Biomimetic Engineering Preparation of High Mechanical and Flame Retardant Elastomers by Introducing Sacrificial Bonds in Covalently Cross-Linked Chloroprene Rubber
Abstract
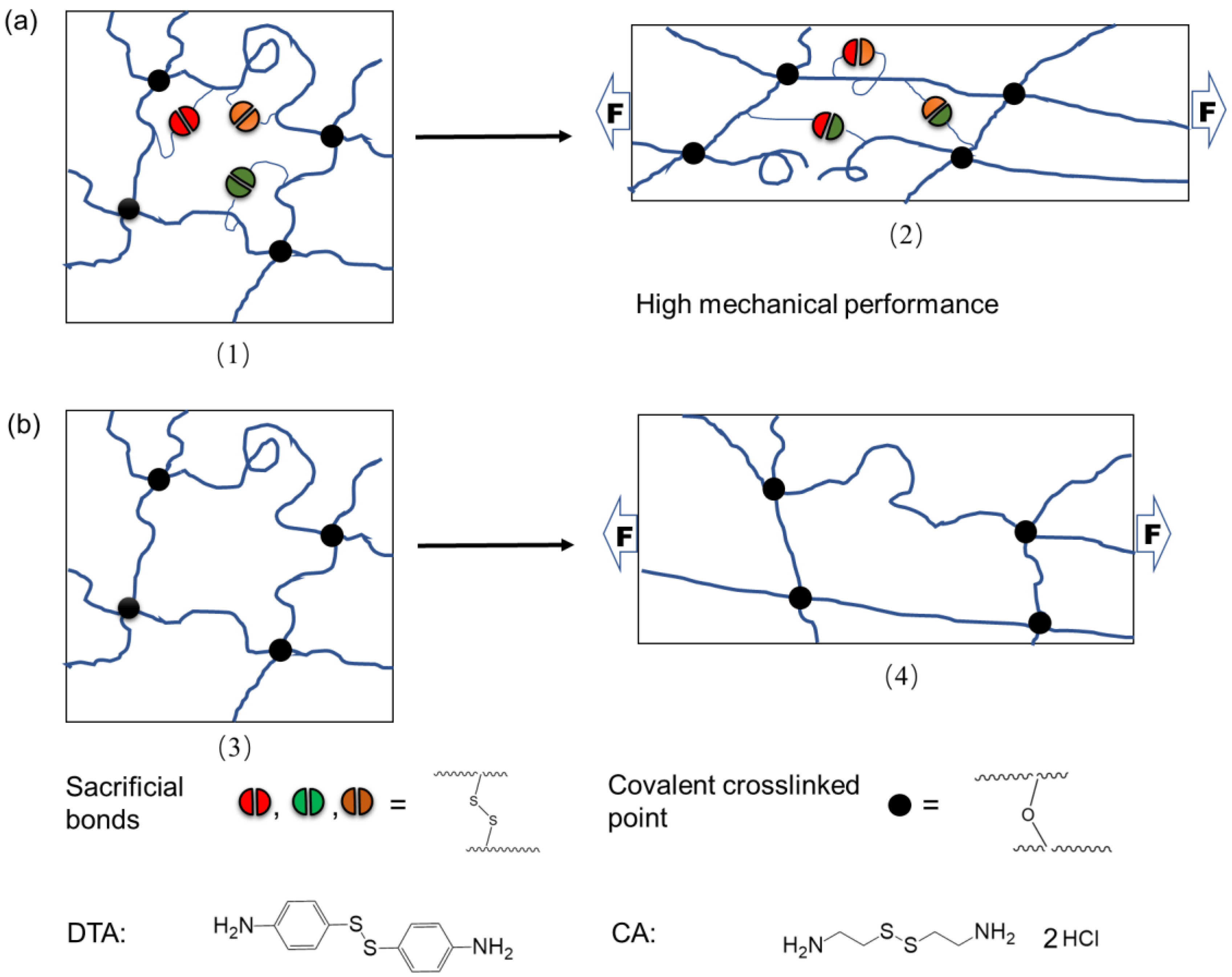
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of CR Vulcanizates
2.3. Measurement and Characterization
3. Results and Discussion
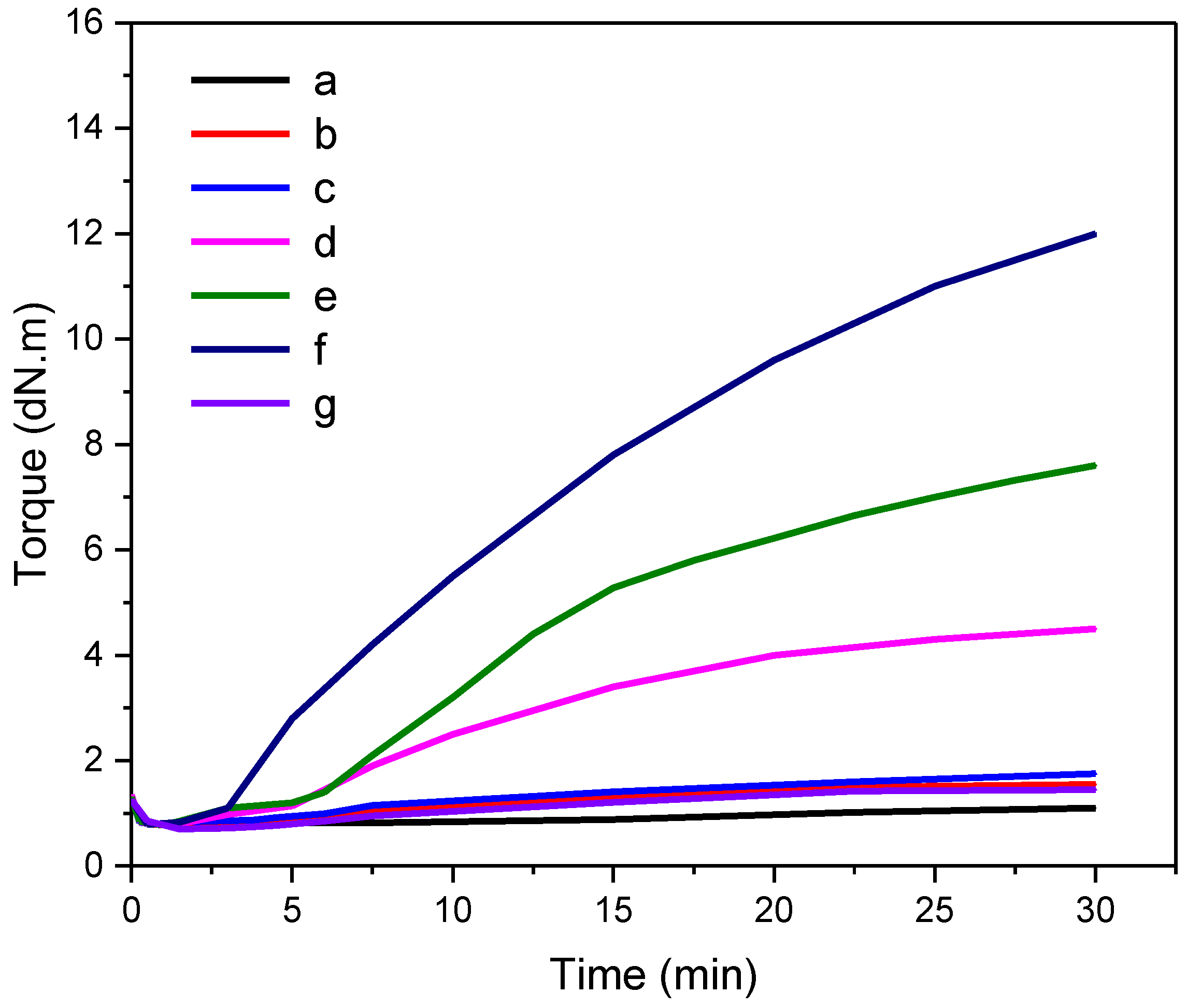
3.1. The Vulcanization Characteristics of CR
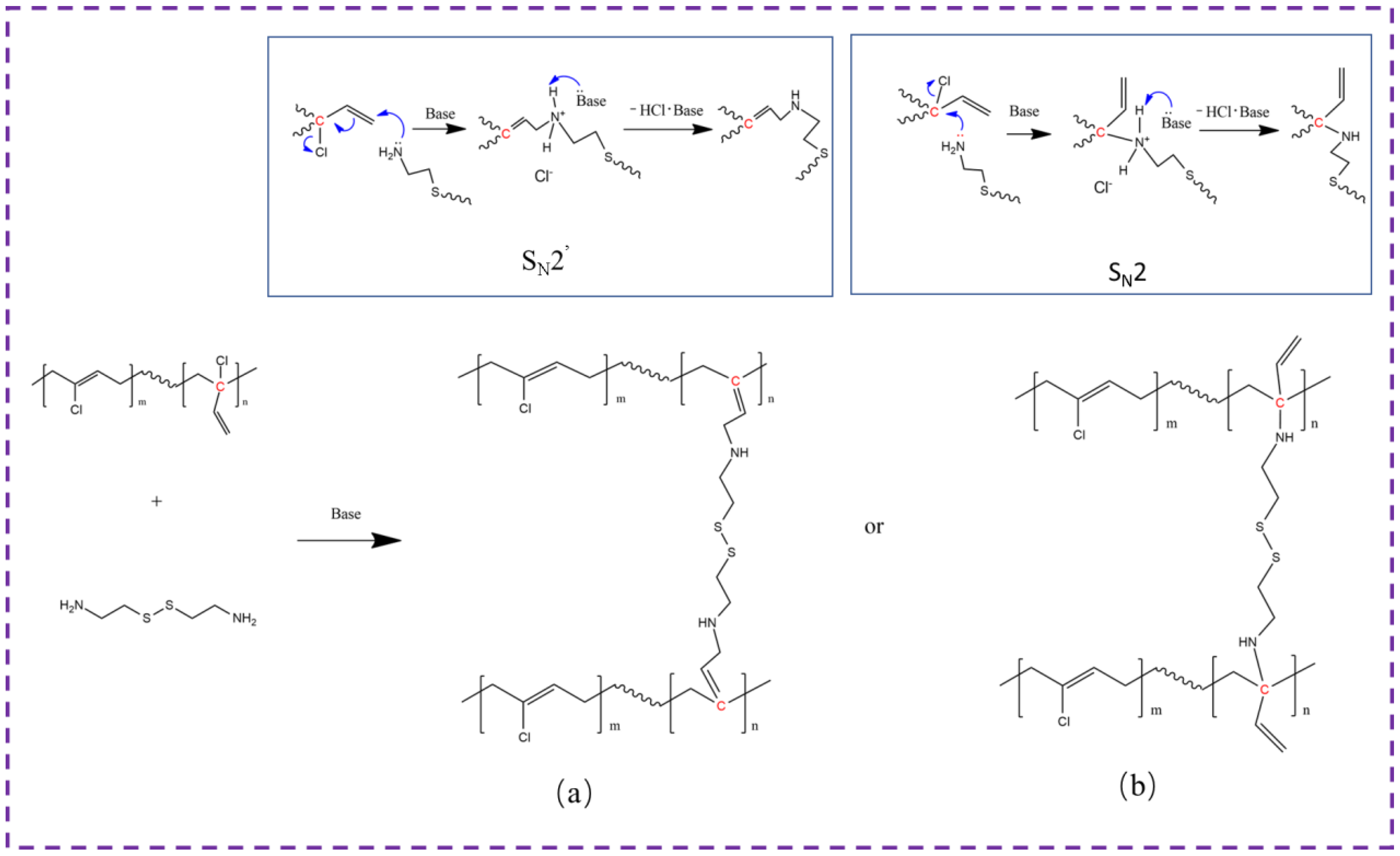
3.2. Vulcanization Performance of CR with Dual Networks
3.3. Tensile Properties of CR Vulcanizate
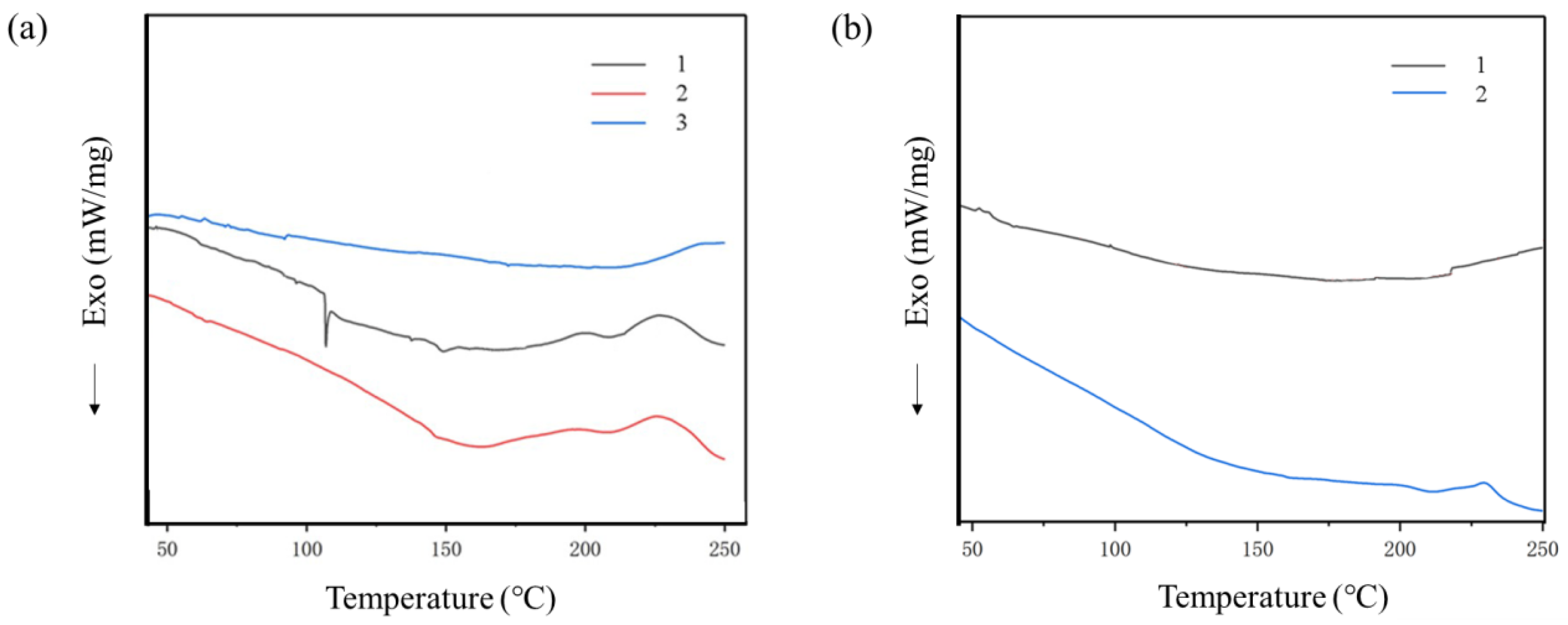
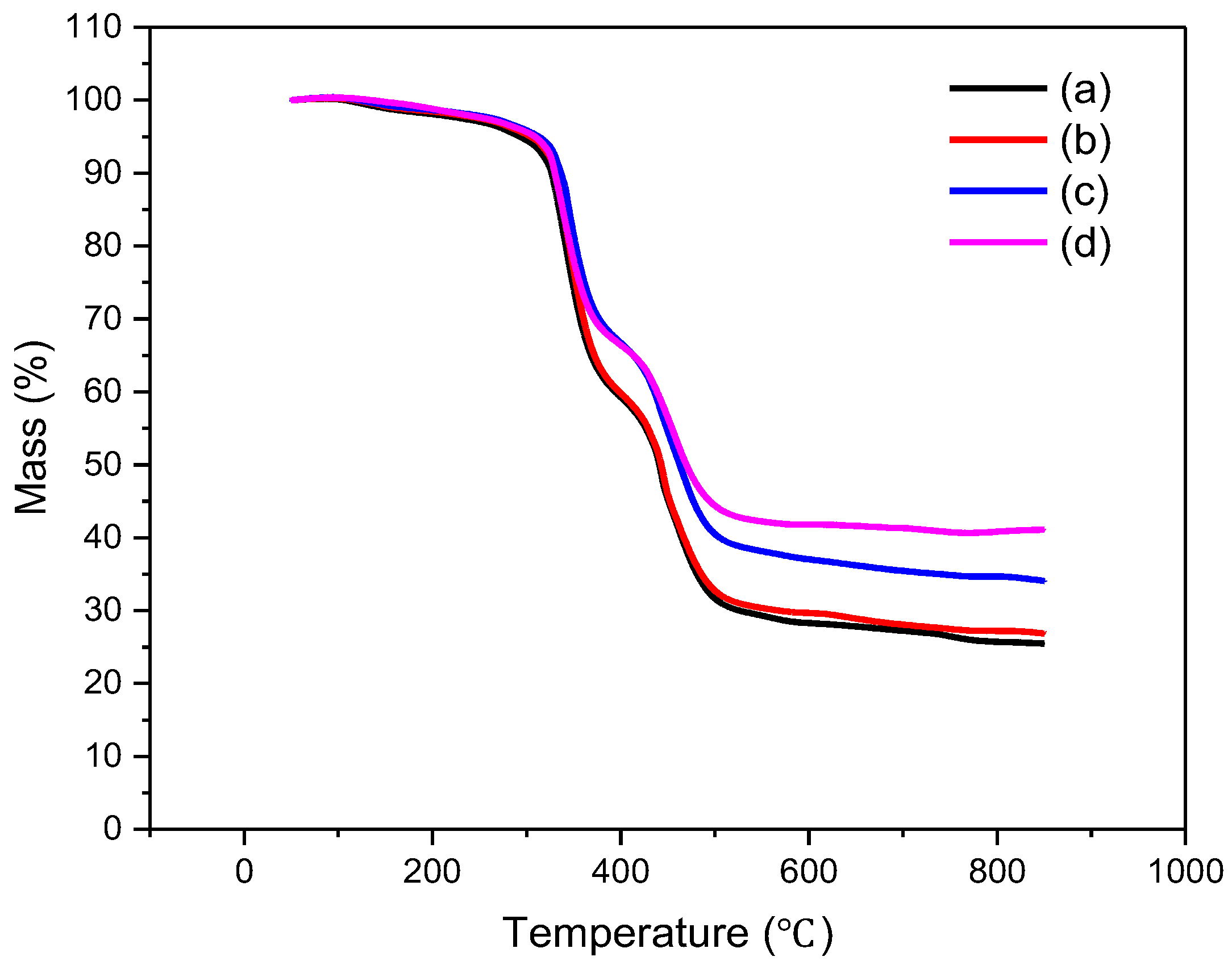
3.4. Thermal Analysis of CR/CA Vulcanizates
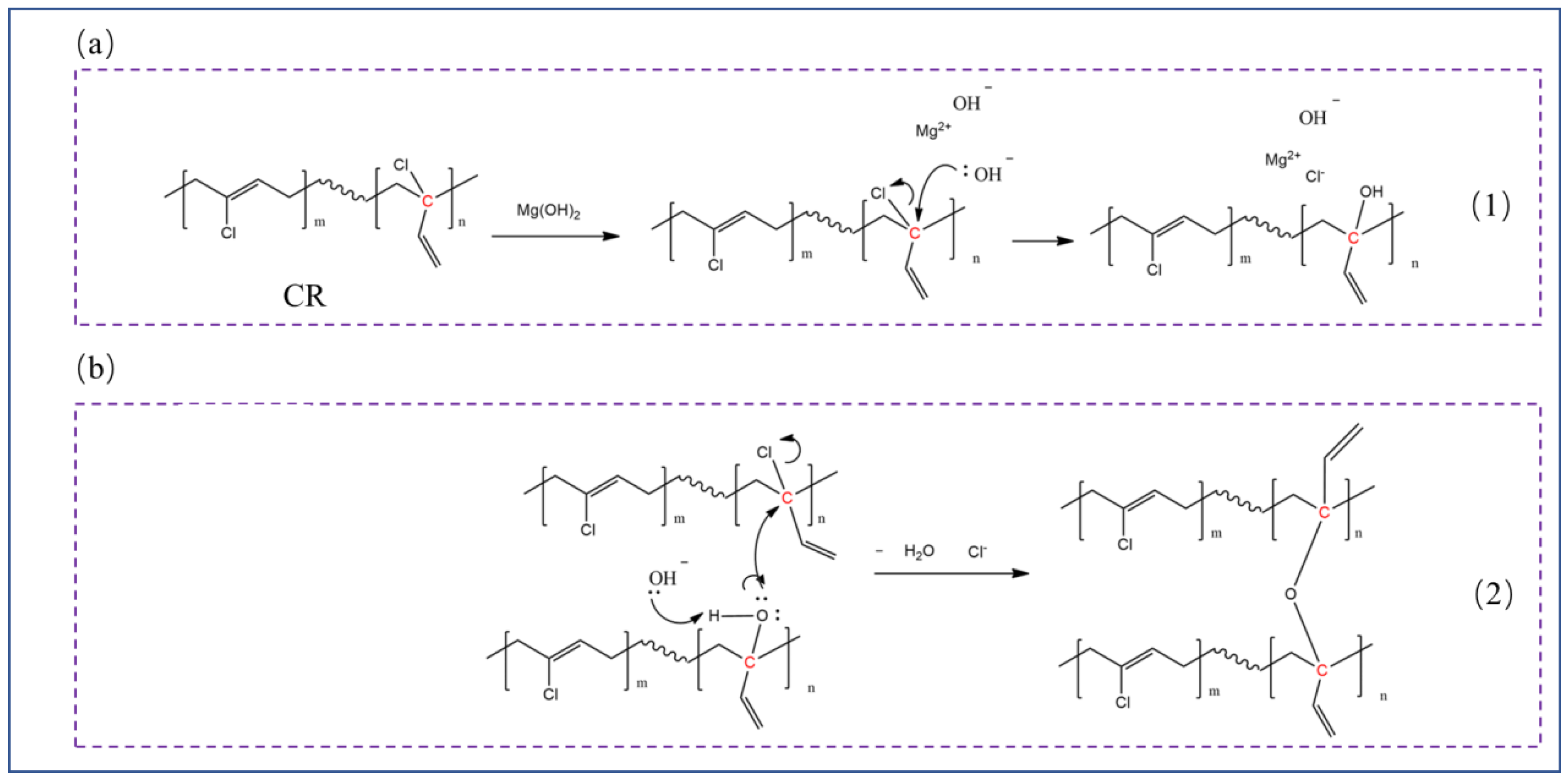
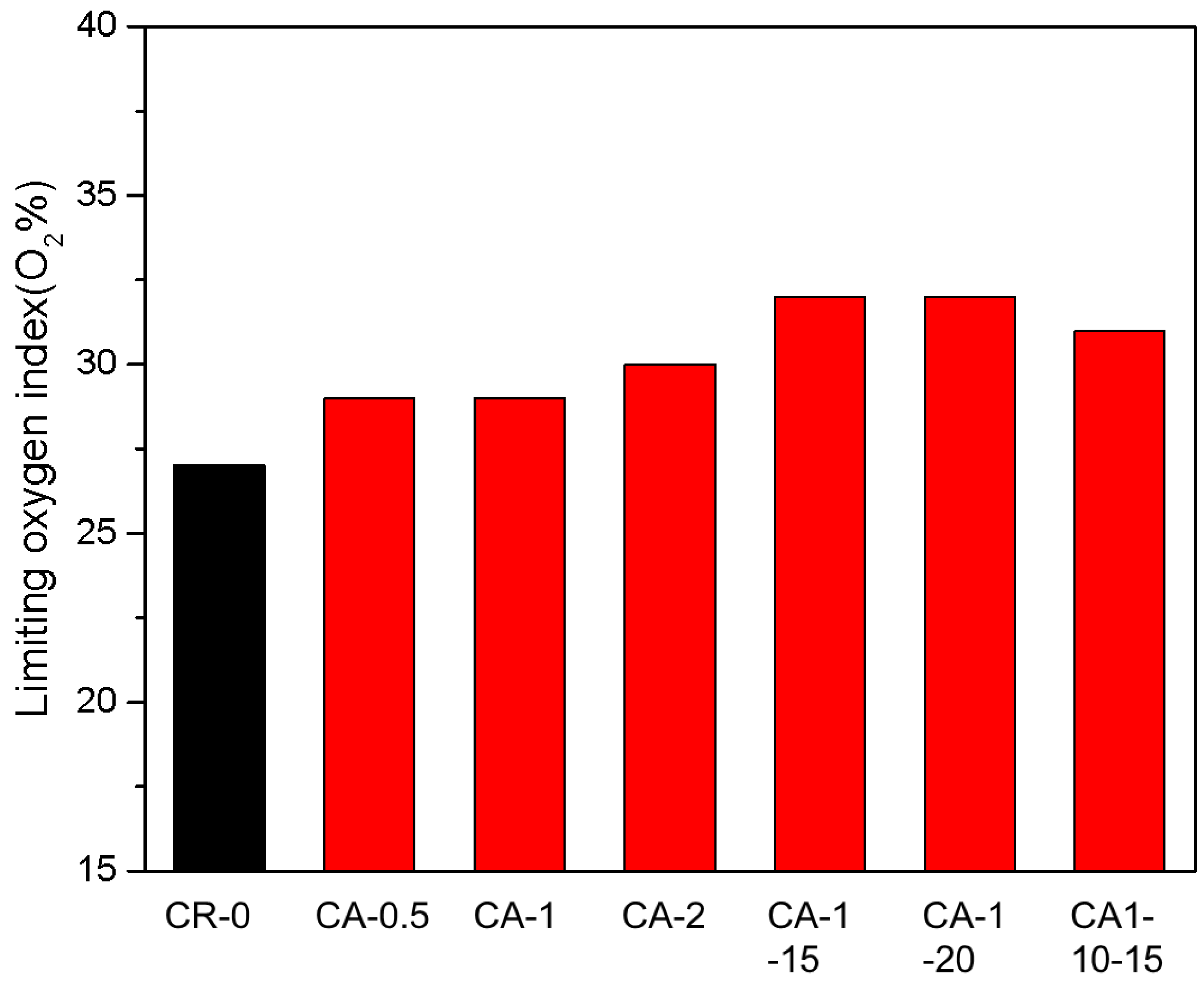
3.5. Flame Retardancy of CR Vulcanizates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fantner, G.E.; Hassenkam, T.; Kindt, J.H.; Weaver, J.C.; Birkedal, H.; Pechenik, L.; Cutroni, J.A.; Cidade, G.A.G.; Stucky, G.D.; Morse, D.E.; et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 2005, 4, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of β-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef]
- Priemel, T.; Degtyar, E.; Dean, M.N.; Harrington, M.J. Rapid self-assembly of complex biomolecular architectures during mussel byssus biofabrication. Nat. Commun. 2017, 8, 14539. [Google Scholar] [CrossRef] [Green Version]
- Fantner, G.E.; Oroudjev, E.; Schitter, G.; Golde, L.S.; Thurner, P.; Finch, M.M.; Turner, P.; Gutsmann, T.; Morse, D.E.; Hansma, H.; et al. Sacrificial Bonds and Hidden Length: Unraveling Molecular Mesostructures in Tough Materials. Biophys. J. 2006, 90, 1411–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Zhang, L.; Tang, Z.; Guo, B. Bioinspired engineering of sacrificial bonds into rubber networks towards high-performance and functional elastomers. Compos. Commun. 2018, 8, 65–73. [Google Scholar] [CrossRef]
- Heim, M.; Keerl, D.; Scheibel, T. Spider Silk: From Soluble Protein to Extraordinary Fiber. Angew. Chem. Int. Ed. 2009, 48, 3584–3596. [Google Scholar] [CrossRef]
- Yarger, J.L.; Cherry, B.R.; van der Vaart, A. Uncovering the structure–function relationship in spider silk. Nat. Rev. Mater. 2018, 3, 18008. [Google Scholar] [CrossRef]
- Filippidi, E.; Cristiani, T.R.; Eisenbach, C.D.; Waite, J.H.; Israelachvili, J.N.; Ahn, B.K.; Valentine, M.T. Toughening elastomers using mussel-inspired iron-catechol complexes. Science 2017, 358, 502–505. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Yu, S.; Zuo, H.; Xu, X.; Ning, N.; Yu, B.; Zhang, L.; Tian, M. Self-Healable Silicone Elastomer Based on the Synergistic Effect of the Coordination and Ionic Bonds. ACS Appl. Polym. Mater. 2021, 3, 2667–2677. [Google Scholar] [CrossRef]
- Nakajima, T. Generalization of the sacrificial bond principle for gel and elastomer toughening. Polym. J. 2017, 49, 477–485. [Google Scholar] [CrossRef]
- Guo, M.; Pitet, L.M.; Wyss, H.M.; Vos, M.; Dankers, P.Y.W.; Meijer, E.W. Tough Stimuli-Responsive Supramolecular Hydrogels with Hydrogen-Bonding Network Junctions. J. Am. Chem. Soc. 2014, 136, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, J.; Shan, G.; Pan, P. High strength of hybrid double-network hydrogels imparted by inter-network ionic bonds. J. Mater. Chem. B 2019, 7, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Qiu, J.; Lu, C.; Jin, S.; Zhang, G.; Sakai, E. Multi-Sacrificial Bonds Enhanced Double Network Hydrogel with High Toughness, Resilience, Damping, and Notch-Insensitivity. Polymers 2020, 12, 2263. [Google Scholar] [CrossRef]
- Wu, S.; Qiu, M.; Tang, Z.; Liu, J.; Guo, B. Carbon Nanodots as High-Functionality Cross-Linkers for Bioinspired Engineering of Multiple Sacrificial Units toward Strong yet Tough Elastomers. Macromolecules 2017, 50, 3244–3253. [Google Scholar] [CrossRef]
- Ducrot, E.; Chen, Y.; Bulters, M.; Sijbesma, R.P.; Creton, C. Toughening Elastomers with Sacrificial Bonds and Watching Them Break. Science 2014, 344, 186–189. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, B.; Zhang, L.; Hu, G.-H. Progress in bio-inspired sacrificial bonds in artificial polymeric materials. Chem. Soc. Rev. 2017, 46, 6301–6329. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Tang, Z.; Huang, J.; Guo, B.; Huang, G. Bioinspired Engineering of Two Different Types of Sacrificial Bonds into Chemically Cross-Linked cis-1,4-Polyisoprene toward a High-Performance Elastomer. Macromolecules 2016, 49, 8593–8604. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, J.; Guo, B.; Zhang, L.; Liu, F. Bioinspired Engineering of Sacrificial Metal–Ligand Bonds into Elastomers with Supramechanical Performance and Adaptive Recovery. Macromolecules 2016, 49, 1781–1789. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.; Jie, S.; Li, B.-G. Preparation of Styrene–Butadiene Rubber Vitrimers with High Strength and Toughness through Imine and Hydrogen Bonds. Ind. Eng. Chem. Res. 2023, 62, 2299–2308. [Google Scholar] [CrossRef]
- You, Y.; Rong, M.Z.; Zhang, M.Q. Adaptable Reversibly Interlocked Networks from Immiscible Polymers Enhanced by Hierarchy-Induced Multilevel Energy Consumption Mechanisms. Macromolecules 2021, 54, 4802–4815. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Huang, J.; Yang, D.; Qiu, X. Bioinspired Engineering towards Tailoring Advanced Lignin/Rubber Elastomers. Polymers 2018, 10, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brostow, W.; Lohse, S.; Lu, X.; Osmanson, A.T. Nano-Al (OH)3 and Mg (OH)2 as flame retardants for polypropylene used on wires and cables. Emergent Mater. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Wang, H.; Zhao, X.; Li, X.; Gu, X.; Li, Y. Flame-retarding nanoparticles as the compatibilizers for immiscible polymer blends: Simultaneously enhanced mechanical performance and flame retardancy. J. Mater. Chem. A 2019, 7, 4903–4912. [Google Scholar] [CrossRef]
- Jiang, J.; Zhai, J.; Kong, L.; Zhao, D.; Feng, Y. Flame retardant chloroprene rubbers with high tensile strength and elongation at break via dual cross-linked networks. RSC Adv. 2022, 12, 27633–27640. [Google Scholar] [CrossRef]
- Kapgate, B.P.; Das, C. Reinforcing efficiency and compatibilizing effect of sol–gel derived in situ silica for natural rubber/chloroprene rubber blends. RSC Adv. 2014, 4, 58816–58825. [Google Scholar] [CrossRef]
- Ju, Y.; Varma, R.S. Aqueous N-alkylation of amines using alkyl halides: Direct generation of tertiary amines under microwave irradiation. Green Chem. 2004, 6, 219–221. [Google Scholar] [CrossRef]
- Fu, M.; Qu, B. Synergistic flame retardant mechanism of fumed silica in ethylene-vinyl acetate/magnesium hydroxide blends. Polym. Degrad. Stab. 2004, 85, 633–639. [Google Scholar] [CrossRef]
- Kitagawa, M.; Misu, S.; Ichikawa, J.; Matsuhashi, H. Preparation of active MgO by short-time thermal decomposition of Mg(OH)2. Res. Chem. Intermed. 2015, 41, 9463–9473. [Google Scholar] [CrossRef]
- Maiti, K.S. Ultrafast N–H vibrational dynamics of hydrogen-bonded cyclic amide reveal by 2DIR spectroscopy. Chem. Phys. 2018, 515, 509–512. [Google Scholar] [CrossRef]
- Mutar, M. A study in vulcanization of neoprene rubber(WER) by polymethylol resin(RESOL). J. Al-Nahrain Univ. Sci. 2010, 13, 1–6. [Google Scholar] [CrossRef]
- Takahashi, A.; Goseki, R.; Otsuka, H. Thermally Adjustable Dynamic Disulfide Linkages Mediated by Highly Air-STable 2,2,6,6-Tetramethylpiperidine-1-sulfanyl (TEMPS) Radicals. Angew. Chem. Int. Ed. 2017, 56, 2016–2021. [Google Scholar] [CrossRef]
- Rekondo, A.; Martin, R.; Ruizdeluzuriaga, A.; Cabañero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horiz. 2014, 1, 237–240. [Google Scholar] [CrossRef]
- Johnson, P.R. Polychloroprene rubber. Rubber Chem. Technol. 1976, 49, 650–702. [Google Scholar] [CrossRef]



| Ingredient (phr) | Sample ID | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR-0 | CR-1 | CR-2 | CA-0.5 | CA-1 | CA-2 | CA-1-x | CA1-10-20 | DTA-0.5 | DTA-1 | DTA-2 | DTA-3 | DTA1-x | DTA1-10-20 | |
| CR | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 0 | 10 | 0 | 10 | 10 | 10 | x | 10 | 10 | 10 | 10 | 10 | x | 10 | |
| 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CA | 0 | 0 | 0 | 0.5 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| DTA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 1 | 2 | 3 | 0 | 0 |
| ZnO | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 20 |
| antioxidant 1010 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Zhai, J.; Zhang, Y.; Feng, Y. Biomimetic Engineering Preparation of High Mechanical and Flame Retardant Elastomers by Introducing Sacrificial Bonds in Covalently Cross-Linked Chloroprene Rubber. Polymers 2023, 15, 3367. https://doi.org/10.3390/polym15163367
Jiang J, Zhai J, Zhang Y, Feng Y. Biomimetic Engineering Preparation of High Mechanical and Flame Retardant Elastomers by Introducing Sacrificial Bonds in Covalently Cross-Linked Chloroprene Rubber. Polymers. 2023; 15(16):3367. https://doi.org/10.3390/polym15163367
Chicago/Turabian StyleJiang, Jianliang, Junxue Zhai, Yiqun Zhang, and Yakai Feng. 2023. "Biomimetic Engineering Preparation of High Mechanical and Flame Retardant Elastomers by Introducing Sacrificial Bonds in Covalently Cross-Linked Chloroprene Rubber" Polymers 15, no. 16: 3367. https://doi.org/10.3390/polym15163367




