Thioether-Containing Zirconium(Alkoxy)Siloxanes: Synthesis and Study of Dielectric and Mechanical Properties of Silica-Filled Polydimethylsiloxane Compositions Cured by Them
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization Methods
2.2. Initial Materials
2.3. Composition Components Preparation
2.3.1. PDMS and PEOS Preparation
2.3.2. Thioether-Containing Silanes Preparation
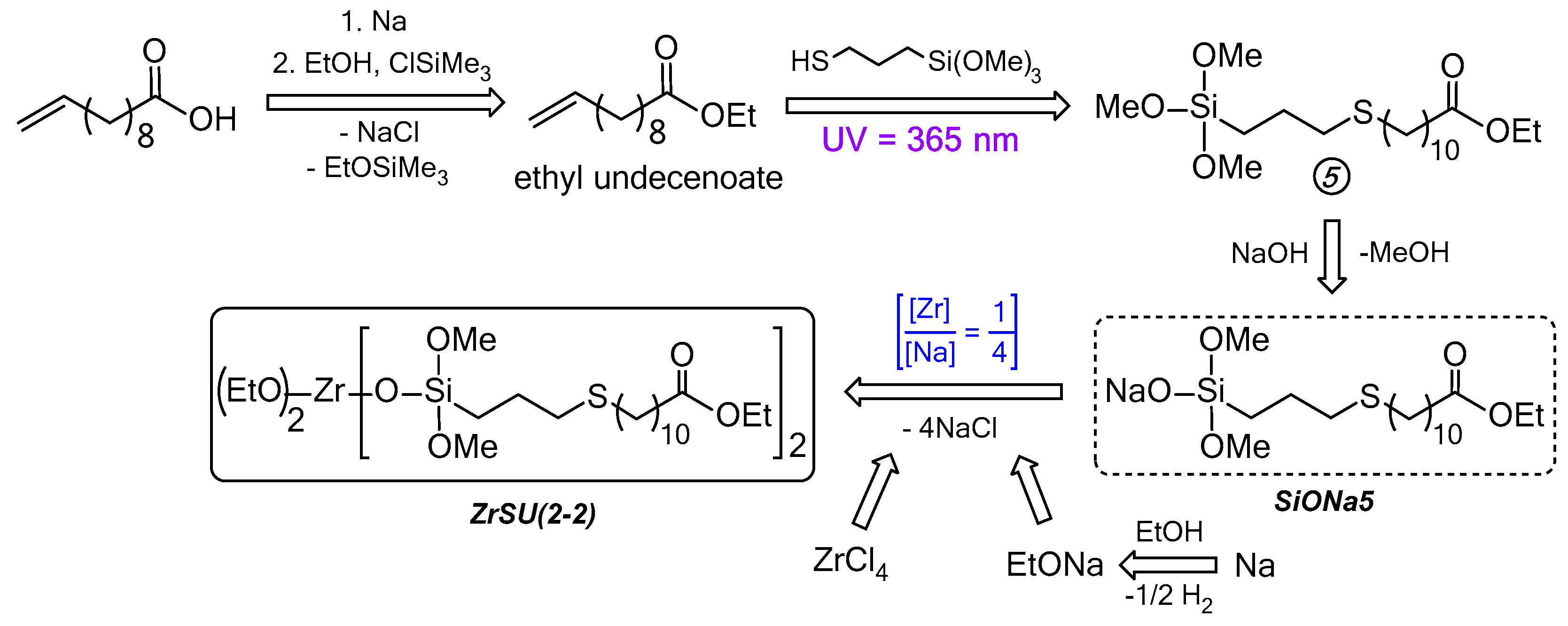
2.3.3. Thioether-Containing Zr-Siloxanes Preparation
2.3.4. General Procedure for Preparation of Silicon Compositions
3. Results
3.1. Compositions Cured with Methoxy Silyl Derivatives of Sulfur-Containing Zr-Siloxanes
3.2. Compositions Cured with Ethoxy Silyl Derivatives of Sulfur-Containing Zr-Siloxanes
3.3. Compositions Cured with a Mixture of Zi-Siloxanes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Sheng, J.; O’Neill, C.T.; Walsh, C.J.; Wood, R.J.; Ryu, J.H.; Jaydev, P.D.; Yip, M.C. Robotic artificial muscles: Current progress and future perspectives. IEEE Trans. Robot. 2019, 35, 761–781. [Google Scholar] [CrossRef]
- Bezsudnov, I.V.; Khmelnitskaia, A.G.; Kalinina, A.A.; Ponomarenko, S.A. Dielectric elastomer actuators: Materials and design. Russ. Chem. Rev. 2023, 92, RCR5070. [Google Scholar] [CrossRef]
- Zhu, R.; Wallrabe, U.; Wapler, M.C.; Woias, P.; Mescheder, U. Dielectric electroactive polymer membrane actuator with ring-type electrode as driving component of a tactile actuator. Proc. Eng. 2016, 168, 1537–1540. [Google Scholar] [CrossRef]
- Madsen, F.B.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. The current state of silicone-based dielectric elastomer transducers. Macromol. Rap. Comm. 2016, 37, 378–413. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S. Dielectric Elastomers. In Soft Actuators; Asaka, K., Okuzaki, H., Eds.; Springer: Tokyo, Japan, 2014; Volume 13, pp. 183–195. [Google Scholar] [CrossRef]
- Tanaka, T. Gels. Sci. Am. 1981, 244, 124-S-17. [Google Scholar] [CrossRef]
- Pelrine, R.; Eckerle, J.; Chiba, S. Review of artificial muscle approaches. In Proceedings of the Third International Symposium on Micro Machine and Human Science, Nagoya, Japan, 14–16 October 1992; pp. 14–16. [Google Scholar]
- Carpi, F.; Anderson, I.; Bauer, S.; Frediani, G.; Gallone, G.; Gei, M.; Graaf, C.; Jean-Mistral, C.; Kaal, W.; Kofod, G.; et al. Standards for dielectric elastomer transducers. Smart Mater. Struct. 2015, 24, 105025. [Google Scholar] [CrossRef]
- Kofod, G.; Sommer-Larsen, P.; Kornbluh, R.; Pelrine, R. Actuation response of polyacrylate dielectric elastomers. J. Intell. Mater. Syst. Struct. 2003, 14, 787–793. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Cao, B.; Xue, J.; Wang, L.; Wang, J.; Ding, L. Effect of multiphase content on temperature—Dependent electrical conductivity in silicone rubber composites. High Volt. 2023, 8, 283–292. [Google Scholar] [CrossRef]
- Tuichai, W.; Karaphun, A.; Ruttanapun, C. Improved dielectric properties of PVDF polymer composites filled with Ag nanomaterial deposited reduced graphene oxide (rGO) hybrid particles. Mater. Res. Bull. 2022, 145, 111552. [Google Scholar] [CrossRef]
- McCoul, D.; Rosset, S.; Schlatter, S.; Shea, H. Inkjet 3D printing of UV and thermal cure silicone elastomers for dielectric elastomer actuators. Smart Mater. Struct. 2017, 26, 125022. [Google Scholar] [CrossRef]
- Liu, L.; Fan, J.; Zhang, Z.; Shi, L.; Liu, Y.; Leng, J. Analysis of the novel strain responsive actuators of silicone dielectric elastomer. Adv. Mater. Res. 2008, 47, 298–301. [Google Scholar] [CrossRef]
- Yu, L.; Madsen, F.B.; Hvilsted, S.; Skov, A.L. Dielectric elastomers, with very high dielectric permittivity, based on silicone and ionic interpenetrating networks. RSC Adv. 2015, 5, 49739–49747. [Google Scholar] [CrossRef]
- Hemeda, O.M.; Henaish, A.M.A.; Salem, B.I.; El Sbakhy, F.S.; Hamad, M.A. The dielectric and magnetic properties of RTV silicon rubber Ni–Cr ferrite composites. Appl. Phys. A 2020, 126, 121. [Google Scholar] [CrossRef]
- Wang, W.; Ren, G.; Zhou, M.; Deng, W. Preparation and characterization of CCTO/PDMS delectric elastomers with high dielectric constant and low dielectric loss. Polymers 2021, 13, 1075. [Google Scholar] [CrossRef] [PubMed]
- Wanga, X.; Xiaa, Z.; Zhao, C.; Huang, P.; Zhao, S.; Gao, M.; Nie, J. Microstructured flexible capacitive sensor with high sensitivity based on carbon fiber-filled conductive silicon rubber. Sens. Actuators A Phys. 2020, 312, 112147. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, L.; Yao, X.; Zheng, Z.; Fang, D. Monotonic strain sensing behavior of self-assembled carbon nanotubes/graphene silicone rubber composites under cyclic loading. Compos. Sci. Technol. 2020, 200, 108474. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Verdugo Manzanares, R.; Araujo-Morera, J.; González, S.; Verdejo, R.; López-Manchado, M.Á.; Hernández Santana, M. Understanding the molecular dynamics of dual crosslinked networks by dielectric spectroscopy. Polymers 2021, 13, 3234. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Wu, K.; Wang, L.; Wang, J.; Ding, L. Crystal structure dependent tensile properties of silicone rubber: Influence of aluminium hydroxide. Polym. Test. 2022, 113, 107679. [Google Scholar] [CrossRef]
- Zhang, X.; Le, M.-Q.; Nguyen, V.-C.; Mogniotte, J.-F.; Capsal, J.-F.; Grinberg, D.; Cottinet, P.-J.; Petit, L. Characterization of micro-ZnO/PDMS composite structured via dielectrophoresis—Toward medical application. Mater. Des. 2021, 208, 109912. [Google Scholar] [CrossRef]
- Kopylov, V.M.; Kostyleva, E.I.; Kostylev, I.M.; Koviazin, A.V. Silica fillers for silicone rubber. Int. Polym. Sci. Technol. 2011, 38, 35–47. [Google Scholar] [CrossRef]
- Faiza; Khattak, A.; Rehman, A.U.; Ali, A.; Mahmood, A.; Imran, K.; Ulasyar, A.; Zad, H.S.; Ullah, N.; Khan, A. Multi-stressed nano and micro-silica/silicone rubber composites with improved dielectric and high-voltage insulation properties. Polymers 2021, 13, 1400. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, H.; Zhu, Z.; Yang, H.; Zhang, Q. Enhanced dielectric, electromechanical and hydrophobic behaviors of coreshell AgNWs@SiO2/PDMS composites. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 59–67. [Google Scholar] [CrossRef]
- Dong, X.; Niu, C.; Qi, M. Enhancement of electrorheological performance of electrorheological elastomers by improving TiO2 particles/silicon rubber interface. J. Mater. Chem. C 2016, 4, 6806–6815. [Google Scholar] [CrossRef]
- Perju, E.; Cuervo-Reyes, E.; Shova, S.; Opris, D.M. Synthesis of novel cyclosiloxane monomers containing push–pull moieties and their anionic ring opening polymerization. RSC Adv. 2018, 8, 7569–7578. [Google Scholar] [CrossRef]
- Liao, Y.; Weng, Y.; Wang, J.; Zhou, H.; Lin, J.; He, S. Silicone rubber composites with high breakdown strength and low dielectric loss based on polydopamine coated mica. Polymers 2019, 11, 2030. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, M.; Dunki, S.J.; Quinsaat, J.-E.Q.; Ko, Y.S.; Opris, D.M. Synthesis of silicone elastomers containing trifluoropropyl groups and their use in dielectric elastomer transducers. RSC Adv. 2015, 5, 104516–104523. [Google Scholar] [CrossRef]
- Sheima, Y.; von Szczepanski, J.; Danner, P.M.; Künniger, T.; Remhof, A.; Frauenrath, H.; Opris, D.M. Transient elastomers with high dielectric permittivity for actuators, sensors, and beyond. ACS Appl. Mater. Interf. 2022, 14, 40257–40265. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, S.; Shiau, H.-S.; Colby, R.H. Linear viscoelastic and dielectric properties of phosphonium siloxane ionomers. ACS Macro Lett. 2013, 2, 970–974. [Google Scholar] [CrossRef]
- Sheima, Y.; Yuts, Y.; Frauenrath, H.; Opris, D.M. Polysiloxanes modified with different types and contents of polar groups: Synthesis, structure, and thermal and dielectric properties. Macromolecules 2021, 54, 5737–5749. [Google Scholar] [CrossRef]
- Opris, D.M. Polar elastomers as novel materials for electromechanical actuator applications. Adv. Mater. 2017, 30, 1703678. [Google Scholar] [CrossRef]
- Caspari, P.; Dunki, S.J.; Nuesch, F.A.; Opris, D.M. Dielectric elastomer actuators with increased dielectric permittivity and low leakage current capable of suppressing electromechanical instability. J. Mater. Chem. C 2018, 6, 2043–2053. [Google Scholar] [CrossRef]
- Tarasenkov, A.N.; Parshina, M.S.; Tebeneva, N.A.; Borisov, K.M.; Goncharuk, G.P.; Shevchenko, V.G.; Ponomarenko, S.A.; Muzafarov, A.M. Metalloalkoxysiloxanes-cured polydimethylsiloxane compositions filled with silica component for special applications: Dielectric and mechanical properties. Express Polym. Lett. 2022, 16, 846–870. [Google Scholar] [CrossRef]
- Meshkov, I.B.; Kalinina, A.A.; Gorodov, V.V.; Bakirov, A.V.; Krasheninnikov, S.V.; Chvalun, S.N.; Muzafarov, A.M. New principles of polymer composite preparation. Mq copolymers as an active molecular filler for polydimethylsiloxane rubbers. Polymers 2021, 13, 2848. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, V.V.; Rebrov, E.A.; Myakushev, V.D.; Strelkova, T.V.; Ozerin, A.N.; Ozerina, L.A.; Chenskaya, T.B.; Sheiko, S.S.; Sharipov, E.Y.; Muzafarov, A.M. From a hyperbranched polyethoxysiloxane toward molecular forms of silica: A polymer-based approach to the monitoring of silica properties. ACS Symp. Ser. 2000, 729, 503–515. [Google Scholar] [CrossRef]
- Tarasenkov, A.N.; Tebeneva, N.A.; Parshina, M.S.; Meshkov, I.B.; Vasilenko, N.G.; Cherkaev, G.V.; Goncharuk, G.P.; Katsoulis, D.E.; Muzafarov, A.M. New functional metallosiloxanes with partially siloxy substituted metall atom and their use in silicone compositions. J. Organomet. Chem. 2020, 906, 121034. [Google Scholar] [CrossRef]
- Tebeneva, N.A.; Meshkov, I.B.; Tarasenkov, A.N.; Polshchikova, N.V.; Kalinina, A.A.; Buzin, M.I.; Serenko, O.A.; Zubavichus, Y.V.; Katsoulis, D.E.; Muzafarov, A.M. Polyfunctional branched metallosiloxane oligomers and composites based on them. J. Organomet. Chem. 2018, 868, 112–121. [Google Scholar] [CrossRef]
- Dyre, J.C.; Schrøder, T.B. Universality of ac conduction in disordered solids. Rev. Mod. Phys. 2000, 72, 873. [Google Scholar] [CrossRef]
- Dissado, L.A.; Hill, R.M. Anomalous low-frequency dispersion. Near direct current conductivity in disordered low-dimensional materials. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1984, 80, 291–319. [Google Scholar] [CrossRef]
- Jonscher, A.K. Low-frequency dispersion in carrier-dominated dielectric. Philos. Mag. B 1978, 38, 587–601. [Google Scholar] [CrossRef]
- Zha, J.-W.; Zhu, Y.-H.; Li, W.-K.; Bai, J.; Dang, Z.-M. Low dielectric permittivity and high thermal conductivity silicone rubber composites with micro-nano-sized particles. Appl. Phys. Lett. 2012, 101, 062905. [Google Scholar] [CrossRef]
- Wijaya, K.; Saputri, W.D.; Aziz, I.T.A.; Wangsa; Heraldy, E.; Hakim, L.; Suseno, A.; Utami, M. Mesoporous silica preparation using sodium bicarbonate as template and application of the silica for hydrocracking of used cooking oil into biofuel. Silicon 2022, 14, 1583–1591. [Google Scholar] [CrossRef]






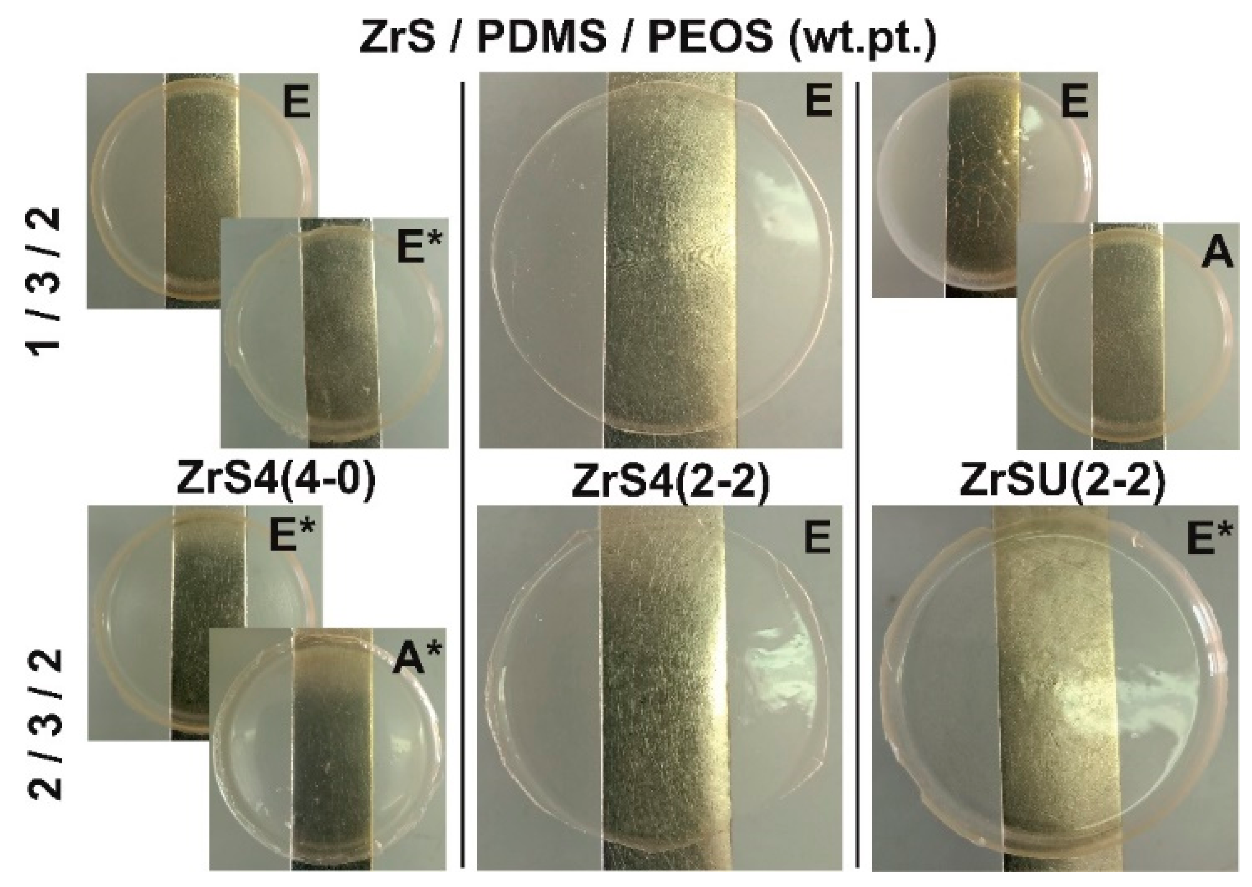







| No. | Initial Ratio MS/PDMS-E/PEOS wt.pt. | ε′ | ε″ | σ′·1011 S/cm | tanδ | MPa/% | Characterization | |
|---|---|---|---|---|---|---|---|---|
| f = 0.1/1/10/100 Hz | ||||||||
| 1 | ZrS1(4-0) | 1/3/- | - | - | - | - | - | Transparent, waxy |
| 2 | 1/3/1 | - | - | - | - | - | ||
| 3 | 1/3 * /- | - | - | - | - | - | White, irreversibly deformed | |
| 4 | 2/3 * /1 | 10.6/6.1/4.4/4.1 | 7.2/2.3/0.7/0.2 | 0.04/0.13/0.39/0.97 | 0.68/0.38/0.16/0.05 | 1.2 ± 0.1 70 ± 11 | Opalescent, heterogeneous | |
| 5 | 1/3 * /2 | 4.7/4.4/3.8/3.4 | 0.1/0.3/0.4/0.2 | 0.001/0.02/0.22/0.96 | 0.02/0.07/0.11/0.06 | 1.2 ± 0.1 161 ± 10 | White, heterogeneous | |
| 6 | ZrS2(4-0) | 1/3/- | - | - | - | - | - | Not cured |
| 7 | 1/3 * /- | 2.5/2.3/2.3/2.3 | 0.2/0.1/0.02/0.01 | 0.001/0.004/0.01/0.06 | 0.08/0.03/0.01/0.004 | 0.4 ± 0.03 100 ± 15 | Transparent, yellowish, homogeneous | |
| 8 | 1/3 * /1 | 5.0/4.3/3.7/3.4 | 0.8/0.4/0.3/0.2 | 0.005/0.02/0.19/0.93 | 0.16/0.09/0.08/0.06 | 1.6 ± 0.2 261 ± 38 | ||
| 9 | 2/3 * /1 | 16.4/7.2/4.5/3.8 | 28.0/5.5/1.32/0.4 | 0.16/0.31/0.79/2.12 | 1.70/0.76/0.29/0.11 | 3.7 ± 0.1 205 ± 5 | ||
| 10 | 1/3 * /2 | 20.7/10.4/5.9/4.2 | 24.6/6.4/2.2/0.9 | 0.14/0.36/1.30/5.22 | 1.19/0.61/0.37/0.21 | 2.1 ± 0.1 264 ± 16 | ||
| 11 | 2/3 * /2 | 34.1/17.0/6.7/4.6 | 222.7/30.2/5.5/1.3 | 1.24/1.71/3.28/7.07 | 6.53/1.78/0.82/0.28 | 3.3 ± 0.1 89 ± 19 | ||
| 12 | ZrS3(4-0) | 1/3/- | - | - | - | - | - | Heterogeneous, waxy |
| 13 | 1/3 * /- | 3.5/3.5/3.3/3.1 | 0.04/0.06/0.1/0.1 | 0.0002/0.003/0.07/0.80 | 0.01/0.02/0.03/0.03 | 0.6 ± 0.1 121 ± 18 | Transparent, yellowish, homogeneous | |
| 14 | 1/3 * /1 | 4.6/3.9/3.6/3.2 | 1.0/0.4/0.3/0.2 | 0.01/0.02/0.16/0.11 | 0.22/0.10/0.08/0.06 | 1.8 ± 0.3 189 ± 45 | ||
| 15 | 2/3 * /1 | 10.5/8.9/7.3/5.7 | 2.7/1.2/1.1/1.1 | 0.02/0.07/0.65/6.19 | 0.26/0.13/0.15/0.19 | 2.2 ± 0.4 165 ± 29 | ||
| 16 | 1/3 * /2 | 11.7/6.0/4.7/3.9 | 9.6/2.3/0.8/0.5 | 0.05/0.13/0.45/2.49 | 0.82/0.38/0.17/0.13 | 2.9 ± 0.3 325 ± 41 | ||
| 17 | 2/3 * /2 | 40.4/17.3/7.7/4.8 | 223.0/29.7/5.8/1.6 | 1.24/1.69/3.48/9.03 | 5.5/1.72/0.75/0.33 | 2.7 ± 0.2 158 ± 21 | ||
| 18 | 2/3 * /3 | 18.6/13.4/10.5/7.6 | 16.1/3.6/2.1/2.1 | 0.09/0.20/1.25/1.19 | 0.87/0.27/0.2/0.28 | 2.2 ± 0.2 266 ± 23 | Opalescent, yellowish, homogeneous | |
| 19 | 2/3 * /3 ~100 μm | 8.8/7.0/6.1/4.9 | 5.4/1.2/0.7/1.0 | 0.03/0.07/0.43/5.92 | 0.61/0.17/0.11/0.20 | 2.9 ± 0.1 310 ± 40 | ||
| 20 | Zr-Ph(4-0) | 1/3/2 | 10.3/7.1/4.5/3.8 | 2.9/2.0/1.2/0.3 | 0.02/0.11/0.69/1.68 | 0.28/0.28/0.25/0.08 | 5.6 ± 0.3 14 ± 2 | Transparent, yellowish, homogeneous |
| 21 | 1/3 * /2 | 8.6/5.1/3.8/3.4 | 6.5/2.0/0.6/0.2 | 0.04/0.11/0.33/0.87 | 0.76/0.38/0.15/0.05 | 5.2 ± 0.5 347 ± 32 | ||
| 22 | Ecoflex TM | 3.4/3.2/3.1/3.1 | 0.2/0.1/0.05/0.03 | 0.001/0.004/0.03/0.17 | 0.90/0.37/0.12/0.04 | - | White | |
| (a) Compositions Characteristics Obtained Using ZrS4(4-0) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Initial Ratio ZrS4(4-0)/PDMS/PEOS wt.pt. | ε′ | ε″ | σ′·1011 S/cm | tanδ | MPa/% | Characterization | |
| f = 0.1/1/10/100 Hz | ||||||||
| 1 | 2/3(E *)/1 | 14.3/7.0/4.5/3.6 | 16.1/3.7/1.3/0.4 | 0.12/0.40/2.22/11.47 | 1.12/0.53/0.28/0.90 | 4.4 ± 0.1 92 ± 10 | Transparent, yellowish, homogeneous | |
| 2 | 2/3(A *)/1 | 26.1/8.7/5.1/4.0 | 94.3/13.3/2.4/0.6 | 0.52/0.76/1.43/3.29 | 3.61/1.53/0.47/0.15 | 4.5 ± 0.3 65 ± 5 | ||
| 3 | 1/3(E *)/2 | 19.3/10.3/6.0/3.7 | 48.2/8.1/2.7/0.9 | 0.27/0.46/1.59/5.24 | 2.50/0.78/0.44/0.24 | 1.6 ± 0.1 174 ± 29 | Transparent, yellowish, homogeneous, light plasticity effect | |
| 4 | 1/3(A *)/2 | 5.6/3.8/3.4/3.3 | 4.1/0.9/0.2/0.1 | 0.02/0.05/0.12/0.31 | 0.74/0.23/0.06/0.02 | 5.5 ± 0.2 42 ± 3 | Transparent, yellowish, homogeneous | |
| 5 | 1/3(E)/2 | - | - | - | - | - | Opalescent, yellowish, homogeneous, brittle | |
| 6 | 1/3(A)/2 | - | - | - | - | - | ||
| 7 | 2/3(E *)/2 | 126.6/23.4/9.0/5.0 | 405.1/58.9/9.1/2.2 | 2.25/3.34/5.42/12.06 | 3.20/2.52/1.01/0.42 | 2.5 ± 0.3 4 ± 1 | ||
| 8 | 2/3(A *)/2 | 50.8/16.1/7.0/4.4 | 109.8/22.0/4.5/1.3 | 0.61/1.25/2.71/7.24 | 2.16/1/37/0.65/0.28 | 5.7 ± 0.8 71 ± 18 | Transparent, yellowish, homogeneous | |
| 9 | 1/3(E *)/3 | 8.4/6.9/4.5/3.7 | 1.2/1.3/1.2/0.3 | 0.04/0.34/2.18/11.53 | 0.14/0.19/0.26/0.08 | 2.5 ± 0.2 295 ± 34 | ||
| 10 | 1/3(A *)/3 | 7.8/4.2/3.5/3.4 | 8.0/1.6/0.3/0.1 | 0.05/0.09/0.19/0.43 | 1.03/0.37/0.09/0.02 | 6.0 ± 0.4 47 ± 4 | ||
| 11 | 2/3(E *)/3 | 16.7/9.3/5.0/4.0 | 17.6/4.9/1.8/0.4 | 0.13/0.55/2.63/17.40 | 1.05/0.53/0.36/0.10 | 4.2 ± 0.1 74 ± 7 | ||
| 12 | 2/3(A *)/3 | 37.5/16.2/7.4/4.5 | 153.5/22.0/5.0/1.4 | 0.85/1.25/2.98/8.07 | 4.09/1.36/0.67/0.32 | 6.1 ± 0.3 83 ± 13 | ||
| 13 b | 2/3(A *)/3 | 34.3/13.7/6.4/4.3 | 88.4/16.1/3.7/0.9 | 0.49/0.91/2.23/5.22 | 2.57/1.17/0.59/0.21 | 4.5 ± 0.3 57 ± 3 | ||
| 14 | 2/3(A *)/5 | 27.2/12.7/7.1/4.4 | 51.4/9.9/3.3/1.1 | 0.29/0.56/1.95/6.02 | 1.89/0.78/0.46/0.25 | 5.7 ± 0.3 22 ± 3 | Transparent, yellowish, homogeneous, plasticity effect | |
| (b) Compositions Characteristics Obtained Using ZrS4(2-2). | ||||||||
| No. | Initial ratio ZrS4(2-2)/PDMS/PEOS wt.pt. | ε′ | ε″ | σ′·1011 S/cm | tanδ | MPa/% | Characterization | |
| f = 0.1/1/10/100 Hz | ||||||||
| 1 a | 1/3(E)/1 | 13.5/6.2/4.3/3.6 | 22.0/4.0/1.0/0.3 | 0.12/0.23/0.60/1.70 | 1.63/0.64/0.23/0.08 | 4.1 ± 0.2 d 322 ± 53 | Transparent, yellowish, homogeneous | |
| 2 a | 0.5/3(E)/2 | 13.7/6.5/4.3/3.6 | 16.0/3.7/1.1/0.4 | 0.09/0.21/0.63/1.98 | 1.17/0.57/0.24/0.09 | 2.4 ± 0.2 249 ± 61 | ||
| 3 | 0.5/3(G)/2 | 11.6/5.4/4.1/3.6 | 17.7/3.1/0.7/0.2 | 0.10/0.17/0.43/1.20 | 1.53/0.57/0.18/0.06 | 3.2 ± 0.2 19 ± 4 | Transparent, yellowish, homogeneous, brittle | |
| 4 | 2/3(E)/1 | 16.8/6.6/4.4/3.8 | 22.7/4.9/1.1/0.3 | 0.13/0.28/0.66/1.74 | 1.35/0.74/0.25/0.08 | 9.0 ± 0.5 23 ± 5 | Transparent, yellowish, homogeneous | |
| 5 | 2/3(G)/1 | 13.0/5.7/4.2/3.7 | 17.8/3.4/0.8/0.3 | 0.10/0.19/0.48/1.31 | 1.37/0.59/0.19/0.07 | 5.2 ± 0.2 6 ± 1 | ||
| 6 | 1/3(E)/2 | 26.1/7.9/4.8/3.9 | 45.1/8.4/1.7/0.5 | 0.25/0.48/1.02/2.78 | 1.73/1.07/0.35/0.12 | 4.6 ± 0.1 d 166 ± 51 | ||
| 7 a | 1/3(G)/2 | 16.6/6.5/4.5/3.8 | 27.0/4.7/1.1/0.3 | 0.15/0.27/0.65/1.85 | 1.62/0.72/0.24/0.08 | 4.8 ± 0.1 28 ± 2 | ||
| 8 a | 1/3(A)/2 | 11.2/5.9/4.4/3.9 | 16.9/3.2/0.8/0.3 | 0.09/0.18/0.46/1.42 | 1.51/0.54/0.18/0.06 | 6.0 ± 0.4 26 ± 3 | ||
| 9 a b | 1/3(A)/2 | 9.4/5.0/4.0/3.7 | 9.2/2.0/0.5/0.2 | 0.05/0.11/0.30/0.88 | 0.98/0.40/0.12/0.04 | 6.6 ± 0.6 14 ± 2 | Opalescent, yellowish, homogeneous | |
| 10 a | 0.5/3(E)/3 | 31.1/9.7/5.4/4.2 | 58.2/11.6/2.2/0.6 | 0.32/0.66/1.34/3.52 | 1.87/1.19/0.42/0.15 | 2.7 ± 0.03 8 ± 1 | Transparent, yellowish, homogeneous, light plasticity effect | |
| 11 | 0.5/3(G)/3 | 15.0/5.8/4.1/3.6 | 32.0/4.9/1.0/0.3 | 0.18/0.28/0.60/1.54 | 2.14/0.85/0.25/0.08 | 3.8 ± 0.2 12 ± 6 | Transparent, yellowish, homogeneous, brittle | |
| 12 a | 2/3(E)/2 | 32.4/12.0/5.7/4.4 | 55.5/13.0/2.6/0.7 | 0.31/0.74/1.57/3.87 | 1.71/1.08/0.46/0.16 | 5.0 ± 1.0 4 ± 1 | Transparent, yellowish, homogeneous | |
| 13 a b | 2/3(E)/2 | 29.6/11.8/5.6/4.2 | 36.9/10.8/2.5/0.7 | 0.21/0.61/1.48/3.86 | 1.25/0.92/0.44/0.16 | 6.2 ± 1.0 7 ± 1 | Opalescent, yellowish, homogeneous, brittle | |
| 14 | 2/3(G)/2 | 26.7/7.9/5.0/4.2 | 33.7/7.3/1.5/0.4 | 0.19/0.42/0.89/2.23 | 1.26/0.93/0.30/0.10 | 3.0 ± 0.6 2 ± 1 | Transparent, yellowish, homogeneous, brittle | |
| 15 | 2/3(A *)/2 | 13.6/6.0/4.4/4.0 | 16.1/3.3/0.8/0.2 | 0.09/0.19/0.46/1.29 | 1.18/0.55/0.18/0.06 | 3.4 ± 0.2 3 ± 1 | ||
| 16 | 1/3(E)/3 | 37.1/12.4/6.1/4.6 | 71.0/14.6/2.9/0.8 | 0.39/0.83/1.76/4.56 | 1.91/1.17/0.49/0.18 | 5.0 ± 0.1 c 13 ± 1 | Transparent, yellowish, homogeneous | |
| 17 a b | 1/3(E)/3 | 53.3/18.3/9.2/6.0 | 132.5/21.1/5.1/1.8 | 0.74/1.20/3.03/9.92 | 2.49/1.15/0.55/0.30 | 2.4 ± 0.1 20 ± 3 | Opalescent, yellowish, homogeneous | |
| 18 a | 1/3(G)/3 | 27.5/8.5/5.3/4.3 | 54.2/9.6/1.9/0.5 | 0.30/0.54/1.11/3.06 | 1.97/1.12/0.35/0.13 | 6.2 ± 0.1 18 ± 1 | Transparent, yellowish, homogeneous | |
| 19 e | Zr-Ph(2-2) | 1/3(E)/2 | 14.4/5.8/4.1/3.6 | 18.2/3.7/0.8/0.2 | 0.10/0.21/0.50/1.31 | 1.27/0.64/0.21/0.07 | 6.5 ± 0.1 35 ± 3 | Transparent, yellowish, homogeneous |
| 20 e | 2/3(E)/2 | 12.8/5.6/4.0/3.6 | 13.3/3.1/0.7/0.2 | 0.074/0.18/0.44/1.19 | 1.05/0.56/0.18/0.06 | 8.3 ± 0.2 14 ± 1 | ||
| 21 e | Zr-Vin(2-2) | 1/3(E)/3 | 40.3/10.0/5.4/4.3 | 82.4/14.9/2.5/0.6 | 0.46/0.85/1.52/3.45 | 2.05/1.49/0.47/0.14 | 7.6 ± 0.7 9 ± 1 | |
| 22 e | 2/3(E)/2 | 13.0/5.4/3.9/3.6 | 12.5/3.0/0.7/0.2 | 0.07/0.17/0.40/1.08 | 0.96/0.56/0.17/0.05 | 5.9 ± 0.6 7 ± 1 | ||
| (c) Compositions Characteristics Obtained Using ZrSU(2-2). | ||||||||
| No. | Initial ratio ZrSU(2-2)/PDMS/PEOS wt.pt. | ε′ | ε″ | σ′·1011 S/cm | tanδ | MPa/% | Characterization | |
| f = 0.1/1/10/100 Hz | ||||||||
| 1 | 1/3(E)/1 | - | - | - | - | - | Transparent, yellowish, homogeneous, plasticity effect | |
| 2 | 2/3(E *)/1 | 7.6/4.7/3.8/3.6 | 10.9/1.9/0.4/0.1 | 0.06/0.11/0.26/0.58 | 1.45/0.41/0.12/0.03 | 2.7 ± 0.2 d 374 ± 50 | Transparent, yellowish, homogeneous | |
| 3 | 1/3(E)/2 | - | - | - | - | - | Transparent, yellowish, heterogeneous morphology | |
| 4 | 1/3(G)/2 | 6.6/4.4/3.7/3.5 | 4.6/1.2/0.3/0.1 | 0.03/0.07/0.19/0.43 | 0.70/0.26/0.08/0.02 | 2.1 ± 0.1 15 ± 0.4 | Transparent, yellowish, homogeneous | |
| 5 | 1/3(A)/2 | 6.7/4.5/3.8/3.7 | 6.6/1.3/0.3/0.1 | 0.04/0.07/0.18/0.42 | 0.99/0.30/0.08/0.02 | 2.7 ± 0.2 28 ± 4 | ||
| 6 | 1/3(E *)/2 | 7.7/4.9/3.9/3.6 | 4.1/1.4/0.4/0.1 | 0.02/0.08/0.25/0.62 | 0.53/0.29/0.11/0.01 | 3.5 ± 0.4 278 ± 45 | ||
| 7 | 2/3(E *)/2 | 8.0/4.8/4.0/3.8 | 10.9/1.9/0.4/0.9 | 0.06/0.11/0.23/0.53 | 1.36/0.39/0.10/0.02 | 4.1 ± 0.1 d 142 ± 52 | ||
| 8 | 2/3(A *)/2 | 6.9/4.8/4.2/4.0 | 5.9/1.2/0.3/0.1 | 0.03/0.07/0.18/0.45 | 0.85/0.26/0.07/0.02 | 5.0 ± 0.1 62 ± 9 | ||
| 9 | 1/3(E)/3 | - | - | - | - | - | Transparent, yellowish, heterogeneous morphology | |
| 10 | 1/3(G)/3 | - | - | - | - | - | ||
| No. | Initial Ratio MS1+MS2/PDMS/PEOS wt.pt. | ε′ | ε″ | σ′·1011 S/cm | tanδ | MPa/% | Characterization | |
|---|---|---|---|---|---|---|---|---|
| f = 0.1/1/10/100 Hz | ||||||||
| 1 | ZrS4(4-0) + Zr-Ph(2-2) | 0.5+0.5/3(G)/2 | 22.8/7.4/4.7/3.9 | 34.5/6.8/1.4/0.4 | 0.19/0.38/0.85/2.22 | 1.52/0.92/0.31/0.10 | 6.5 ± 0.1 d 49 ± 7 | Transparent, yellowish, homogeneous |
| 2 | 0.25+0.25/3(G)/3 | 70.9/20.2/7.2/5.1 | 105.4/28.4/5.1/1.3 | 0.58/1.61/3.04/6.93 | 1.49/1.40/0.71/0.24 | 6.8 ± 0.4 12 ± 1 | ||
| 3 | 0.25+0.25/3(E)/3 | 94.1/28.8/8.5/5.5 | 240.7/45.3/8.2/1.8 | 1.34/2.57/4.90/10.40 | 2.56/1.57/0.96/0.33 | 4.3 ± 0.2 d 202 ± 51 | ||
| 4 | ZrS4(4-0) + Zr-Vin(2-2) | 0.25+0.25/3(E)/3 | 7.9/4.8/3.9/3.7 | 7.2/1.6/0.4/0.1 | 0.06/0.23/1.77/15.60 | 0.91/0.32/0.10/0.03 | 3.9 ± 0.1 d 24 ± 1 | Transparent, yellowish, homogeneous |
| 5 | 0.25+0.25/3(G)/3 | 6.1/4.4/3.9/3.8 | 4.5/0.9/0.2/0.1 | 0.04/0.20/16.21 | 0.73/0.21/0.05/0.02 | 7.7 ± 0.3 7 ± 1 | ||
| 6 | 0.25+0.25/3(G)/3 | 6.1/4.6/3.9/3.7 | 1.9/0.8/0.3/0.1 | 0.03/0.21/1.71/15.45 | 0.31/0.18/0.07/0.02 | 3.5 ± 0.1 23 ± 2 | Opalescent, yellowish, homogeneous | |
| 7 | ZrS4(4-0) + Zr-Ph(1-3) | 0.75+0.25/3(E)/2 | 35.4/13.8/5.9/3.9 | 184.9/26.0/4.5/1.2 | 1.03/1.47/2.72/6.66 | 5.23/1.88/0.77/0.31 | 3.9 ± 0.1 18 ± 3 | Transparent, yellowish, homogeneous |
| 8 a | ZrS4(4-0) + ZrS4(2-2) | 0.5+0.5/3(G)/2 | 18.2/7.3/4.4/3.6 | 21.1/4.9/1.4/0.4 | 0.12/0.28/0.82/2.03 | 1.16/0.67/0.31/0.10 | 3.1 ± 0.1 24 ± 2 | Transparent, yellowish, homogeneous |
| 9 | ZrS4(2-2) + Zr-Ph(4-0) | 0.5+0.5/3(G)/2 | 14.9/5.9/4.3/3.8 | 33.0/5.0/1.0/0.3 | 0.18/0.28/0.60/1.52 | 2.22/0.84/0.23/0.07 | 4.9 ± 0.1 d 81 ± 6 | Transparent, yellowish, homogeneous |
| 10 | 0.25+0.25/3(G)/3 | 16.7/6.7/4.6/3.9 | 23.7/4.7/1.1/0.4 | 0.13/0.26/0.67/1.95 | 1.41/0.69/0.25/0.09 | 3.6 ± 0.1 95 ± 35 | ||
| 11 | 0.5+0.5/3(G)/3 | 21.0/7.3/4.7/4.1 | 34.2/6.4/1.3/0.4 | 0.19/0.36/0.80/2.07 | 1.63/0.88/0.28/0.10 | 5.3 ± 0.1 c 20 ± 5 | ||
| 12 | ZrS4(2-2) + Zr-Vin(2-2) | 0.5+0.5/3(G)/2 | 25.1/8.0/4.8/4.0 | 46.2/8.6/1.7/0.5 | 0.26/0.49/1.03/2.64 | 1.84/1.07/0.36/0.12 | 6.8 ± 0.3 c 21 ± 2 | Transparent, yellowish, homogeneous |
| 13 | 0.25+0.25/3(G)/3 | 42.0/10.7/6.0/4.6 | 92.9/16.1/2.9/0.8 | 0.52/0.92/1.71/4.51 | 2.22/1.52/0.48/0.17 | 5.6 ± 0.1 d 41 ± 3 | ||
| 14 a b | 0.25+0.25/3(G)/3 | 38.4/11.7/6.2/4.6 | 63.0/13.3/2.8/0.9 | 0.35/0.75/1.69/5.01 | 1.64/1.14/0.45/0.17 | 3.5 ± 0.1 66 ± 8 | ||
| 15 | 0.25+0.25/3(E)/3 | 109.5/30.1/10.1/5.8 | 261.5/43.7/9.7/2.3 | 1.45/2.48/5.81/12.6 | 2.39/1.45/0.96/0.38 | 5.1 ± 0.3 c 12 ± 2 | ||
| 16 a b | 0.25+0.25/3(E)/3 | 66.2/21.7/10.0/6.3 | 125.4/24.0/5.7/2.1 | 0.70/1.36/3.43/11.70 | 1.89/1.11/0.58/0.33 | 4.1 ± 0.2 195 ± 18 | Opalescent, yellowish, homogeneous | |
| 17 | ZrSU(2-2) + Zr-Ph(2-2) | 0.5+0.5/3(G)/2 | 14.1/5.8/4.2/3.8 | 21.7/3.8/0.8/0.2 | 0.12/0.21/0.49/1.26 | 1.54/0.66/0.20/0.06 | 6.5 ± 0.1 d 119 ± 4 | Transparent, yellowish, homogeneous |
| 18 | 0.25+0.25/3(G)/3 | 21.1/7.9/4.7/3.9 | 32.0/7.1/1.5/0.4 | 0.18/0.40/0.91/2.30 | 1.51/0.90/0.32/0.10 | 6.3 ± 0.1 c 18 ± 7 | ||
| 23 | ZrSU(2-2) + Zr-Vin(2-2) | 0.5+0.5/3(G)/2 | 19.2/6.3/4.2/3.7 | 24.7/5.1/1.1/0.3 | 0.14/0.29/0.63/1.55 | 1.28/0.81/0.25/0.07 | 6.7 ± 0.1 d 63 ± 14 | Transparent, yellowish, homogeneous |
| 24 | 0.25+0.25/3(G)/3 | 30.7/8.1/5.1/4.2 | 52.7/9.5/1.8/0.4 | 0.29/0.54/1.06/2.51 | 1.71/1.16/0.35/0.11 | 6.5 ± 0.2 c 21 ± 6 | ||
| 25 | 0.25+0.25/3(A*)/3 | 8.1/5.2/4.1/3.8 | 3.5/1.5/0.5/0.15 | 0.02/0.09/0.28/0.83 | 0.44/0.29/0.11/0.04 | 4.3 ± 0.3 78 ± 10 | Transparent, yellowish, homogeneous | |
| 26 | 0.25+0.25/3(E)/3 | 69.7/16.7/6.7/4.9 | 142.9/28.4/4.7/1.1 | 0.79/1.61/2.82/6.23 | 2.1/1.70/0.70/0.22 | 5.4 ± 0.1 d 34 ± 15 | ||
| 27 b | 0.25+0.25/3(E)/3 | 31.9/14.1/8.2/5.4 | 89.2/14.2/3.6/1.5 | 0.50/0.80/2.12/8.47 | 2.80/1.00/0.43/0.27 | 2.2 ± 0.1 147 ± 9 | Opalescent, yellowish, homogeneous | |
| 28 | ZrSU(2-2) + ZrS4(2-2) | 0.5+0.5/3(G)/2 | 10.5/5.3/4.1/3.8 | 17.5/2.9/0.6/0.2 | 0.10/0.17/0.38/0.96 | 1.67/0.54/0.15/0.04 | 6.5 ± 0.2 c 24 ± 4 | Transparent, yellowish, homogeneous |
| 29 | 0.25+0.25/3(G)/3 | 14.9/5.9/4.3/3.8 | 33.0/5.0/1.0/0.3 | 0.18/0.28/0.60/1.76 | 2.22/0.85/0.23/0.07 | 6.7 ± 0.2 16 ± 2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasenkov, A.N.; Parshina, M.S.; Goncharuk, G.P.; Borisov, K.M.; Golubev, E.K.; Meshkov, I.B.; Cherkaev, G.V.; Shevchenko, V.G.; Ponomarenko, S.A.; Muzafarov, A.M. Thioether-Containing Zirconium(Alkoxy)Siloxanes: Synthesis and Study of Dielectric and Mechanical Properties of Silica-Filled Polydimethylsiloxane Compositions Cured by Them. Polymers 2023, 15, 3361. https://doi.org/10.3390/polym15163361
Tarasenkov AN, Parshina MS, Goncharuk GP, Borisov KM, Golubev EK, Meshkov IB, Cherkaev GV, Shevchenko VG, Ponomarenko SA, Muzafarov AM. Thioether-Containing Zirconium(Alkoxy)Siloxanes: Synthesis and Study of Dielectric and Mechanical Properties of Silica-Filled Polydimethylsiloxane Compositions Cured by Them. Polymers. 2023; 15(16):3361. https://doi.org/10.3390/polym15163361
Chicago/Turabian StyleTarasenkov, Alexander N., Maria S. Parshina, Galina P. Goncharuk, Kirill M. Borisov, Evgeniy K. Golubev, Ivan B. Meshkov, Georgiy V. Cherkaev, Vitaliy G. Shevchenko, Sergey A. Ponomarenko, and Aziz M. Muzafarov. 2023. "Thioether-Containing Zirconium(Alkoxy)Siloxanes: Synthesis and Study of Dielectric and Mechanical Properties of Silica-Filled Polydimethylsiloxane Compositions Cured by Them" Polymers 15, no. 16: 3361. https://doi.org/10.3390/polym15163361
APA StyleTarasenkov, A. N., Parshina, M. S., Goncharuk, G. P., Borisov, K. M., Golubev, E. K., Meshkov, I. B., Cherkaev, G. V., Shevchenko, V. G., Ponomarenko, S. A., & Muzafarov, A. M. (2023). Thioether-Containing Zirconium(Alkoxy)Siloxanes: Synthesis and Study of Dielectric and Mechanical Properties of Silica-Filled Polydimethylsiloxane Compositions Cured by Them. Polymers, 15(16), 3361. https://doi.org/10.3390/polym15163361








