton, N.; Alary, C. Extraction of microplastics from sediment matrices: Experimental comparative analysis. J. Hazard. Mater. 2021, 420, 126571. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Lindsay, D.J.; Tsuchiya, M.; Matsui, R.; Kitahashi, T.; Fujikura, K.; Fukushima, T. A small, stainless-steel sieve optimized for laboratory beaker-based extraction of microplastics from environmental samples. Methodsx 2019, 6, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Tsuchiya, M.; Lindsay, D.J.; Kitahashi, T.; Fujikura, K.; Fukushima, T. A new small device made of glass for separating microplastics from marine and freshwater sediments. PeerJ 2019, 7, e7915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enders, K.; Lenz, R.; Sul, J.A.I.D.; Tagg, A.S.; Labrenz, M. When every particle matters: A QuEChERS approach to extract microplastics from environmental samples. Methodsx 2020, 7, 100784. [Google Scholar] [CrossRef]
- Zobkov, M.B.; Esiukova, E.E. Evaluation of the Munich Plastic Sediment Separator efficiency in extraction of microplastics from natural marine bottom sediments. Limnol. Oceanogr. Methods 2017, 15, 967–978. [Google Scholar] [CrossRef]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 2017, 230, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Rhein, F.; Scholl, F.; Nirschl, H. Magnetic seeded filtration for the separation of fine polymer particles from dilute suspensions: Microplastics. Chem. Eng. Sci. 2019, 207, 1278–1287. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic Extraction of Microplastics from Environmental Samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Budhiraja, V.; Mušič, B.; Krzan, A. Magnetic Extraction of Weathered Tire Wear Particles and Polyethylene Microplastics. Polymers 2022, 14, 5189. [Google Scholar] [CrossRef]
- Ramage, S.J.F.F.; Pagaling, E.; Haghi, R.K.; Dawson, L.A.; Yates, K.; Prabhu, R.; Hillier, S.; Devalla, S. Rapid extraction of high- and low-density microplastics from soil using high-gradient magnetic separation. Sci. Total Environ. 2022, 831, 154912. [Google Scholar] [CrossRef]
- Rhein, F.; Kaiser, S.; Rhein, M.; Nirschl, H. Agglomerate processing and recycling options in magnetic seeded filtration. Chem. Eng. Sci. 2021, 238, 116577. [Google Scholar] [CrossRef]
- Rhein, F.; Nirschl, H.; Kaegi, R. Separation of Microplastic Particles from Sewage Sludge Extracts Using Magnetic Seeded Filtration. Water Res. X 2022, 17, 100155. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of microplastics from water by magnetic nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838. [Google Scholar] [CrossRef] [PubMed]
- Felsing, S.; Kochleus, C.; Buchinger, S.; Brennholt, N.; Stock, F.; Reifferscheid, G. A new approach in separating microplastics from environmental samples based on their electrostatic behavior. Environ. Pollut. 2018, 234, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Enders, K.; Tagg, A.S.; Labrenz, M. Evaluation of Electrostatic Separation of Microplastics from Mineral-Rich Environmental Samples. Front. Environ. Sci. 2020, 8, 112. [Google Scholar] [CrossRef]
- Kurzweg, L.; Schirrmeister, S.; Hauffe, M.; Adomat, Y.; Socher, M.; Harre, K. Application of electrostatic separation and differential scanning calorimetry for microplastic analysis in river sediments. Front. Environ. Sci. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Andrade, J.M.; Ferreiro, B.; López-Mahía, P.; Muniategui-Lorenzo, S. Standardization of the minimum information for publication of infrared-related data when microplastics are characterized. Mar. Pollut. Bull. 2020, 154, 111035. [Google Scholar] [CrossRef] [PubMed]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.-K.K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef]
- Castelvetro, V.; Corti, A.; Biale, G.; Ceccarini, A.; Degano, I.; La Nasa, J.; Lomonaco, T.; Manariti, A.; Manco, E.; Modugno, F.; et al. New methodologies for the detection, identification, and quantification of microplastics and their environmental degradation by-products. Environ. Sci. Pollut. Res. 2021, 28, 46764–46780. [Google Scholar] [CrossRef]
- Šunta, U.; Prosenc, F.; Trebše, P.; Bulc, T.G.; Kralj, M.B. Adsorption of acetamiprid, chlorantraniliprole and flubendiamide on different type of microplastics present in alluvial soil. Chemosphere 2020, 261, 127762. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Goedecke, C.; Eisentraut, P.; Piechotta, C.; Braun, U. Microplastic analysis using chemical extraction followed by LC-UV analysis: A straightforward approach to determine PET content in environmental samples. Environ. Sci. Eur. 2020, 32, 85. [Google Scholar] [CrossRef]
- Vermeiren, P.; Muñoz, C.; Ikejima, K. Microplastic identification and quantification from organic rich sediments: A validated laboratory protocol. Environ. Pollut. 2020, 262, 114298. [Google Scholar] [CrossRef] [PubMed]
- El Hayany, B.; El Fels, L.; Quénéa, K.; Dignac, M.-F.; Rumpel, C.; Gupta, V.K.; Hafidi, M. Microplastics from lagooning sludge to composts as revealed by fluorescent staining- image analysis, Raman spectroscopy and pyrolysis-GC/MS. J. Environ. Manag. 2020, 275, 111249. [Google Scholar] [CrossRef] [PubMed]
- Peez, N.; Janiska, M.-C.; Imhof, W. The first application of quantitative 1H NMR spectroscopy as a simple and fast method of identification and quantification of microplastic particles (PE, PET, and PS). Anal. Bioanal. Chem. 2019, 411, 823–833. [Google Scholar] [CrossRef] [PubMed]
- David, J.; Steinmetz, Z.; Kučerík, J.; Schaumann, G.E. Quantitative Analysis of Poly(ethylene terephthalate) Microplastics in Soil via Thermogravimetry–Mass Spectrometry. Anal. Chem. 2018, 90, 8793–8799. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Kintzi, A.; Muñoz, K.; Schaumann, G.E. A simple method for the selective quantification of polyethylene, polypropylene, and polystyrene plastic debris in soil by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2020, 147, 104803. [Google Scholar] [CrossRef]
- Eltemsah, Y.S.; Bøhn, T. Acute and chronic effects of polystyrene microplastics on juvenile and adult Daphnia magna. Environ. Pollut. 2019, 254, 112919. [Google Scholar] [CrossRef]
- Suman, T.Y.; Jia, P.-P.; Li, W.-G.; Junaid, M.; Xin, G.-Y.; Wang, Y.; Pei, D.-S. Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater. 2020, 400, 123220. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.L.; An, X.L.; Yang, X.R.; Christie, P.; Ke, X.; Wu, L.H.; Zhu, Y.G. Exposure of Soil Collembolans to Micro-plastics Perturbs Their Gut Microbiota and Alters Their Isotopic Composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ. Pollut. 2020, 259, 113896. [Google Scholar] [CrossRef] [PubMed]
- Lahive, E.; Walton, A.; Horton, A.A.; Spurgeon, D.J.; Svendsen, C. Microplastic particles reduce reproduction in the terrestrial worm Enchytraeus crypticus in a soil exposure. Environ. Pollut. 2019, 255, 113174. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M.K.; Hwang, J.-S.; Han, J.E.; et al. Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo. Sci. Total Environ. 2022, 807, 150817. [Google Scholar] [CrossRef]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Lwanga, E.H. Low density-microplastics detected in sheep faeces and soil: A case study from the intensive vegetable farming in Southeast Spain. Sci. Total Environ. 2021, 755, 142653. [Google Scholar] [CrossRef]
- Alma, A.M.; de Groot, G.S.; Buteler, M. Microplastics incorporated by honeybees from food are transferred to honey, wax and larvae. Environ. Pollut. 2023, 320, 121078. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, L.; Cao, T.; Chen, J.; Lv, M.; Wei, S.; Lu, S.; Tian, X. Microplastics are transferred by soil fauna and regulate soil function as material carriers. Sci. Total Environ. 2023, 857, 159690. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A. Microplastics could be a threat to plants in terrestrial systems directly or indirectly. Environ. Pollut. 2020, 267, 115653. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, X.; Song, Z.; Wei, N.; Li, D. Terrestrial plants as a potential temporary sink of atmospheric microplastics during transport. Sci. Total Environ. 2020, 742, 140523. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Arenas, R.; Beltrán-Sanahuja, A.; Navarro-Quirant, P.; Sanz-Lazaro, C. The effect of sewage sludge containing microplastics on growth and fruit development of tomato plants. Environ. Pollut. 2021, 268, 115779. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, R.; Li, Q.; Zhou, J.; Wang, G. Physiological response of cucumber (Cucumis sativus L.) leaves to polystyrene nanoplastics pollution. Chemosphere 2020, 255, 127041. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Praveena, S.M.; Ariffin, N.I.S.; Nafisyah, A.L. Microplastics in Malaysian bottled water brands: Occurrence and potential human exposure. Environ. Pollut. 2022, 315, 120494. [Google Scholar] [CrossRef]
- Conti, G.O.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Sivagami, M.; Selvambigai, M.; Devan, U.; Velangani, A.A.J.; Karmegam, N.; Biruntha, M.; Arun, A.; Kim, W.; Govarthanan, M.; Kumar, P. Extraction of microplastics from commonly used sea salts in India and their toxicological evaluation. Chemosphere 2020, 263, 128181. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Jiang, Y.; Zhang, Y.; Fan, Y.; Rao, W.; Qian, X. Microplastics in infant milk powder. Environ. Pollut. 2023, 323, 121225. [Google Scholar] [CrossRef]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of microplastics and natural fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; dos Santos Galvão, L.; de Weger, L.A.; Hiemstra, P.S.; Vijver, M.G.; Mauad, T. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health? Sci. Total Environ. 2020, 749, 141676. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.E.; Hare, J.T.; Khamis, Z.I.; Hua, T.; Sang, Q.-X.A. Exposure of Human Lung Cells to Polystyrene Microplastics Significantly Retards Cell Proliferation and Triggers Morphological Changes. Chem. Res. Toxicol. 2021, 34, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef]
- Santos, D.; Félix, L.; Luzio, A.; Parra, S.; Cabecinha, E.; Bellas, J.; Monteiro, S.M. Toxicological effects induced on early life stages of zebrafish (Danio rerio) after an acute exposure to microplastics alone or co-exposed with copper. Chemosphere 2020, 261, 127748. [Google Scholar] [CrossRef]
- Khalid, A.; Zalouk-Vergnoux, A.; Benali, S.; Mincheva, R.; Raquez, J.-M.; Bertrand, S.; Poirier, L. Are bio-based and biodegradable microplastics impacting for blue mussel (Mytilus edulis)? Mar. Pollut. Bull. 2021, 167, 112295. [Google Scholar] [CrossRef]
- de Souza, S.S.; Freitas, Í.N.; Gonçalves, S.d.O.; da Luz, T.M.; Araújo, A.P.d.C.; Rajagopal, R.; Balasubramani, G.; Rahman, M.; Malafaia, G. Toxicity induced via ingestion of naturally-aged polystyrene microplastics by a small-sized terrestrial bird and its potential role as vectors for the dispersion of these pollutants. J. Hazard. Mater. 2022, 434, 128814. [Google Scholar] [CrossRef]
- Lackmann, C.; Velki, M.; Šimić, A.; Müller, A.; Braun, U.; Ečimović, S.; Hollert, H. Two types of microplastics (polystyrene-HBCD and car tire abrasion) affect oxidative stress-related biomarkers in earthworm Eisenia andrei in a time-dependent manner. Environ. Int. 2022, 163, 107190. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. Microplastic particles increase arsenic toxicity to rice seedlings. Environ. Pollut. 2020, 259, 113892. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Lwanga, E.H.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rede, D.; Delerue-Matos, C.; Fernandes, V.C. The Microplastics Iceberg: Filling Gaps in Our Understanding. Polymers 2023, 15, 3356. https://doi.org/10.3390/polym15163356
Rede D, Delerue-Matos C, Fernandes VC. The Microplastics Iceberg: Filling Gaps in Our Understanding. Polymers. 2023; 15(16):3356. https://doi.org/10.3390/polym15163356
Chicago/Turabian StyleRede, Diana, Cristina Delerue-Matos, and Virgínia Cruz Fernandes. 2023. "The Microplastics Iceberg: Filling Gaps in Our Understanding" Polymers 15, no. 16: 3356. https://doi.org/10.3390/polym15163356
APA StyleRede, D., Delerue-Matos, C., & Fernandes, V. C. (2023). The Microplastics Iceberg: Filling Gaps in Our Understanding. Polymers, 15(16), 3356. https://doi.org/10.3390/polym15163356







