Effect of Bleaching Processes on Physicochemical and Functional Properties of Cellulose and Carboxymethyl Cellulose from Young and Mature Coconut Coir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Preparation
2.3. Extraction of Cellulose from Young and Mature Coconut Coir
2.4. Carboxymethyl Cellulose (CMC) Synthesized from Young and Mature Coconut Coir
2.5. The Determination of Lignin Content
2.6. The Kappa Number of Pulp
2.7. Color Characteristics of Cellulose and CMC Samples
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. X-ray Diffraction (XRD) of Cellulose and CMC Samples
2.10. Scanning Electron Microscopy (SEM) of Cellulose and CMC Samples
2.11. Determination of the Degree of Substitution (DS) of CMCy and CMCm
2.12. Water/Oil Absorption Capacity
2.13. Water/Oil Holding Capacity
2.14. Water/Oil Retention Capacity
2.15. Water Solubility
2.16. Viscosity of CMC Samples
3. Results and Discussion
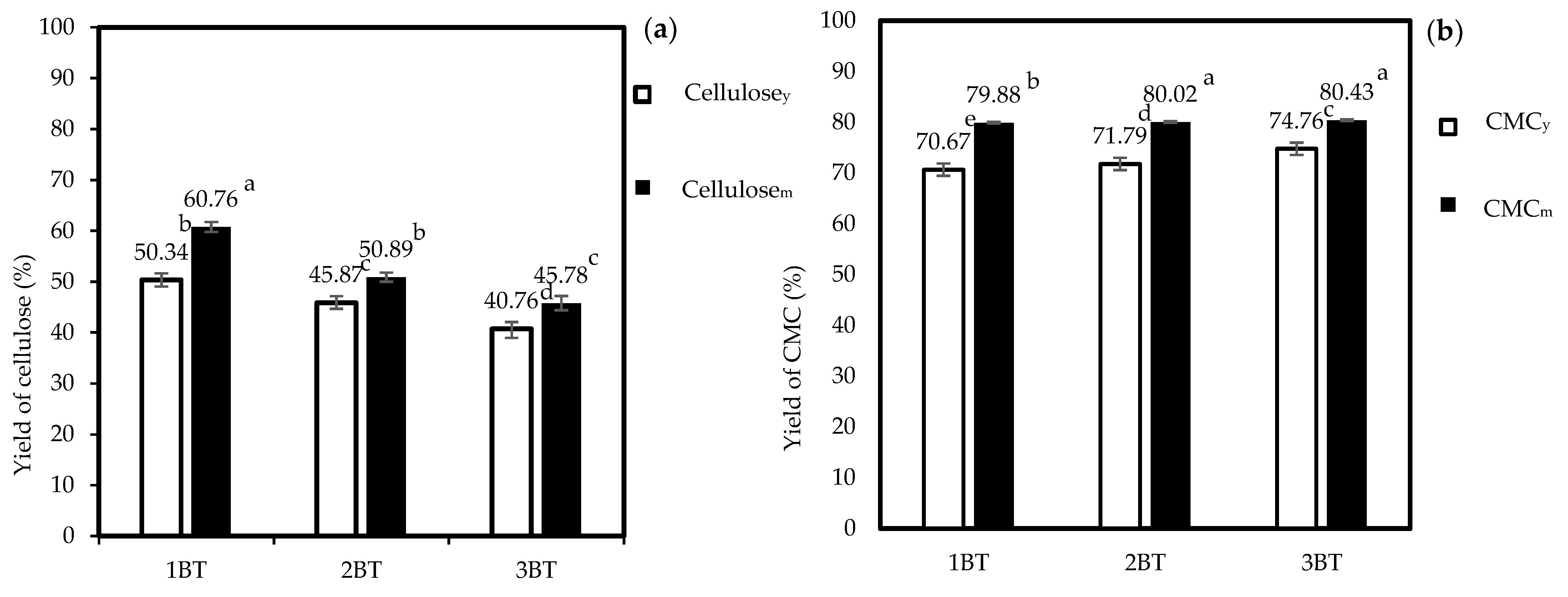
3.1. Percent Yield of Cellulose and CMC Samples
3.2. Lignin Content and Kappa Number of Cellulose
3.3. Appearance and Color of Cellulose and CMC Samples
3.4. Fourier Transform Infrared Spectroscopy (FTIR) of Cellulose and CMC Samples
3.5. X-ray Diffraction (XRD) of Cellulose and CMC Samples
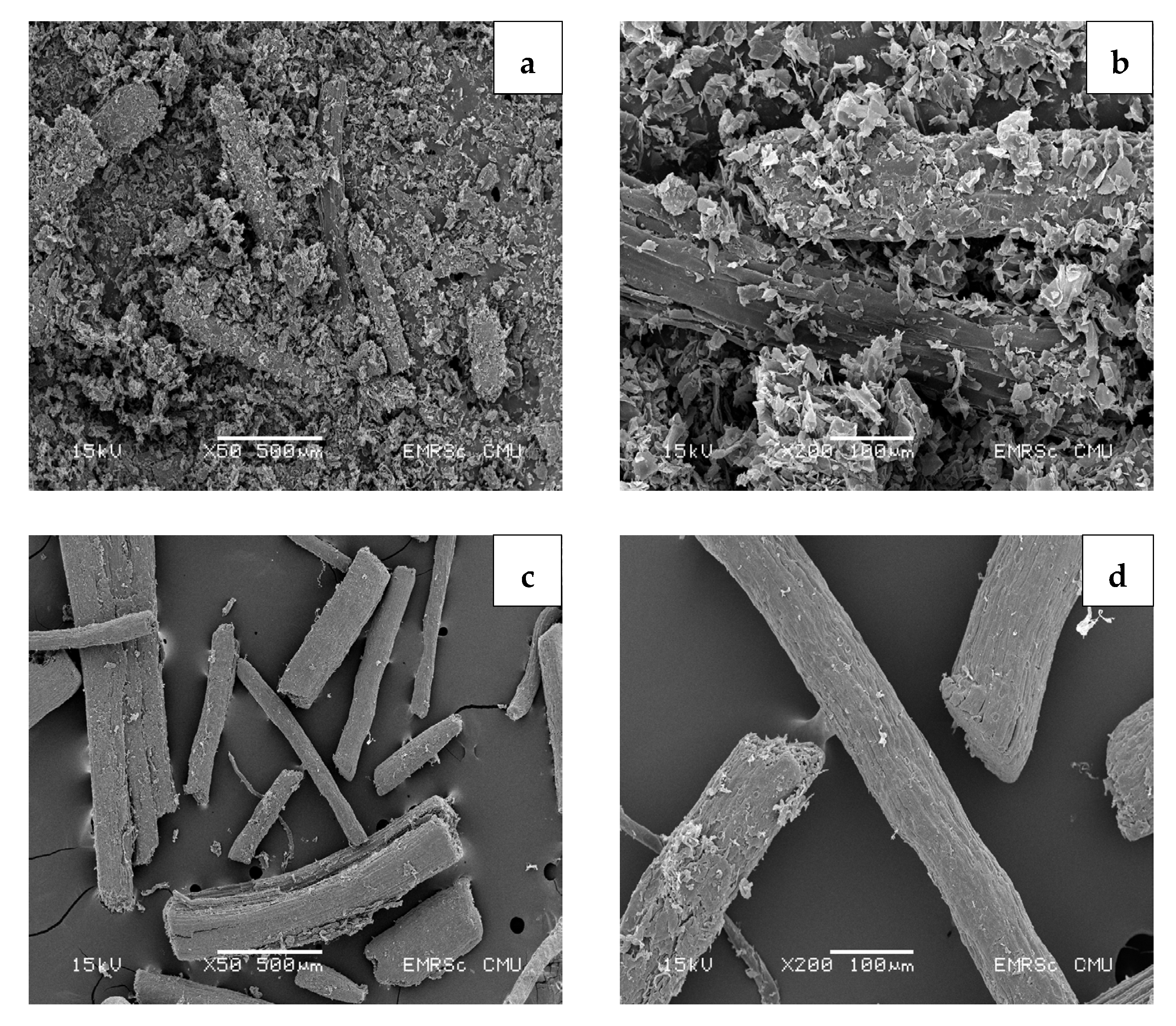
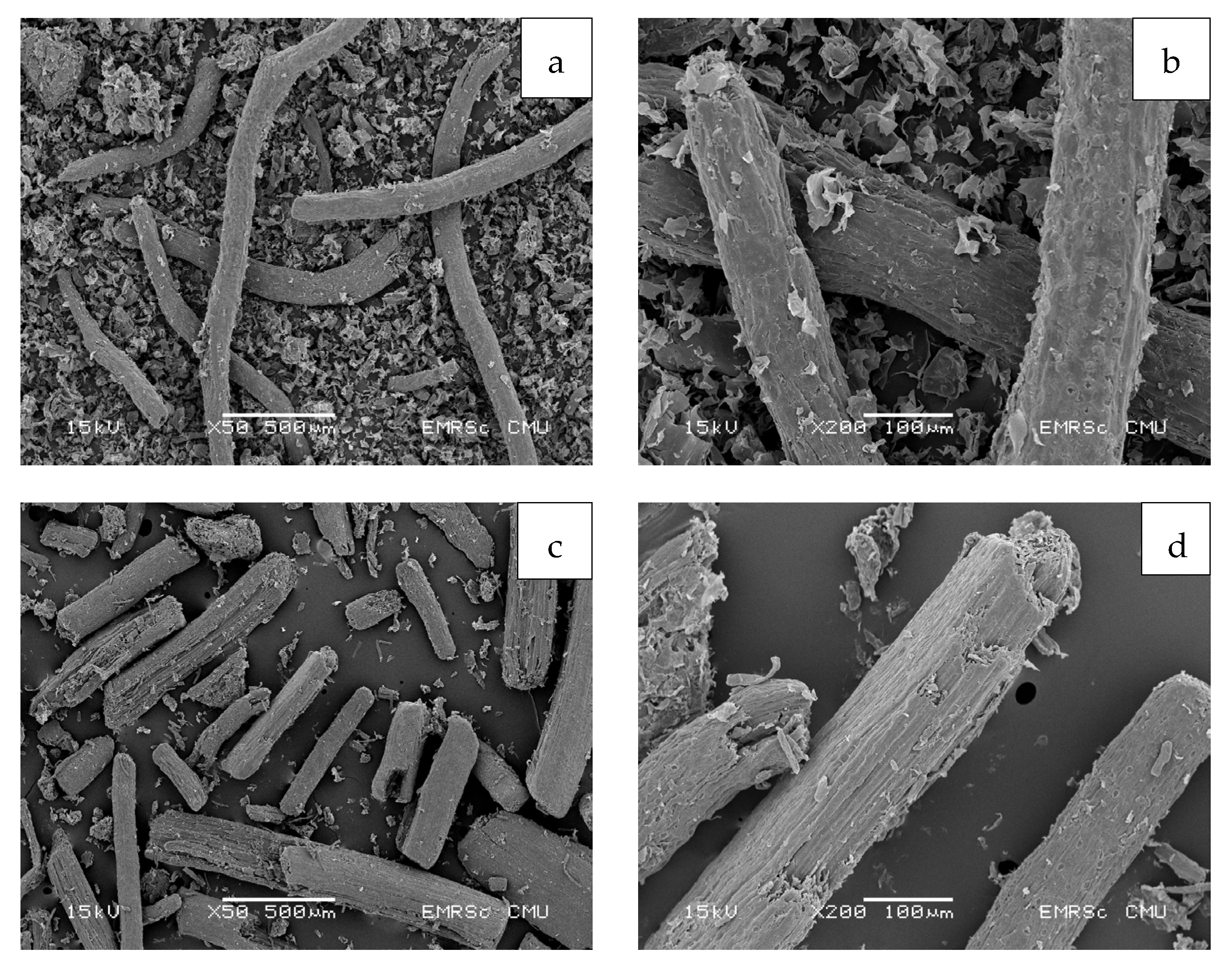
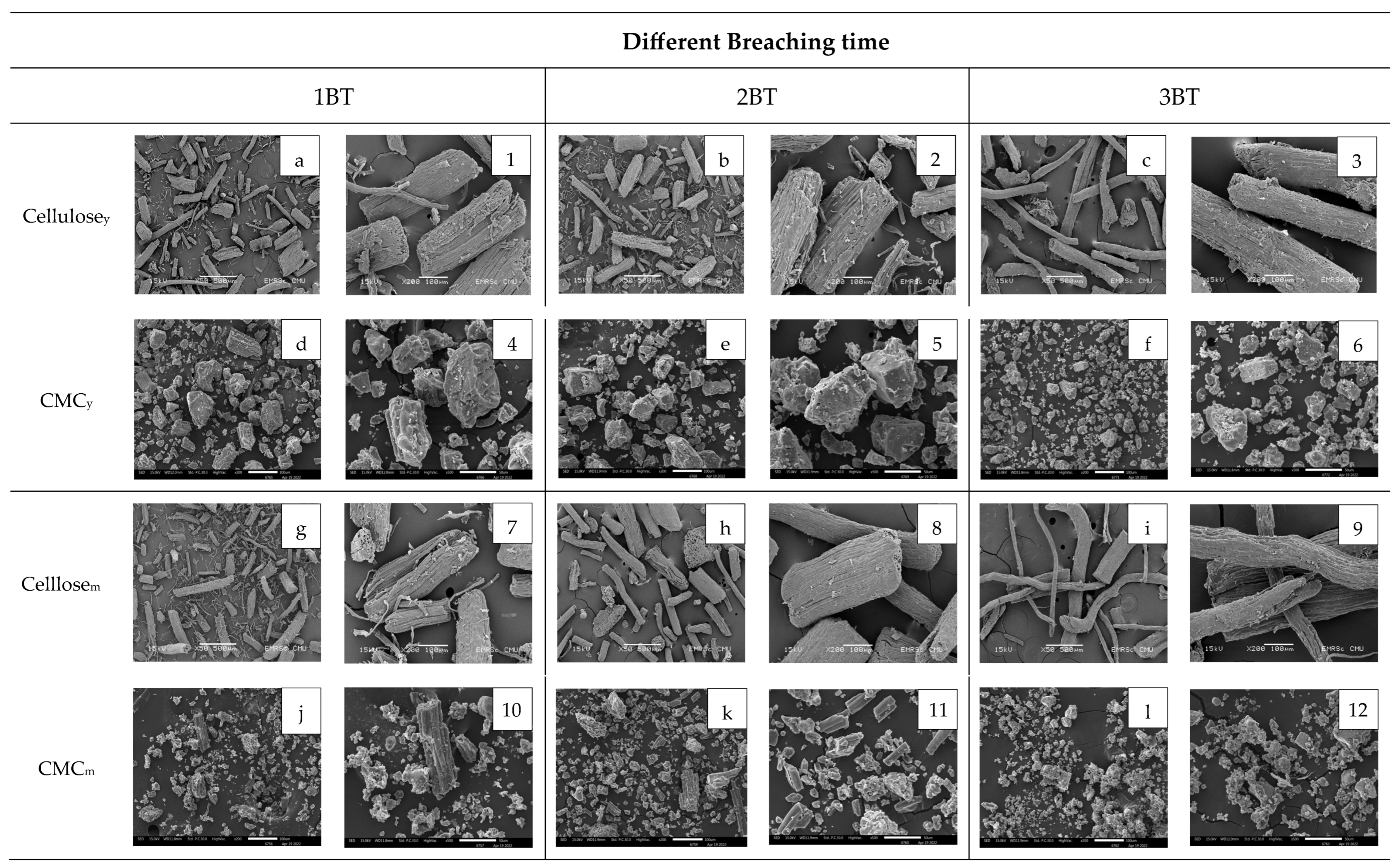
3.6. Morphology of Cellulose and CMC Samples
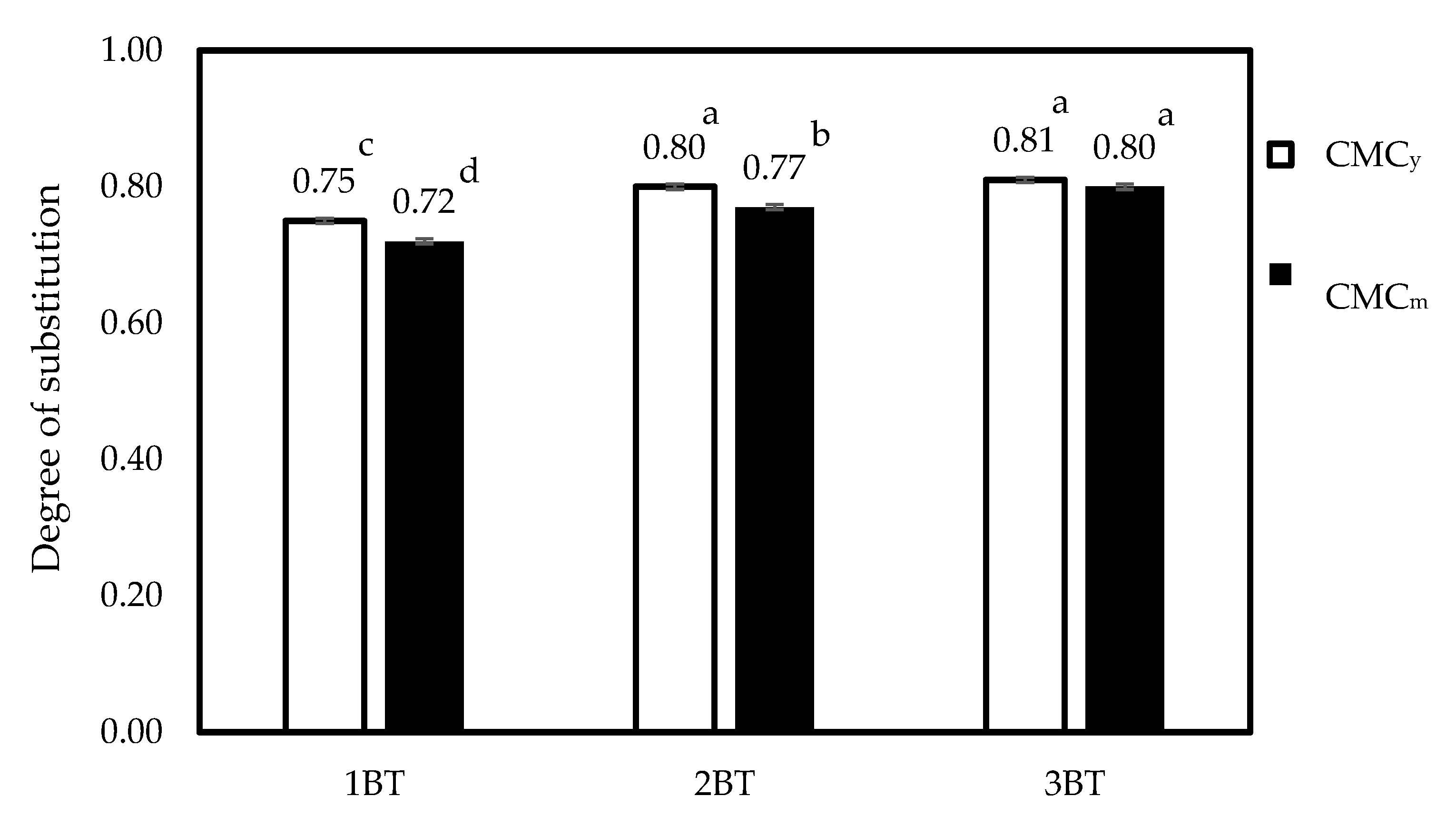
3.7. Degree of Substitution (DS) of CMCy and CMCm with Different Bleaching Times
3.8. Functional Properties of Cellulosey and Cellulosem
3.9. Functional Properties of CMCy and CMCm
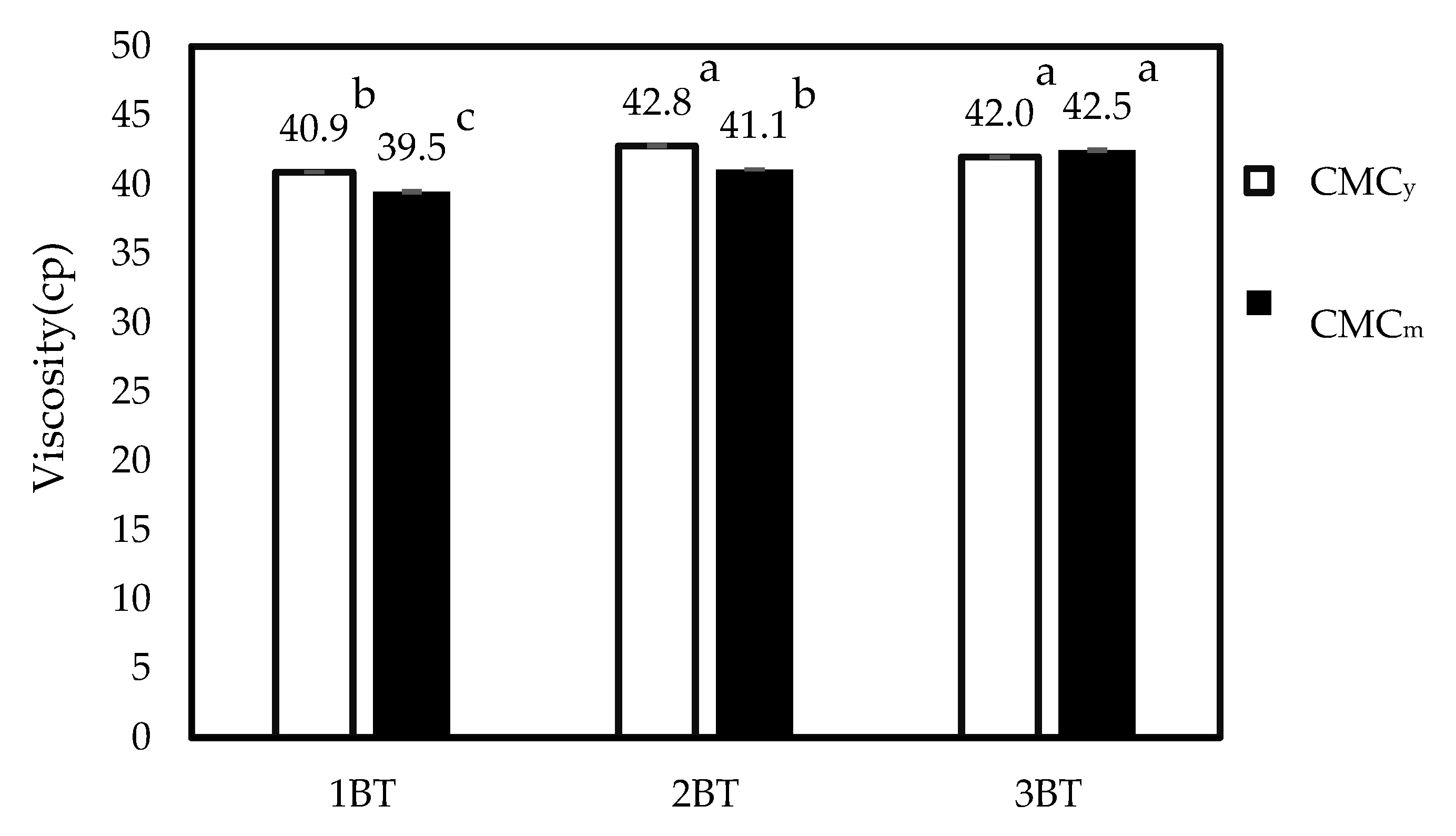
3.10. Effect of Bleaching Times on Viscosity of CMC Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prades, A.; Salum, U.N.; Pioch, D. New era for the coconut sector. What prospects for research? OCL 2016, 23, D607. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.; Gope, P.C.; Shandilya, A.; Gupta, A.; Maheshwari, M.K. Coir fibre reinforcement and application in polymer composites: A review. J. Mater. Environ. Sci. 2013, 4, 263–276. [Google Scholar]
- Ebrahimi, A.; Naranjani, B.; Milani, S.; Dadras Javan, F. Laminar convective heat transfer of shear-thinning liquids in rectangular channels with longitudinal vortex generators. Chem. Eng. Sci. 2017, 173, 264–274. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S.; Samart, C. Biodiesel production in an autoclave reactor using waste palm oil and coconut coir husk derived catalyst. Renew. Energy 2019, 134, 125–134. [Google Scholar] [CrossRef]
- Abifarin, J.K.; Obada, D.O.; Dauda, E.T.; Dodoo-Arhin, D. Experimental data on the characterization of hydroxyapatite synthesized from biowastes. Data Br. 2019, 26, 104485. [Google Scholar] [CrossRef] [PubMed]
- Sathiparan, N.; Rupasinghe, M.N.; Pavithra, B.H.M. Performance of coconut coir reinforced hydraulic cement mortar for surface plastering application. Constr. Build Mater. 2017, 142, 23–30. [Google Scholar] [CrossRef]
- Rawangkul, R.; Khedari, J.; Hirunlabh, J.; Zeghmati, B. Characteristics and performance analysis of a natural desiccant prepared from coconut coir. Sci. Asia 2010, 36, 216–222. [Google Scholar] [CrossRef]
- Aggarwal, S.; Nirmala, C. Utilization of coir fibers as an eco-friendly substitute for costly gelling agents for in vitro orchid seed germination. Sci. Hortic. 2012, 133, 89–92. [Google Scholar] [CrossRef]
- Suriyatem, R.; Noikang, N.; Kankam, T.; Jantanasakulwong, K.; Leksawasdi, N.; Phimolsiripol, Y.; Insomphun, C.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; et al. Physical properties of carboxymethyl cellulose from palm bunch and bagasse agricultural wastes: Effect of delignification with hydrogen peroxide. Polymers 2020, 12, 1505. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Alam, K.; Ahmed, M.; Akter, S.; Islam, N.; Eun, J.B. Effect of carboxymethylcellulose and starch as thickening agents on the quality of tomato ketchup. Pak. J. Nutr. 2009, 8, 1144–1149. [Google Scholar]
- Rachtanapun, P.; Eitssayeam, S.; Pengpat, K. Study of carboxymethyl cellulose from papaya peels as binder in ceramics. Adv. Mat. Res. 2010, 93–94, 17–21. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Homsaard, N.; Kodsangma, A.; Phongthai, S.; Leksawasdi, N.; Phimolsiripol, Y.; Seesuriyachan, P.; Chaiyaso, T.; Chotinan, S.; Jantrawut, P.; et al. Effects of storage temperature on the quality of eggs coated by cassava starch blended with carboxymethyl cellulose and paraffin wax. Poult. Sci. 2022, 101, 101509. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; et al. Carboxymethyl bacterial cellulose from Nata de Coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Saenjaiban, A.; Singtisan, T.; Suppakul, P.; Jantanasakulwong, K.; Punyodom, W.; Rachtanapun, P. Novel color change film as a time-temperature indicator using polydiacetylene/silver nanoparticles embedded in carboxymethyl cellulose. Polymers 2020, 12, 2306. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT—J. Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Tavares, K.M.; Campos, A.; Mitsuyuki, M.C.; Luchesi, B.R.; Marconcini, J.M. Corn and cassava starch with carboxymethyl cellulose films and its mechanical and hydrophobic properties. Carbohydr. Polym. 2019, 223, 115055. [Google Scholar] [CrossRef]
- More, A. Carboxymethyl Cellulose (CMC) Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023–2028. Available online: https://www.imarcgroup.com/carboxymethyl-cellulose-market (accessed on 2 February 2023).
- Candido, R.G.; Gonçalves, A.R. Synthesis of cellulose acetate and carboxymethylcellulose from sugarcane straw. Carbohydr. Polym. 2016, 152, 679–686. [Google Scholar] [CrossRef]
- Yaşar, F.; Toğrul, H.; Arslan, N. Flow properties of cellulose and carboxymethyl cellulose from orange peel. J. Food Eng. 2007, 81, 187–199. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Rafie, M.; Abdel-Mohdy, F.; Abdel-Halim, E.; Emam, H.E. Carboxymethyl cellulose for green synthesis and stabilization of silver nanoparticles. Carbohydr. Polym. 2010, 82, 933–941. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Utilization of carboxymethyl cellulose from durian rind agricultural waste to improve physical properties and stability of rice starch-based film. J. Polym. Environ. 2018, 27, 286–298. [Google Scholar] [CrossRef]
- Mondal, M.I.; Yeasmin, M.S.; Rahman, M.S. Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Rojsitthisak, P.; Khunthon, S.; Noomun, K.; Limpanart, S. Response surface method to optimize the preparation of carboxymethyl cellulose from corn peel agricultural waste. Sci. Asia 2017, 43, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, characterization, and application of carboxymethyl cellulose from asparagus stalk end. Polymers 2020, 13, 81. [Google Scholar] [CrossRef]
- Rachtanapun, P. Blended films of carboxymethyl cellulose from papaya peel/corn starch film blends. Agric. Nat. Resour. 2009, 43, 259–266. [Google Scholar]
- Eliza, M.Y.; Shahruddin, M.; Noormaziah, J.; Rosli, W.W. Carboxymethyl cellulose (CMC) from oil palm empty fruit bunch (OPEFB) in the new solvent dimethyl sulfoxide (DMSO)/tetrabutylammonium Fluoride (TBAF). J. Phys. Conf. Ser. 2015, 622, 012026. [Google Scholar] [CrossRef]
- Mulyatno, H.A.; Pratama, O.I.; Inayati, I. Synthesis of Carboxymethyl cellulose (CMC) from banana tree stem: Influence of ratio of cellulose with sodium chloroacetate to properties of carboxymethyl cellulose. J. Chem. Eng. 2017, 1, 2622–3430. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from Mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2015, 23, 57–91. [Google Scholar] [CrossRef]
- Daud, Z.; Hatta, M.Z.M.; Kassim, A.S.M.; Awang, H.; Aripin, A.M.A. Exploring of agro waste (pineapple leaf, corn stalk, and napier grass) by chemical composition and morphological study. BioResources 2014, 9, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Duda, K.; Edukondalu, L.; Kumar, S.; Satyanarayana, C.H.V.V.; Lakshmipathy, R.; Madava, M. Isolation and characterization of cellulose fibre from turmeric (Curcuma longa L.) leaves. J. Pharmacogn. Phytochem. 2019, 8, 4001–4003. [Google Scholar]
- Yamazaki, E.; Murakami, K.; Kurita, O. Easy preparation of dietary fiber with the high water-holding capacity from food sources. Plant. Foods Hum. Nutr. 2005, 60, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P. Water absorption, retention and the swelling characteristics of cassava starch grafted with polyacrylic acid. Carbohydr. Polym. 2014, 103, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, R.; Billigraham, P.; Sathishkumar, T.P. Characterization studies on new cellulosic fiber extracted from leucaena leucocephala tree. J. Nat. Fibers. 2023, 20, 2157922. [Google Scholar] [CrossRef]
- Norajit, K.; Kim, K.M.; Ryu, G.H. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Huang, C.M.; Chia, P.X.; Lim, C.S.; Nai, J.Q.; Ding, D.Y.; Seow, P.B.; Wong, C.W.; Chan, E.W. Synthesis and characterisation of carboxymethyl cellulose from various agricultural wastes. Cellul. Chem. Technol. 2017, 51, 665–672. [Google Scholar]
- Barba, C.; Montané, D.; Rinaudo, M.; Farriol, X. Synthesis and characterization of carboxymethylcelluloses (CMC) from non-wood fibers I.Accessibility of cellulose fibers and CMC synthesis. Cellulose 2002, 9, 319–326. [Google Scholar] [CrossRef]
- Alam, A.B.M.F.; Mondal, M.I.H. Utilization of cellulosic wastes in textile and garment industries. I. Synthesis and grafting characterization of carboxymethyl cellulose from knitted rag. J. Appl. Polym. Sci. 2013, 128, 1206–1212. [Google Scholar] [CrossRef]
- Afsahi, G.; Rojalin, T.; Vuorinen, T. Chemical characteristics and stability of eucalyptus kraft pulps bleached with tertiary amine catalyzed hypochlorous acid. Cellulose 2018, 26, 2047–2054. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Ferdous, T.; Quaiyyum, M.A.; Jahan, M.S. Chlorine dioxide bleaching of nineteen non-wood plant pulps. Nord. Pulp Pap. Res. J. 2020, 35, 569–576. [Google Scholar] [CrossRef]
- Celino, A.; Goncalves, O.; Jacquemin, F.; Freour, S. Qualitative and quantitative assessment of water sorption in natural fibres using ATR-FTIR spectroscopy. Carbohydr. Polym. 2014, 101, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adinugraha, M.P.; Marseno, D.W. Synthesis and characterization of sodium carboxymethylcellulose from cavendish banana pseudo stem (Musa cavendishii LAMBERT). Carbohydr. Polym. 2005, 62, 164–169. [Google Scholar] [CrossRef]
- Hu, Z.; Zhai, R.; Li, J.; Zhang, Y.; Lin, J. Preparation and characterization of nanofibrillated cellulose from bamboo fiber via ultrasonication assisted by repulsive effect. Int. J. Polym. Sci. 2017, 2017, 9850814. [Google Scholar] [CrossRef]
- Tavares, K.M.; Campos, A.; Luchesi, B.R.; Resende, A.A.; Oliveira, J.E.; Marconcini, J.M. Effect of carboxymethyl cellulose concentration on mechanical and water vapor barrier properties of corn starch films. Carbohydr. Polym. 2020, 246, 116521. [Google Scholar] [CrossRef] [PubMed]
- Penjumras, P.; Rahman, R.B.A.; Talib, R.A.; Abdan, K. Extraction and characterization of cellulose from durian rind. J. Agric. Sci. 2014, 2, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.X.; Xu, H.P.; Wang, N.; Yang, L.Y.; Wang, Y.G.; Yu, D.; Ouyang, X.K. Fabrication of carboxymethylcellulose/metal-organic framework beads for removal of Pb(II) from aqueous solution. J. Mater. Chem. A 2019, 12, 942. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Wang, M.; Liao, C.; Fang, T.; Li, J.; Zhou, R. Isolation of cellulose from rice straw and its conversion into cellulose acetate catalyzed by phosphotungstic acid. Carbohydr. Polym. 2013, 94, 71–76. [Google Scholar] [CrossRef]
- Mazlan, M.M.; Kian, L.K.; Fouad, H.; Jawaid, M.; Karim, Z.; Saba, N. Synthesis and characterization of carboxymethyl cellulose from pineapple leaf and kenaf core biomass: A comparative study of new raw materials. Biomass Convers. Biorefin. 2022, 1–11. [Google Scholar] [CrossRef]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of water absorption in natural cellulosic fibres from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
- Martinelli, F.R.B.; Ribeiro, F.R.C.; Marvila, M.T.; Monteiro, S.N.; Filho, F.; Azevedo, A.R.G. A review of the use of coconut fiber in cement composites. Polymers 2023, 15, 1309. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, A.; Pereira, F.V. Isolation and surface modification of cellulose from underutilizedLuffa cylindricasponge: A potential feed stock for local polymer industry in Africa. J. Assoc. Arab Univ. Basic Appl. Sci. 2018, 24, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Anggrahini, S.; Marsono, D.W.; Setiyoko, A.; Wahyuningtyas, A. Carboxymethyl celulose (CMC) from snake fruit (Salaca edulis Reinw) kernel of pondoh super: Synthesis and characterization. Indones. Food. Nutr. Prog. 2017, 12, 101–107. [Google Scholar] [CrossRef] [Green Version]
- El Ghzaoui, A.; Trompette, J.L.; Cassanas, G.; Bardet, L.; Fabregue, E. Comparative rheological behavior of some cellulosic ether derivatives. Langmuir 2001, 17, 1453–1456. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; Barringer, S.A.; Iqbal, T. Flow property measurement of food powders and sensitivity of Jenike’s hopper design methodology to the measured values. J. Food. Eng. 2004, 61, 399–405. [Google Scholar] [CrossRef]
- FAO/WHO Expert Committee on Food Additives. Meeting and World Health Organization, Compendium of food additive specifications, food and agriculture organization of the United Nations, Rome, Italy. Available online: https://www.fao.org/3/a0691e/a0691e.pdf (accessed on 15 March 2023).



| Samples | Bleaching Times | Lignin Content (%) | Kappa Number (%) |
|---|---|---|---|
| Cellulosey | 1 BT | 18.35 ± 0.28 c | 20.89 ± 0.14 b |
| 2 BT | 12.21 ± 0.06 d | 17.67 ± 0.13 d | |
| 3 BT | 11.19 ± 0.28 e | 14.11 ± 0.11 f | |
| Cellulosem | 1 BT | 32.04 ± 0.03 a | 21.23 ± 0.10 a |
| 2 BT | 23.04 ± 0.10 b | 18.21 ± 0.17 c | |
| 3 BT | 18.81 ± 0.08 c | 15.00 ± 0.05 e |
| Samples | Bleaching Times | L* | a* | b* | WI | ∆E |
|---|---|---|---|---|---|---|
| Cellulosey | 1 BT | 9.80 ± 0.08 f | 5.91 ± 0.03 c | 8.24 ± 0.09 b | 11.21 ± 0.05 d | 3.60 ± 0.05 d |
| 2 BT | 13.64 ± 0.06 b | 6.43 ± 0.04 a | 7.54 ± 0.08 d | 13.07± 0.04 c | 3.93 ± 0.04 c | |
| 3 BT | 15.41 ± 3.00 ab | 6.49 ± 0.05 a | 8.15 ± 0.05 c | 15.80 ± 0.09 a | 1.87 ± 0.09 f | |
| Cellulosem | 1 BT | 11.86 ± 0.04 d | 6.22 ± 0.09 b | 5.66 ± 0.04 e | 9.23 ± 0.03 e | 9.80 ± 0.08 a |
| 2 BT | 12.34 ± 0.04 c | 6.84 ± 0.06 a | 10.85 ± 0.08 a | 11.40 ± 0.06 d | 6.51 ± 0.06 b | |
| 3 BT | 16.23 ± 0.06 a | 6.23 ± 0.04 b | 8.72 ± 0.03 b | 14.77 ± 0.08 b | 2.26 ± 0.03 e |
| Samples | Bleaching Times | L* | a* | b* | WI | ∆E |
|---|---|---|---|---|---|---|
| CMCy | 1 BT | 42.17 ± 0.03 b | 9.55 ± 0.01 c | 5.63 ± 0.08 d | 41.11 ± 0.05 c | 13.06 ± 0.05 c |
| 2 BT | 42.62 ± 0.05 b | 9.97 ± 0.37 b | 5.56 ± 0.07 d | 41.49 ± 0.04 b | 15.89 ± 0.04 b | |
| 3 BT | 81.81 ± 0.02 a | 11.08 ± 0.02 a | 11.20 ± 0.04 b | 75.35 ± 0.01 a | 39.60 ± 0.01 a | |
| CMCm | 1 BT | 7.44 ± 0.10 e | 5.14 ± 0.04 e | 9.63 ± 1.19 c | 16.72 ± 0.32 f | 2.75 ± 0.32 f |
| 2 BT | 8.45 ± 0.04 d | 6.29 ± 0.02 d | 9.95 ± 0.06 c | 17.60 ± 0.10 e | 3.84 ± 0.10 e | |
| 3 BT | 10.81 ± 0.03 c | 5.57 ± 0.03 e | 13.64 ± 0.04 a | 29.25 ± 0.08 d | 12.91 ± 0.08 d |
| Samples | Bleaching Times | Water Absorption Capacity (%) | Oil Absorption Capacity (%) | Water Holding Capacity (%) | Oil Holding Capacity (%) | Water Retention Capacity (%) | Oil Retention Capacity (%) | Water Solubility (%) | Moisture Content (%) |
|---|---|---|---|---|---|---|---|---|---|
| Cellulosey | 1 BT | 9.48 ± 0.07 f | 21.12 ± 0.01 c | 16.34 ± 0.43 d | 10.16 ± 0.04 c | 60.12 ± 0.02 c | 3.91 ± 0.02 b | 9.21 ± 0.01 e | 1.21 ± 0.05 f |
| 2 BT | 10.36 ± 0.45 e | 26.21 ± 0.16 b | 17.02 ± 0.02 b | 10.89 ± 0.02 b | 64.28 ± 0.10 b | 2.13 ± 0.02 f | 10.42 ± 0.02 b | 1.36 ± 0.05 e | |
| 3 BT | 10.87 ± 0.02 c | 28.07 ± 0.02 a | 17.71 ± 0.20 a | 11.40 ± 0.35 a | 66.25 ± 0.05 a | 2.83 ± 0.08 d | 10.43 ± 0.02 b | 1.48 ± 0.05 d | |
| Cellulosem | 1 BT | 10.66 ± 0.23 d | 18.12 ± 0.01 f | 15.46 ± 0.34 f | 7.06 ± 0.03 f | 50.12 ± 0.02 f | 3.11 ± 0.02 c | 9.93 ± 0.01 d | 1.52 ± 0.02 c |
| 2 BT | 11.62 ± 0.26 b | 19.91 ± 0.07 e | 15.90 ± 0.01 e | 8.10 ± 0.06 e | 53.12 ± 0.01 e | 4.39 ± 0.16 a | 10.22 ± 0.01 c | 1.79 ± 0.07 b | |
| 3 BT | 11.82 ± 0.14 a | 20.28 ± 0.11 d | 16.57 ± 0.23 c | 8.84 ± 0.09 d | 56.62 ± 0.01 d | 2.72 ± 0.13 e | 11.60 ± 0.01 a | 1.87 ± 0.06 a |
| Samples | Bleaching Times | Water Absorption Capacity (%) | Oil Absorption Capacity (%) | Water Holding Capacity (%) | Oil Holding Capacity (%) | Water Retention Capacity (%) | Oil Retention Capacity (%) | Water Solubility (%) | Moisture Content (%) |
|---|---|---|---|---|---|---|---|---|---|
| Commercial CMC | - | 125.00 ± 0.03 a | 0.50 ± 0.05 e | 75.16 ± 0.04 a | 4.51 ± 0.02 e | 98.09 ± 0.07 a | 0.43 ± 0.01 e | 100.00 ± 0.02 a | 0.91± 0.02 g |
| CMCy | 1 BT | 41.94 ± 0.07 d | 1.21 ± 0.02 d | 12.09 ± 0.02 d | 9.07 ± 0.02 d | 48.19 ± 0.07 e | 1.11 ± 0.01 d | 80.11 ± 0.01 f | 1.78 ± 0.02 a |
| 2 BT | 41.64 ± 0.24 d | 1.27 ± 0.03 d | 13.05 ± 0.01 b | 9.12 ± 0.02 d | 50.19 ± 0.12 d | 1.16 ± 0.01 c | 82.22 ± 0.01 e | 1.73 ± 0.03 b | |
| 3 BT | 41.25 ± 0.02 d | 1.38 ± 0.01 c | 13.47 ± 0.40 b | 9.68 ± 0.07 b | 50.44 ± 0.05 d | 1.17 ± 0.02 c | 88.51 ± 0.34 c | 1.71 ± 0.05 c | |
| CMCm | 1 BT | 42.93 ± 0.05 b | 3.12 ± 0.02 b | 11.87 ± 0.03 e | 9.24 ± 0.12 c | 50.23 ± 0.10 d | 1.20 ± 0.01 b | 84.34 ± 0.01 d | 1.16 ± 0.02 d |
| 2 BT | 42.55 ± 0.11 c | 3.32 ± 0.01 b | 12.09 ± 0.01 d | 9.83 ± 0.02 b | 52.90 ± 0.01 c | 1.28 ± 0.02 b | 88.12 ± 0.01 c | 1.15 ± 0.07 e | |
| 3 BT | 42.10 ± 0.01 c | 3.49 ± 0.01 a | 12.55 ± 0.15 c | 9.90 ± 0.08 a | 53.08 ± 0.06 b | 1.31 ± 0.04 a | 89.72 ± 0.01 b | 1.14 ± 0.03 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klunklin, W.; Hinmo, S.; Thipchai, P.; Rachtanapun, P. Effect of Bleaching Processes on Physicochemical and Functional Properties of Cellulose and Carboxymethyl Cellulose from Young and Mature Coconut Coir. Polymers 2023, 15, 3376. https://doi.org/10.3390/polym15163376
Klunklin W, Hinmo S, Thipchai P, Rachtanapun P. Effect of Bleaching Processes on Physicochemical and Functional Properties of Cellulose and Carboxymethyl Cellulose from Young and Mature Coconut Coir. Polymers. 2023; 15(16):3376. https://doi.org/10.3390/polym15163376
Chicago/Turabian StyleKlunklin, Warinporn, Sasina Hinmo, Parichat Thipchai, and Pornchai Rachtanapun. 2023. "Effect of Bleaching Processes on Physicochemical and Functional Properties of Cellulose and Carboxymethyl Cellulose from Young and Mature Coconut Coir" Polymers 15, no. 16: 3376. https://doi.org/10.3390/polym15163376
APA StyleKlunklin, W., Hinmo, S., Thipchai, P., & Rachtanapun, P. (2023). Effect of Bleaching Processes on Physicochemical and Functional Properties of Cellulose and Carboxymethyl Cellulose from Young and Mature Coconut Coir. Polymers, 15(16), 3376. https://doi.org/10.3390/polym15163376








