Molecularly Imprinted Polymers for Pharmaceutical Impurities: Design and Synthesis Methods
Abstract
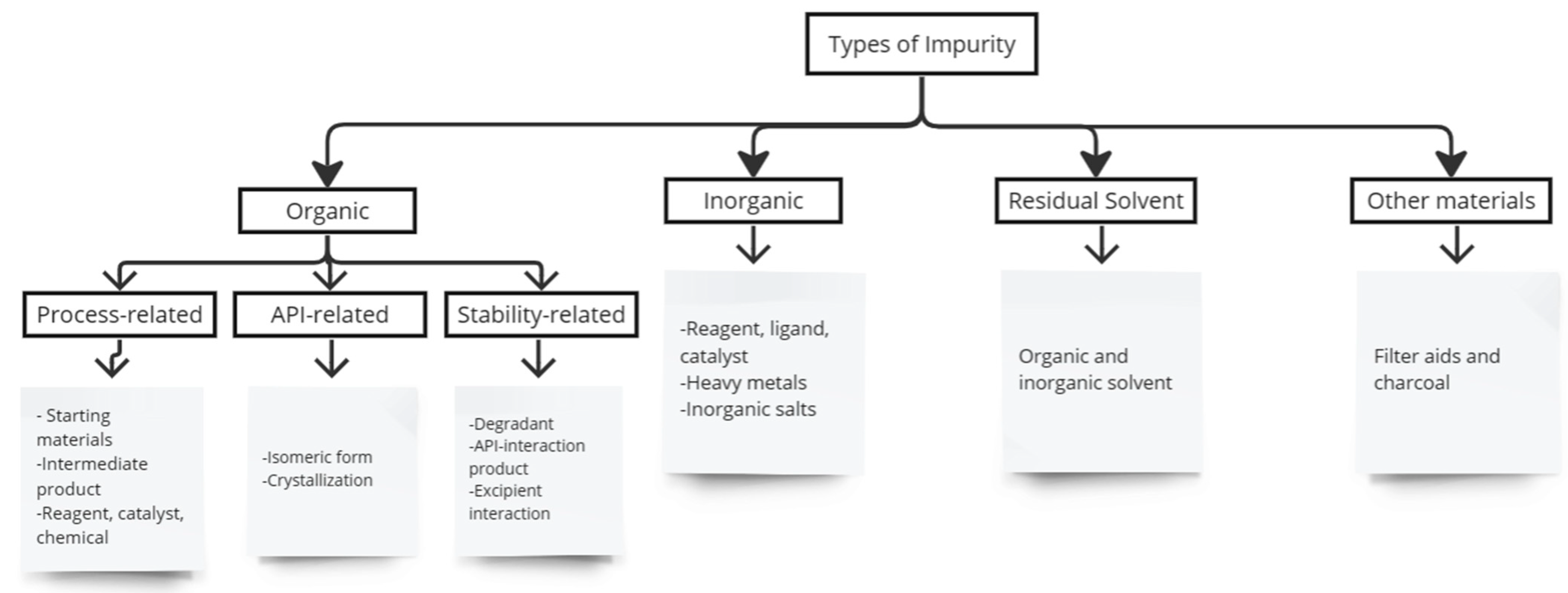
:1. Introduction
2. Design of MIPs for Pharmaceutical Impurities
3. Methods Used to Synthesize MIPs for Pharmaceutical Impurities
3.1. Bulk Polymerization
| Sample | Impurity | Type of Impurity | Template | Binding Capacity | Imprinting Factor | Ref. |
|---|---|---|---|---|---|---|
| Mometasone furoate (APIs) | 4-Dimethylamino pyridine | Organic (genotoxic impurity) from API post-reaction stream | 4-Dimethylamino pyridine | 5.03 mg/g | NM | [37] |
| Diclofenac sodium and torasemide | 2,6-Dichloroaniline | Organic (genotoxic impurities) from synthesis, storage, or transportation of APIs | Aniline (dummy template) | 4.08 mg/g | NM for 2,6-dichloroaniline or p-toluidine Aniline: 1.3 | [45] |
| p-Toluidine | ±6 mg/g | |||||
| Keppra (Kp), mometasone furoate (Meta), and roxithromycin (Roxi) as APIs | 1,3-Diisopropylurea | Organic (genotoxic impurity) from API post-reaction stream | 1,3-Diisopropylurea | NM, but 80% binding for MIP synthesized when base was added | NM | [39] |
| Diphenhydramine hydrochloride | Benzhydrol | Organic (genotoxic impurity) from intermediate of pharmaceuticals | Benzhydrol | 98.3 µmol/g | NM | [55] |
| Fluvoxamine maleate hydrochloride (APIs) | ((2RS)-2-[[2-[[[(1E)-5-methoxy- 1-[4(trifluoromethyl) phenyl] pentylidene]amino] oxy]ethyl]amino] butanedioic acid | Organic | ((2RS)-2-[[2-[[[(1E)-5-methoxy- 1-[4(trifluoromethyl) phenyl] pentylidene]amino] oxy]ethyl]amino] butanedioic acid | 100 µg/mg | NM | [34] |
3.2. Surface-Imprinting Polymerization
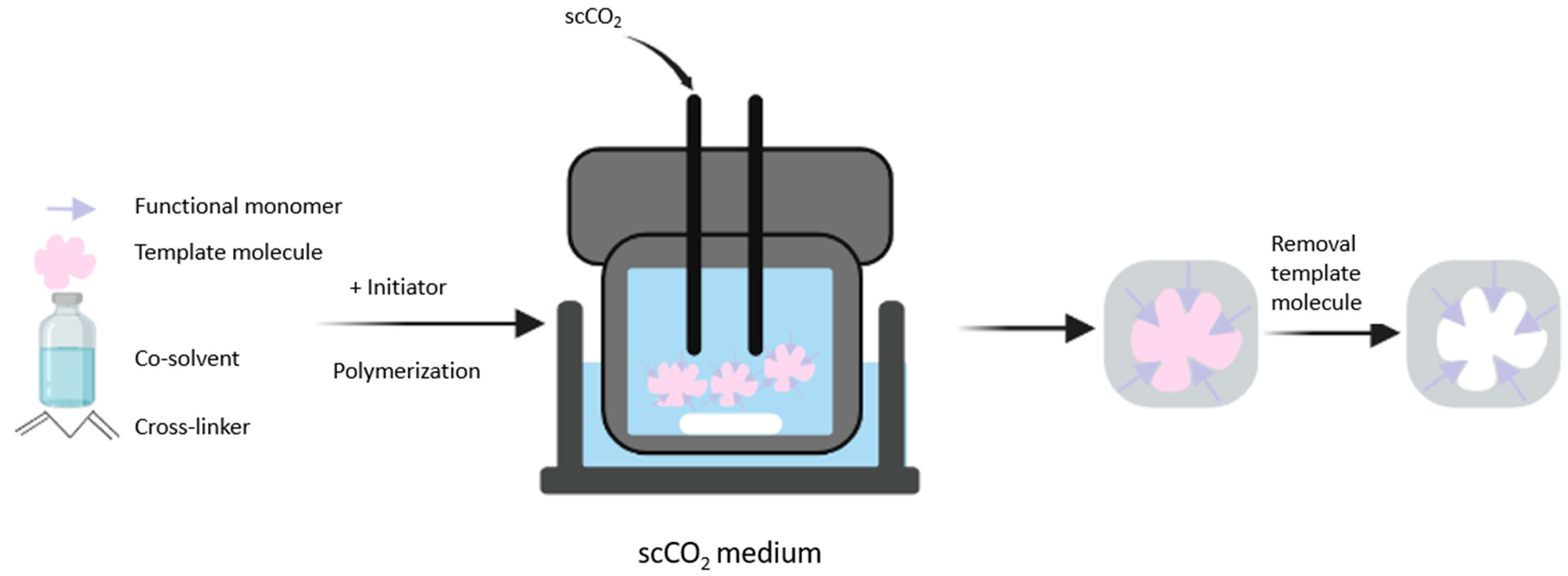
3.3. SCF Technology
4. Conclusions
- Develop MIPs for other types of impurities. Ionic MIPs can be developed to detect and separate heavy metals in pharmaceutical products.
- Compare the analytical performance of MIPs obtained using SCF technology with those obtained using other methods. In addition, compare the costs required for each technique to determine cost-effectiveness and analytical performance.
- Develop MT-MIPs to separate multiple impurities simultaneously and to reduce the time required for analysis.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kątny, M.; Frankowski, M. Impurities in Drug Products and Active Pharmaceutical Ingredients. Crit. Rev. Anal. Chem. 2017, 47, 187–193. [Google Scholar] [CrossRef]
- Patole, S.; Gosar, A.; Shaikh, T. Impurities Characterization in Pharmaceuticals: A Review. Ijppr. Hum. 2019, 14, 1170–1177. [Google Scholar]
- Maggio, R.M.; Calvo, N.L.; Vignaduzzo, S.E.; Kaufman, T.S. Pharmaceutical Impurities and Degradation Products: Uses and Applications of NMR Techniques. J. Pharm. Biomed. Anal. 2014, 101, 102–122. [Google Scholar] [CrossRef]
- ICH Q3B (R2) on Impurities: Impurities in New Drug Products; EMEA European Medicines Agency: Amsterdam, The Netherlands, 2006.
- Parmar, I.; Rathod, H.; Siddique, S. A Review: Recent Trends in Analytical Techniques for Characterization and Structure Elucidation of Impurities in the Drug Substances. Indian J. Pharm. Sci. 2021, 83, 402–415. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.; Afreen, S.; Pratap Singh, D. A Review on Pharmaceutical Impurities and Their Importance. World J. Pharm. Pharm. Sci. 2017, 6, 1337–1354. [Google Scholar]
- Mansouri, I.; Botton, J.; Semenzato, L.; Haddy, N.; Zureik, M. N-Nitrosodimethylamine-Contaminated Valsartan and Risk of Cancer: A Nationwide Study of 1.4 Million Valsartan Users. J. Am. Heart Assoc. 2022, 11, e8067. [Google Scholar] [CrossRef]
- WHO. Investigation of Acute Kidney Injury in Children in Indonesia: Results and Regulatory Actions. Available online: https://www.who.int/indonesia/news/detail/01-03-2023-investigation-of-acute-kidney-injury-in-children-in-indonesia--results-and-regulatory-actions (accessed on 5 June 2023).
- Ghanem, M.P.H. Detection of Diethylene Glycol in Glycerin and Propylene Glycol by Using High Performance Thin Layer Chromatography HPTLC. IOSR J. Pharm. 2011, 1, 29–34. [Google Scholar] [CrossRef]
- Baranwal, M.; Kaur, A.; Kumar, R. Challenges in Utilizing Diethylene Glycol and Ethylene Glycol as Excipient: A Thorough Overview. Pharmaspire 2023, 15, 8–15. [Google Scholar] [CrossRef]
- Wood, C. In-Process Control Testing, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Kalauz, A.; Kapui, I. Determination of Potentially Genotoxic Impurities in Crotamiton Active Pharmaceutical Ingredient by Gas Chromatography. J. Pharm. Biomed. Anal. 2022, 210, 114544. [Google Scholar] [CrossRef]
- Jireš, J.; Gibala, P.; Kalášek, S.; Douša, M.; Doubský, J. The Determination of Two Analogues of 4-(Azidomethyl)-1,1′-Biphenyl as Potential Genotoxic Impurities in the Active Pharmaceutical Ingredient of Several Sartans Containing a Tetrazole Group. J. Pharm. Biomed. Anal. 2021, 205, 114300. [Google Scholar] [CrossRef]
- Generalova, Y.; Sipkina, N.; Alekseeva, G. Determination of Related Impurities in a New Active Pharmaceutical Ingredient—Sodium 4,4′-(Propanediamido)Dibenzoate. Microchem. J. 2021, 168, 106498. [Google Scholar] [CrossRef]
- Vogel, M.; Norwig, J. Analysis of Genotoxic N-Nitrosamines in Active Pharmaceutical Ingredients and Market Authorized Products in Low Abundance by Means of Liquid Chromatography—Tandem Mass Spectrometry. SSRN Electron. J. 2022, 219, 114910. [Google Scholar] [CrossRef]
- Wohlfart, J.; Scherf-Clavel, O.; Kinzig, M.; Sörgel, F.; Holzgrabe, U. The Nitrosamine Contamination of Drugs, Part 3: Quantification of 4-Methyl-1-Nitrosopiperazine in Rifampicin Capsules by LC-MS/HRMS. J. Pharm. Biomed. Anal. 2021, 203, 114205. [Google Scholar] [CrossRef]
- Matmour, D.; Bouaffad, A.; Merad, Y.; Ziani, N.H. From the Limit Test for Trace Elements Control to the Elemental Impurities Analysis by Inductively Coupled Plasma Optical Emission Spectrometry: Application on Six Samples of Metronidazole API. J. Trace Elem. Miner. 2022, 2, 100017. [Google Scholar] [CrossRef]
- Lim, H.H.; Oh, Y.S.; Shin, H.S. Determination of N-Nitrosodimethylamine and N-Nitrosomethylethylamine in Drug Substances and Products of Sartans, Metformin and Ranitidine by Precipitation and Solid Phase Extraction and Gas Chromatography—Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2020, 189, 113460. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.; Yang, X.; Jin, Y.; Liu, X.; Wang, C.; Zhang, Z. Determination of N-Nitrosodimethylamine in Ranitidine Dosage Forms by ESI-LC-MS/MS.; Applications for Routine Laboratory Testing. Iran. J. Pharm. Res. 2021, 20, 255. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, S.; Nasir, N.M.; Faudzi, S.M.M.; Yahaya, N.; Hanapi, N.S.M.; Ibrahim, W.N.W. Solid-Phase Extraction of Active Compounds from Natural Products by Molecularly Imprinted Polymers: Synthesis and Extraction Parameters. Polymers 2021, 13, 3780. [Google Scholar] [CrossRef] [PubMed]
- Lucci, P.; David, S.; Conchione, C.; Milani, A.; Moret, S.; Pacetti, D.; Conte, L. Molecularly Imprinted Polymer as Selective Sorbent for the Extraction of Zearalenone in Edible Vegetable Oils. Foods 2020, 9, 1439. [Google Scholar] [CrossRef]
- Fu, Y.; Pessagno, F.; Manesiotis, P.; Borrull, F.; Fontanals, N.; Maria Marcé, R. Preparation and Evaluation of Molecularly Imprinted Polymers as Selective SPE Sorbents for the Determination of Cathinones in River Water. Microchem. J. 2022, 175, 107100. [Google Scholar] [CrossRef]
- Dil, E.A.; Doustimotlagh, A.H.; Javadian, H.; Asfaram, A.; Ghaedi, M. Nano-Sized Fe3O4@SiO2-Molecular Imprinted Polymer as a Sorbent for Dispersive Solid-Phase Microextraction of Melatonin in the Methanolic Extract of Portulaca Oleracea, Biological, and Water Samples. Talanta 2021, 221, 121620. [Google Scholar] [CrossRef]
- Bashir, K.; Luo, Z.; Chen, G.; Shu, H.; Cui, X.; Li, W.; Lu, W.; Fu, Q. Development of Surface Molecularly Imprinted Polymers as Dispersive Solid Phase Extraction Coupled with HPLC Method for the Removal and Detection of Griseofulvin in Surface Water. Int. J. Environ. Res. Public Health 2020, 17, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, A.T.M.; de Oliveira, H.L.; Silva, C.F.; Fonseca, M.C.; Pereira, T.F.D.; Nascimento, C.S.; de Figueiredo, E.C.; Borges, K.B. Efficient Molecularly Imprinted Polymer as a Pipette-Tip Solid-Phase Sorbent for Determination of Carvedilol Enantiomers in Human Urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Ghaedi, M. Synthesis of Chitosan Based Molecularly Imprinted Polymer for Pipette-Tip Solid Phase Extraction of Rhodamine B from Chili Powder Samples. Int. J. Biol. Macromol. 2019, 139, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Hasanah, A.N.; Maelaningsih, F.S.; Apriliandi, F.; Sabarudin, A. Synthesis and Characterisation of a Monolithic Imprinted Column Using a Methacrylic Acid Monomer with Porogen Propanol for Atenolol Analysis. J. Anal. Methods Chem. 2020, 2020, 3027618. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. Molecular Imprinting Techniques Used for the Preparation of Biosensors. Sensors 2017, 17, 288. [Google Scholar] [CrossRef] [Green Version]
- Hasanah, A.N.; Susanti, I.; Mutakin, M. An Update on the Use of Molecularly Imprinted Polymers in Beta-Blocker Drug Analysis as a Selective Separation Method in Biological and Environmental Analysis. Molecules 2022, 27, 2880. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Kılıç, S.; Esen, C.; Denizli, A. Molecularly Imprinted Polymer-Based Sensors for Protein Detection. Polymers 2023, 15, 629. [Google Scholar] [CrossRef]
- Bakhtiar, S.; Bhawani, S.A.; Shafqat, S.R. Synthesis and Characterization of Molecular Imprinting Polymer for the Removal of 2-Phenylphenol from Spiked Blood Serum and River Water. Chem. Biol. Technol. Agric. 2019, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Sajini, T.; Mathew, B. A Brief Overview of Molecularly Imprinted Polymers: Highlighting Computational Design, Nano and Photo-Responsive Imprinting. Talanta Open 2021, 4, 100072. [Google Scholar] [CrossRef]
- Susanti, I.; Safitri, N.; Pratiwi, R.; Hasanah, A.N. Synthesis of Molecular Imprinted Polymer Salbutamol using Methacrylic Acid Monomer and Trimethyl Propane Trimethacrylate (TRIM) as a Cross-Linker through Suspension Polymerization. Int. J. Appl. Pharm. 2022, 14, 32–39. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Shakeri, M. Removal of Potentioally Genotoxic Impurity from Fluroxamine Maleate Crude Drug by Molecularly Imprinted Polymer. Korean J. Chem. Eng. 2014, 31, 1898–1902. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Abbasi, F. Synthesis of Molecularly Imprinted Polymers Coated on Silica Nanoparticles for Removal of P-Nitrophenol from Crude Pharmaceuticals. Pharm. Chem. J. 2015, 49, 280–286. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Shabestani-Trojeni, M. Application of a Magnetic Molecularly Imprinted Polymer for the Removal of Sulfanilamide as Major Impurity in Eye Drops (Sulfacetamide). Pharm. Chem. J. 2020, 54, 977–983. [Google Scholar] [CrossRef]
- Esteves, T.; Viveiros, R.; Bandarra, J.; Heggie, W.; Casimiro, T.; Ferreira, F.C. Molecularly Imprinted Polymer Strategies for Removal of a Genotoxic Impurity, 4-Dimethylaminopyridine, from an Active Pharmaceutical Ingredient Post-Reaction Stream. Sep. Purif. Technol. 2016, 163, 206–214. [Google Scholar] [CrossRef]
- Luo, Z.; Zeng, A.; Zheng, P.; Guo, P.; Du, W.; Du, K.; Fu, Q. Preparation of Surface Molecularly Imprinted Polymers as the Solid-Phase Extraction Sorbents for the Specific Recognition of Penicilloic Acid in Penicillin. Anal. Meth. 2014, 6, 7865–7874. [Google Scholar] [CrossRef]
- Székely, G.; Bandarra, J.; Heggie, W.; Ferreira, F.C.; Sellergren, B. Design, Preparation and Characterization of Novel Molecularly Imprinted Polymers for Removal of Potentially Genotoxic 1,3-Diisopropylurea from API Solutions. Sep. Purif. Technol. 2012, 86, 190–198. [Google Scholar] [CrossRef]
- Balamurugan, K.; Prakasam, T.; Srinivasan, K.R. Determination of Enantiomeric Impurity of D-Pseudoephedrine Determination of Enantiomeric Impurity of D- Pseudoephedrine Using Mip Column. Indian J. Pharm. 2015, 4, 13–19. [Google Scholar]
- Abdel Ghani, N.T.; Mohamed El Nashar, R.; Abdel-Haleem, F.M.; Madbouly, A. Computational Design, Synthesis and Application of a New Selective Molecularly Imprinted Polymer for Electrochemical Detection. Electroanalysis 2016, 28, 1530–1538. [Google Scholar] [CrossRef]
- Viveiros, R.; Karim, K.; Piletsky, S.A.; Heggie, W.; Casimiro, T. Development of a Molecularly Imprinted Polymer for a Pharmaceutical Impurity in Supercritical CO2: Rational Design Using Computational Approach. J. Clean. Prod. 2017, 168, 1025–1031. [Google Scholar] [CrossRef]
- Guerreiro, A.; Soares, A.; Piletska, E.; Mattiasson, B.; Piletsky, S. Preliminary Evaluation of New Polymer Matrix for Solid-Phase Extraction of Nonylphenol from Water Samples. Anal. Chim. Acta 2008, 612, 99–104. [Google Scholar] [CrossRef]
- Viveiros, R.; Lopes, M.I.; Heggie, W.; Casimiro, T. Green Approach on the Development of Lock-and-Key Polymers for API Purification. Chem. Eng. J. 2017, 308, 229–239. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Xia, Z.; Huang, Y. Preparation of Dummy Molecularly Imprinted Polymers for Selective Extraction of Aromatic Amine Genotoxic Impurities. J. Chromatogr. A 2022, 1685, 463617. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, W.; Tan, L.; Wang, J.; Li, H.; Wang, J. Separation and Detection of Trace Atrazine from Seawater Using Dummy-Template Molecularly Imprinted Solid-Phase Extraction Followed by High-Performance Liquid Chromatography. Mar. Pollut. Bull. 2019, 149, 110502. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liu, J.; Tang, S.; Wang, Y.; Wang, Y.; Jin, R. Optimization of Enrofloxacin-Imprinted Polymers by Computer-Aided Design. J. Mol. Model. 2015, 21, 290. [Google Scholar] [CrossRef]
- Sobiech, M.; Giebułtowicz, J.; Woźnica, M.; Jaworski, I.; Luliński, P. Theoretical and Experimental Model of Molecularly Imprinted Polymer Surface Microenvironment for Selective Stationary Phase—Exemplary of S-Pramipexole for Potential Pharmaceutical Analysis. Microchem. J. 2022, 182, 107875. [Google Scholar] [CrossRef]
- Woźnica, M.; Luliński, P. Design of Selective Molecularly Imprinted Sorbent for the Optimized Solid-Phase Extraction of S-Pramipexole from the Model Multicomponent Sample of Human Urine. J. Sep. Sci. 2019, 42, 1412–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dow, L.K.; Hansen, M.M.; Pack, B.W.; Page, T.J.; Baertschi, S.W. The Assessment of Impurities for Genotoxic Potential and Subsequent Control in Drug Substance and Drug Product. J. Pharm. Sci. 2013, 102, 1404–1418. [Google Scholar] [CrossRef]
- Székely, G.; Fritz, E.; Bandarra, J.; Heggie, W.; Sellergren, B. Removal of Potentially Genotoxic Acetamide and Arylsulfonate Impurities from Crude Drugs by Molecular Imprinting. J. Chromatogr. A 2012, 1240, 52–58. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Huang, C.; Jiao, Y.; Chen, J. A Phenolphthalein-Dummy Template Molecularly Imprinted Polymer for Highly Selective Extraction and Clean-up of Bisphenol A in Complex Biological, Environmental and Food Samples. Polymers 2018, 10, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Wang, J.; Li, Y.; Jin, J.; Zhang, B.; Shah, S.M.; Wang, X.; Chen, J. Highly Selective Dummy Molecularly Imprinted Polymer as a Solid-Phase Extraction Sorbent for Five Bisphenols in Tap and River Water. J. Chromatogr. A 2014, 1343, 33–41. [Google Scholar] [CrossRef]
- Sambe, H.; Hoshina, K.; Hosoya, K.; Haginaka, J. Direct Injection Analysis of Bisphenol A in Serum by Combination of Isotope Imprinting with Liquid Chromatography-Mass Spectrometry. Analyst 2005, 130, 38–40. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Alaeian, M.R. Synthesis of Molecularly Imprinted Polymer for Removal of Effective Impurity (Benzhydrol) from Diphenhydramine Hydrochloride Drug. J. Chin. Chem. Soc. 2014, 61, 643–648. [Google Scholar] [CrossRef]
- Susanti, I.; Hasanah, A.N. How to Develop Molecularly Imprinted Mesoporous Silica for Selective Recognition of Analytes in Pharmaceutical, Environmental, and Food Samples. Polym. Adv. Technol. 2021, 32, 1965–1980. [Google Scholar] [CrossRef]
- He, J.X.; Pan, H.Y.; Xu, L.; Tang, R.Y. Application of Molecularly Imprinted Polymers for the Separation and Detection of Aflatoxin. J. Chem. Res. 2020, 45, 400–410. [Google Scholar] [CrossRef]
- Dong, C.; Shi, H.; Han, Y.; Yang, Y.; Wang, R.; Men, J. Molecularly Imprinted Polymers by the Surface Imprinting Technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Eichenbaum, G.; Johnson, M.; Kirkland, D.; O’Neill, P.; Stellar, S.; Bielawne, J.; DeWire, R.; Areia, D.; Bryant, S.; Weiner, S.; et al. Assessment of the Genotoxic and Carcinogenic Risks of P-Nitrophenol When It Is Present as an Impurity in a Drug Product. Regul. Toxicol. Pharmacol. 2009, 55, 33–42. [Google Scholar] [CrossRef]
- Susanti, I.; Holik, H.A. Review: Application of Magnetic Solid-Phase Extraction (Mspe) in Various Types of Samples. Int. J. Appl. Pharm. 2021, 13, 59–68. [Google Scholar] [CrossRef]
- Wojtowicz, E.J. A Column Chromatographic Method for the Determination of Sulfanilamide in Pharmaceutical Preparations Containing Sulfacetamide or Its Sodium Salt. J. Pharm. Sci. 1970, 59, 240–241. [Google Scholar] [CrossRef]
- Ahmed, S.; Anwar, N.; Sheraz, M.A.; Ahmad, I. Validation of a Stability-Indicating Spectrometric Method for the Determination of Sulfacetamide Sodium in Pure form and Ophthalmic Preparations. J. Pharm. Bioallied Sci. 2017, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Peng, K.; Su, Y.; Song, X.; Qiu, J.; Xiong, R.; He, L. Preparation of Surface Molecularly Imprinted Polymer and Its Application for the Selective Extraction of Teicoplanin from Water. RSC Adv. 2021, 11, 13615–13623. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Casimiro, T.; Aguiar-Ricardo, A.; Simplício, A.L.; Duarte, C.M.M. Supercritical Fluid Polymerisation and Impregnation of Molecularly Imprinted Polymers for Drug Delivery. J. Supercrit. Fluids 2006, 39, 102–106. [Google Scholar] [CrossRef]
- Furtado, A.I.; Viveiros, R.; Casimiro, T. MIP Synthesis and Processing Using Supercritical Fluids. In Molecularly Imprinted Polymers: Methods in Molecular Biology; Martín-Esteban, A., Ed.; Humana Press: Totowa, NJ, USA, 2021; p. 20. [Google Scholar]
- Boyère, C.; Jérôme, C.; Debuigne, A. Input of Supercritical Carbon Dioxide to Polymer Synthesis: An Overview. Eur. Polym. J. 2014, 61, 45–63. [Google Scholar] [CrossRef]
- Viveiros, R.; Bonifácio, V.D.B.; Heggie, W.; Casimiro, T. Green Development of Polymeric Dummy Artificial Receptors with Affinity for Amide-Based Pharmaceutical Impurities. ACS Sustain. Chem. Eng. 2019, 7, 15445–15451. [Google Scholar] [CrossRef]
- Sun, C.; Wang, J.; Huang, J.; Yao, D.; Wang, C.Z.; Zhang, L.; Hou, S.; Chen, L.; Yuan, C.S. The Multi-Template Molecularly Imprinted Polymer Based on SBA-15 for Selective Separation and Determination of Panax Notoginseng Saponins Simultaneously in Biological Samples. Polymers 2017, 9, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Zeng, G.; Ma, X. Multi-Templates Surface Molecularly Imprinted Polymer for Rapid Separation and Analysis of Quinolones in Water. Environ. Sci. Pollut. Res. 2020, 27, 7177–7187. [Google Scholar] [CrossRef]


| Sample | Impurity | Preparation Method | Instrument | Accuracy | Precision | Ref |
|---|---|---|---|---|---|---|
| API Crotamiton | Toluidine | Dissolved in methanol | Gas chromatography with flame ionization detector (GC-FID) | 79.1–107.4% | 2.1–4.3% | [12] |
| APIs of various sartans containing a tetrazole group | 4′-(Azidomethyl)-[1,1′-biphenyl]-2-carbonitrile (GTI-azide-1) and 5-(4′-(azidomethyl)-[1,1′-biphenyl]- 2-yl)-1H-tetrazole (GTI-azide-2) | Dissolved and sonicated | High-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) | GTI-azide-1: 100.9% GTI-azide-2: 100.4% | GTI-azide-1: 0.25% GTI-azide-2: 1.39% | [13] |
| 4,4′-(propanediamido)dibenzoate (malaben) | Impurities A (4-aminobenzoic acid), B (unidentified), C (Etmaben), and D (unidentified) | Dissolved in water | Capillary electrophoresis | NM | NM | [14] |
| APIs and market-authorized tablets | N-nitrosamines | Extraction, removal using cation exchange resin, enrichment using charcoal, and evaporation | Liquid chromatography–tandem mass spectrometry (LC-MS/MS) | 83.8–113.3% | 0.9–14.9% | [15] |
| Rifampicin capsule | 4-Methyl-1-nitrosopiperazine | Dissolved and vortexed | LC-MS/HRMS | NM | NM | [16] |
| Metronidazole APIs | Cd, Pb, As, Hg, Co, Ni, Ag, Cu, Sn, and Cr | Heated at 250 °C | Inductively coupled plasma optical emission spectrometry | NM | <2% | [17] |
| Drug substances of sartans, metformin, ranitidine, and their finished products | N-Nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) | Precipitation using the solubility difference method for irbesartan, pimasartan, olmesartan, and candesartan samples Solid-phase extraction with activated charcoal for valsartan, rosartan, metformin, and ranitidine samples | Gas chromatography–tandem mass spectrometry | NMDA: 95.0–105% NDEA: 93.6–104% | NMDA: 0.4–2.7% NDEA: 0.4–4.2% | [18] |
| Ranitidine dosage forms | NDMA | Ultrasonic extraction | Electrospray ionization–liquid chromatography–tandem mass spectrometry (ESI-LC-MS/MS) | 94.7–102.0% | 4.9% | [19] |
| Design | Template (T) | Monomer (M) | Ratio of T:M | Static Binding Capacity (mmol/g) | Ref. |
|---|---|---|---|---|---|
| With computer simulations | Acetamide | Itaconic acid | 1:3 | ±2.5 | [42] |
| 2-Hydroxyethyl methacrylate | 1:2 | ±1.1 | |||
| Without computer simulations | Methacrylic acid | 1:4 | ±1.7 | [44] | |
| Methacrylamide | 1:4 | ±2.3 |
| Sample | Impurity | Type of Impurity | Solid Matrix | Template | Monomer | Porogen | Binding Capacity | Selectivity Factor | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Paracetamol | p-Nitrophenol (4-NP) | Organic from an intermediate of pharmaceuticals | Silica nanoparticle | p-Nitrophenol | Methacrylic acid | Toluene–acetonitrile (4:1, v/v) | 600 mol/g | 18.48 | [35] |
| Sulphacetamide eye drops | Sulphanilamide | Organic from a degradation product | Fe3O4@SiO2 @MPTS | Sulphanilamide | Methacrylic acid | acetonitrile/toluene (60:40, v/v) | 114.2 µmol/g | NM | [36] |
| Penicillin | Penicilloic acid | Organic (genotoxic impurities) from a degradation product | SiO2 modified by 3-aminopropyl triethoxysilane (APTES) | Penicilloic acid | Methacrylic acid | Acetonitrile/ methanol (1:1) | 22.67 mg/g | NM, but the IF of penicilloic acid was higher than other compounds | [38] |
| Special Strategies | Impurity | Type of Impurity | Template | Monomer | Solvent | Static Binding Capacity | Imprinting Factor | Selectivity Factor | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| - | Acetamide | Organic from the last stages of API manufacturing | Acetamide | Methacrylamide | Supercritical CO2 and acetonitrile (co-solvent) | ±2.3 mmol/g (at 250 ppm) | 1.31 (at 250 ppm) | NM, but the MIP had higher affinity for acetamide than either benzamide or pivalamide | [44] |
| - | Acetamide | Organic from the last stages of API manufacturing | Acetamide | Itaconic acid | Supercritical CO2 | 2.5 mmol/g | NM | NM, but the MIP had higher affinity for acetamide than either benzamide or pivalamide | [42] |
| Dummy template | Acetamide | Organic from the last stages of API manufacturing | Benzamide | Methacrylic acid | Supercritical CO2 | 1.26 mmol/g for acetamide | 2.04 | NM | [67] |
| Acetamide | Organic from the last stages of API manufacturing | Pivalamide | Methacrylic acid | Supercritical CO2 | 1.33 mmol/g for acetamide | 0.88 |
| Polymerization Method | Advantages | Disadvantages |
|---|---|---|
| Bulk polymerization | Easy procedure Requires a small amount of porogen | Grinding involved in this method can damage the recognition site of MIPs MIPs are irregularly shaped |
| Surface-imprinting polymerization | Can increase the binding capacity Improves the mass transfer kinetics Faster adsorption equilibrium | Quite complicated because it involves many steps |
| Supercritical fluid technology | Uses a green and highly pure solvent The MIPs are obtained as dry free-flowing powder The MIPs are ready to use | Requires special equipment for polymerization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanah, A.N.; Susanti, I. Molecularly Imprinted Polymers for Pharmaceutical Impurities: Design and Synthesis Methods. Polymers 2023, 15, 3401. https://doi.org/10.3390/polym15163401
Hasanah AN, Susanti I. Molecularly Imprinted Polymers for Pharmaceutical Impurities: Design and Synthesis Methods. Polymers. 2023; 15(16):3401. https://doi.org/10.3390/polym15163401
Chicago/Turabian StyleHasanah, Aliya Nur, and Ike Susanti. 2023. "Molecularly Imprinted Polymers for Pharmaceutical Impurities: Design and Synthesis Methods" Polymers 15, no. 16: 3401. https://doi.org/10.3390/polym15163401






