Polyisobutylenes with Controlled Molecular Weight and Chain-End Structure: Synthesis and Actual Applications
Abstract
:1. Introduction
2. Analysis of PIBs and Mechanism of IB Polymerization
2.1. Analysis of PIBs
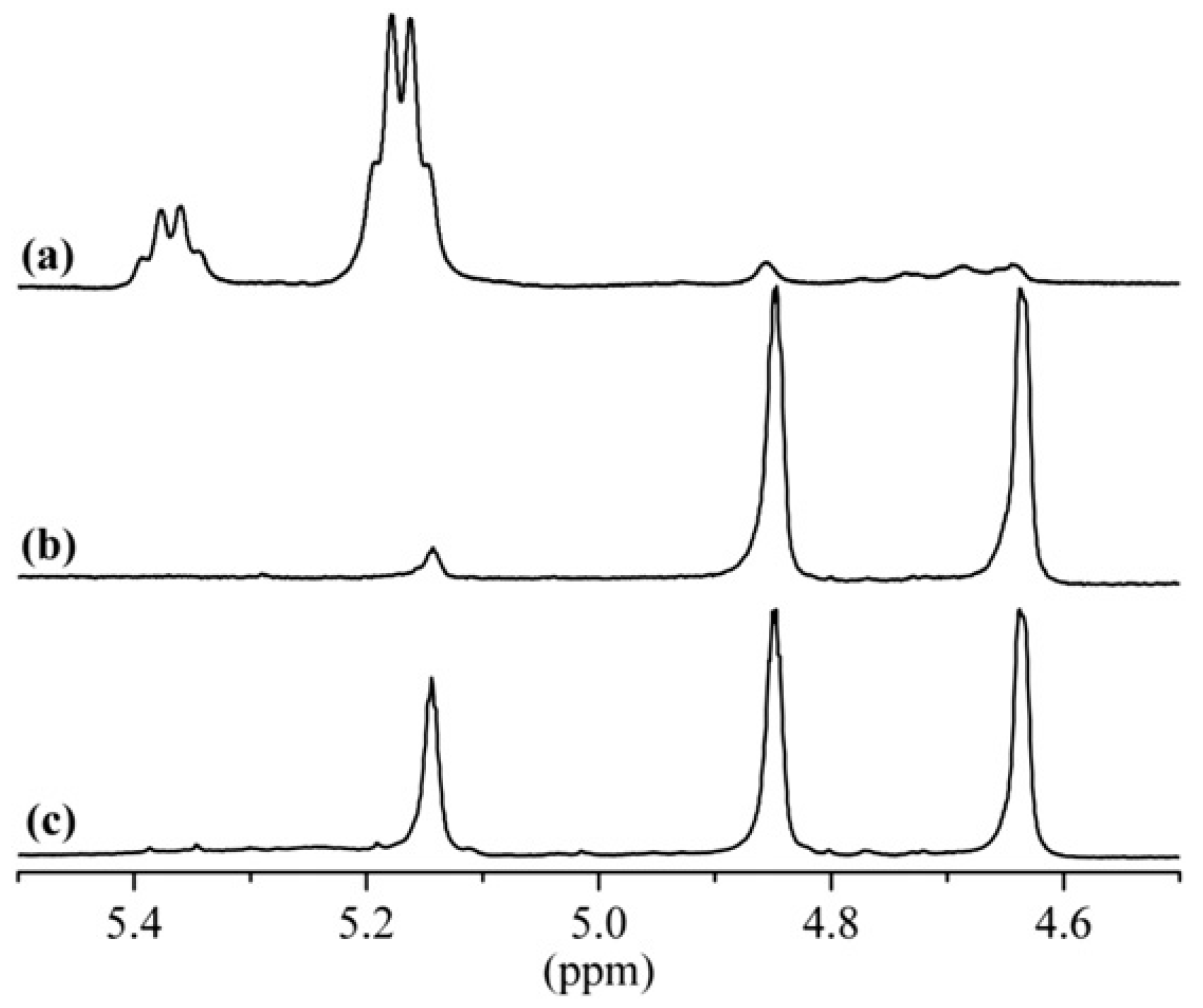
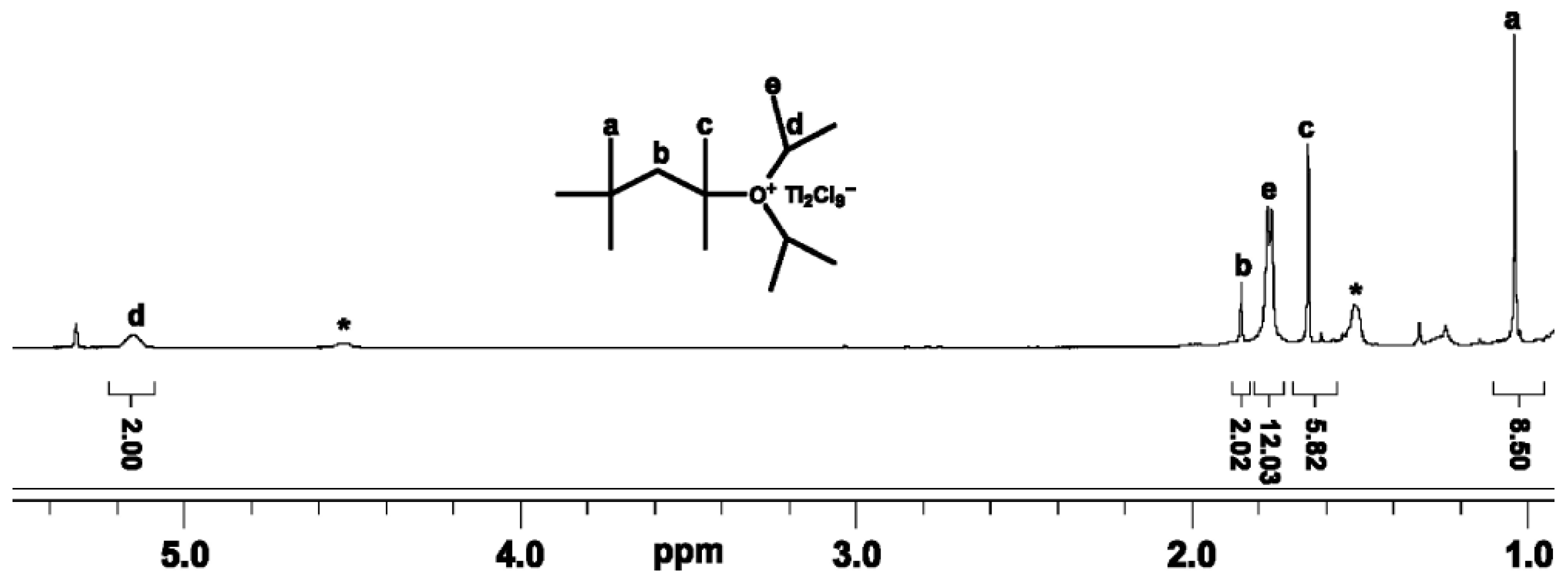
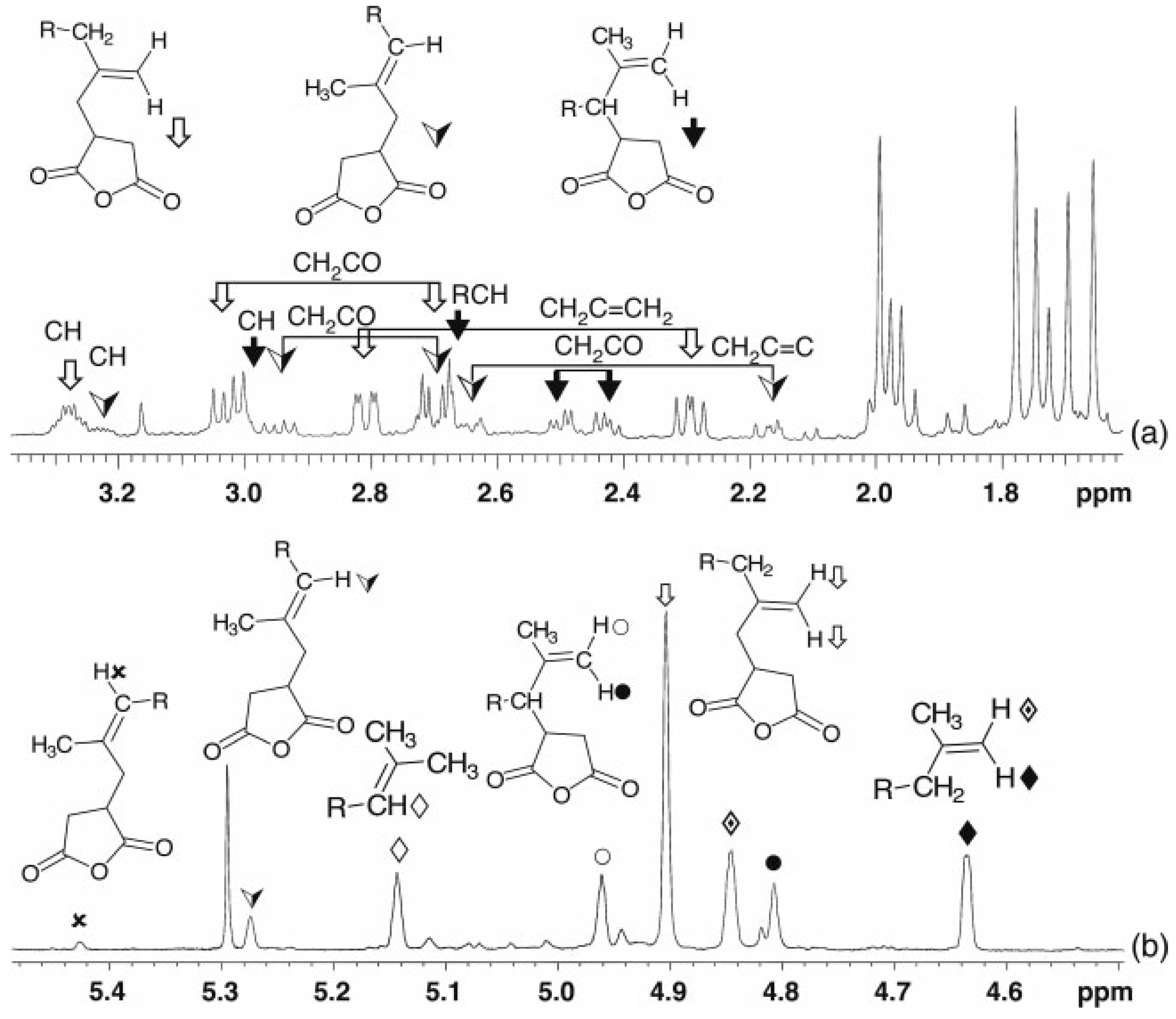
2.1.1. NMR Spectroscopy
2.1.2. Mass Spectrometry
2.1.3. Size Exclusion Chromatography
2.2. Mechanism of Isobutylene Polymerization
3. Industrial Production of PIBs and Actual Studies of Conventional Catalysts
3.1. Industrial Production of PIBs
3.2. Further Development of BF3/ROH Catalysts
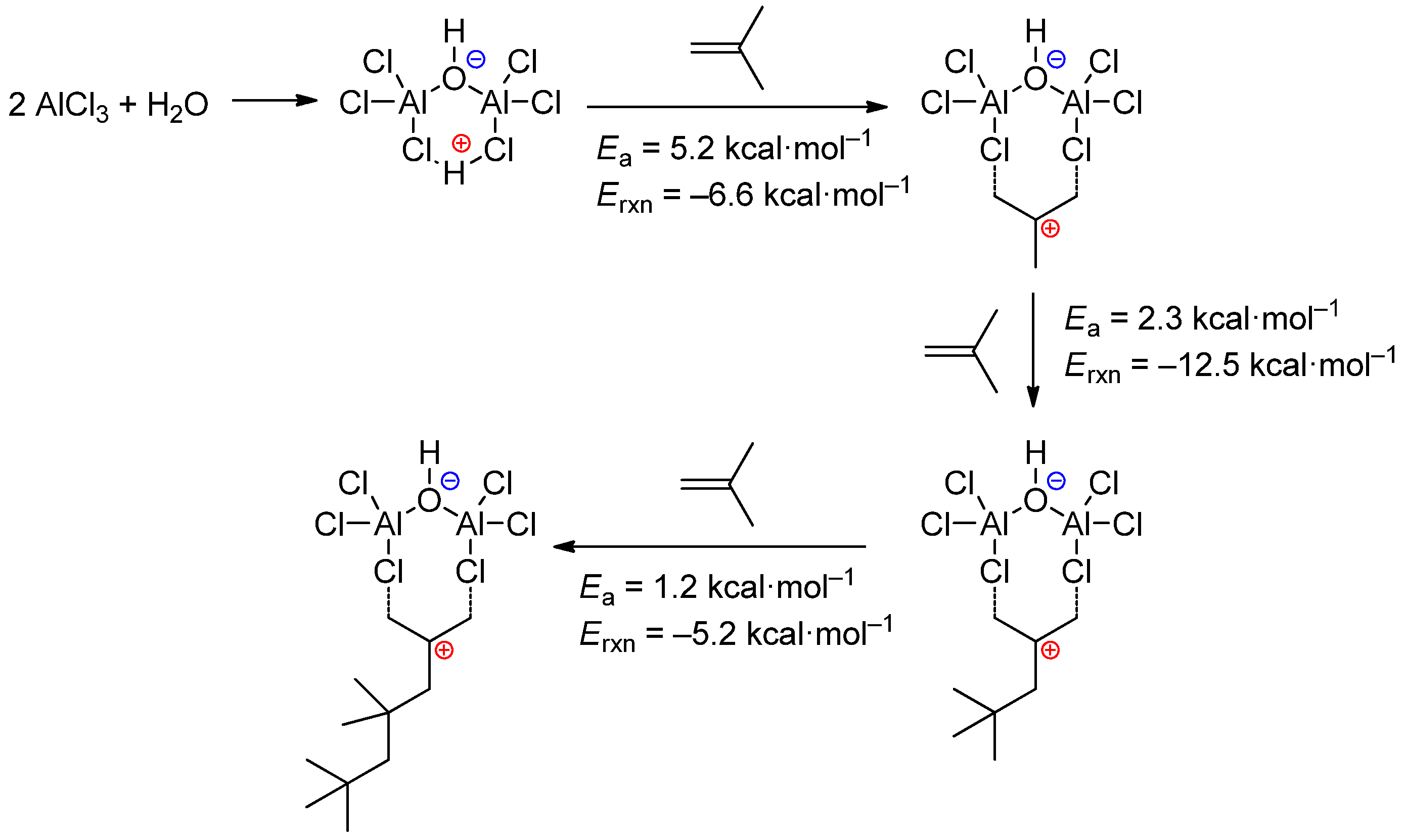
3.3. AlCl3/H2O and Related Systems
3.4. Synthesis of PIB-Cl
4. Three Generations of Modern Catalysts
4.1. First-Generation Catalysts: Metal Chlorides/Ethers
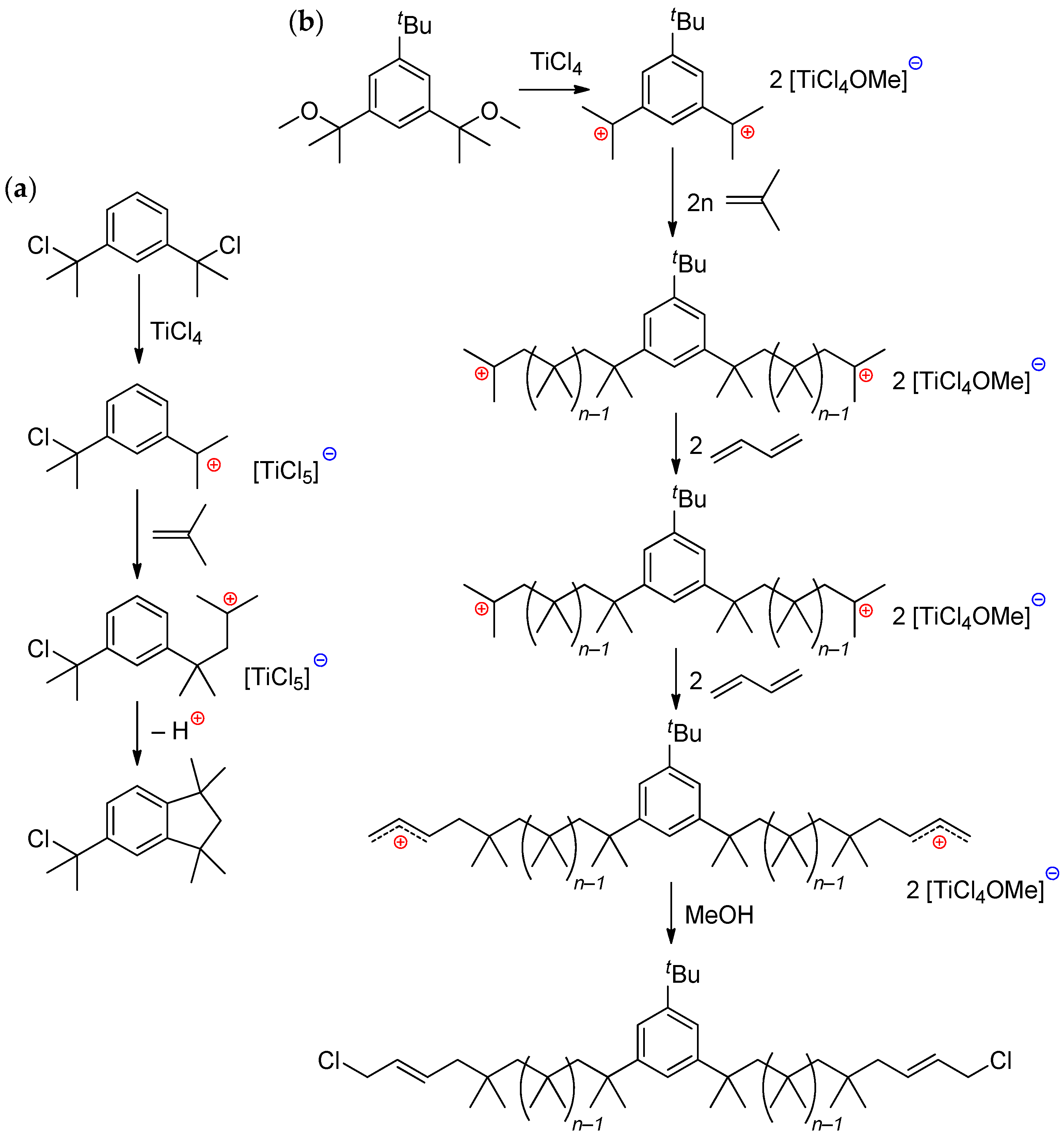
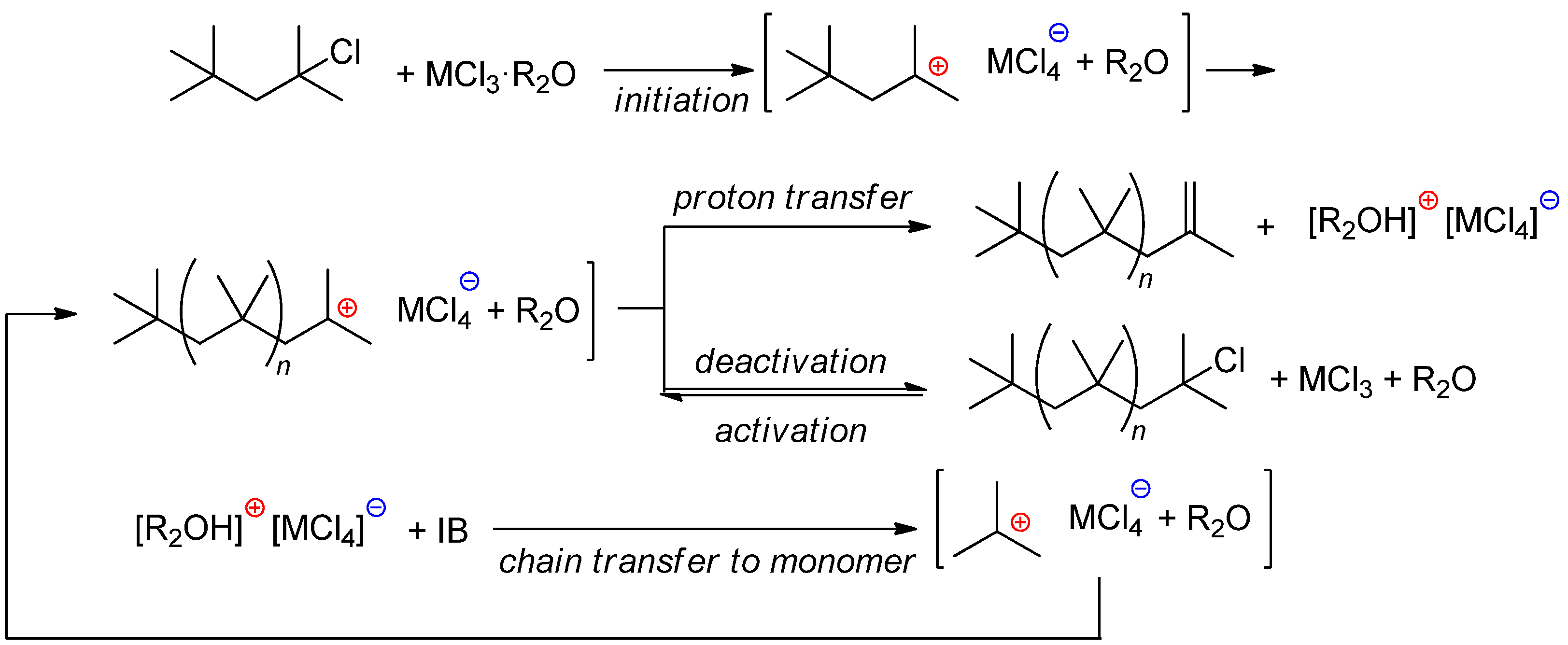
4.1.1. AlCl3/R2O and Related Systems
4.1.2. Other Metal Chlorides/R2O and Related Systems
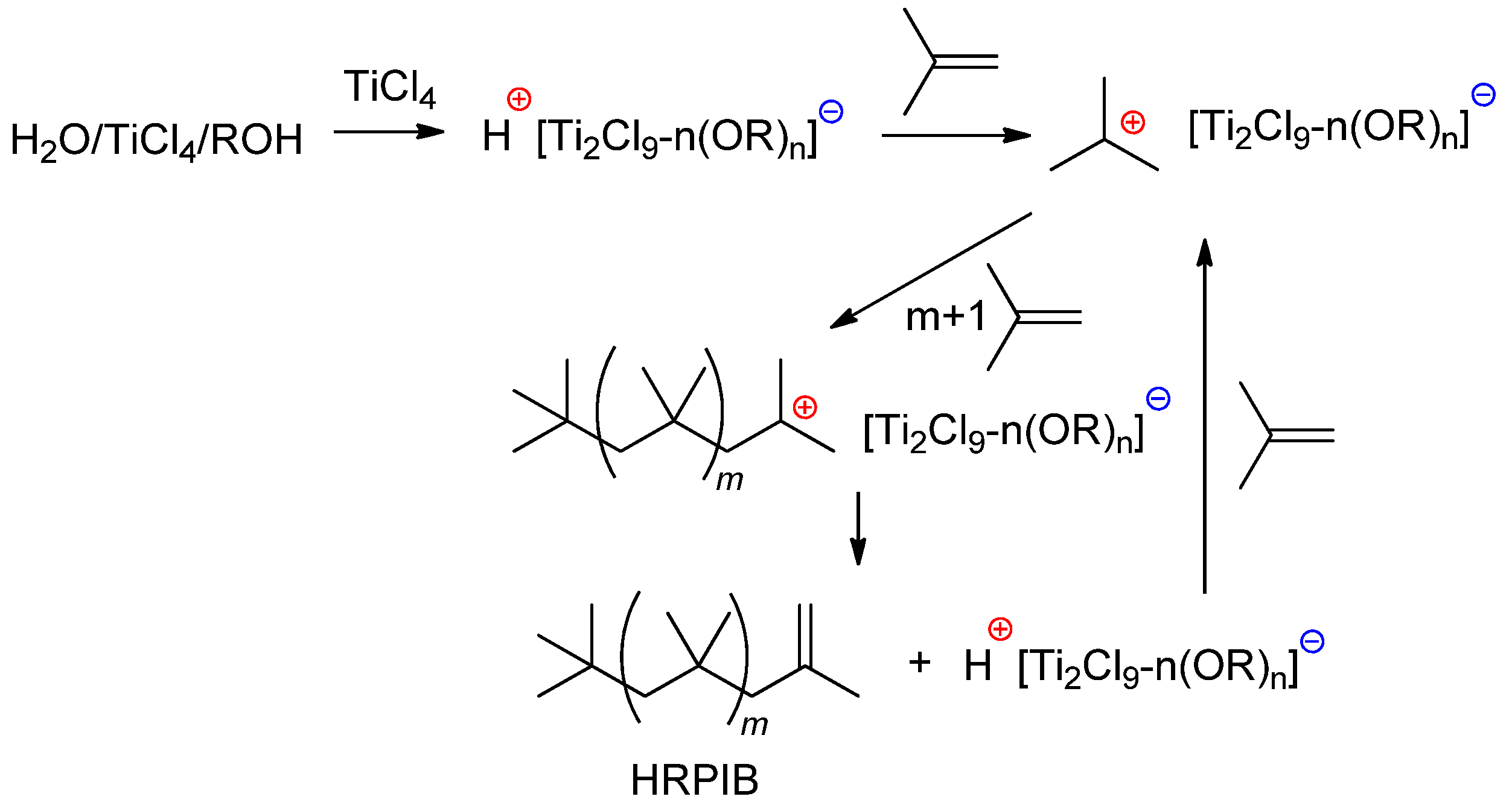
4.1.3. Metal Chlorides/ROH and Related Systems
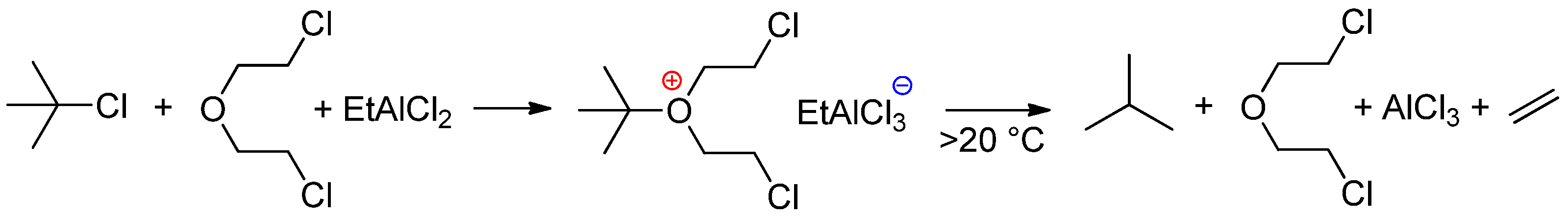
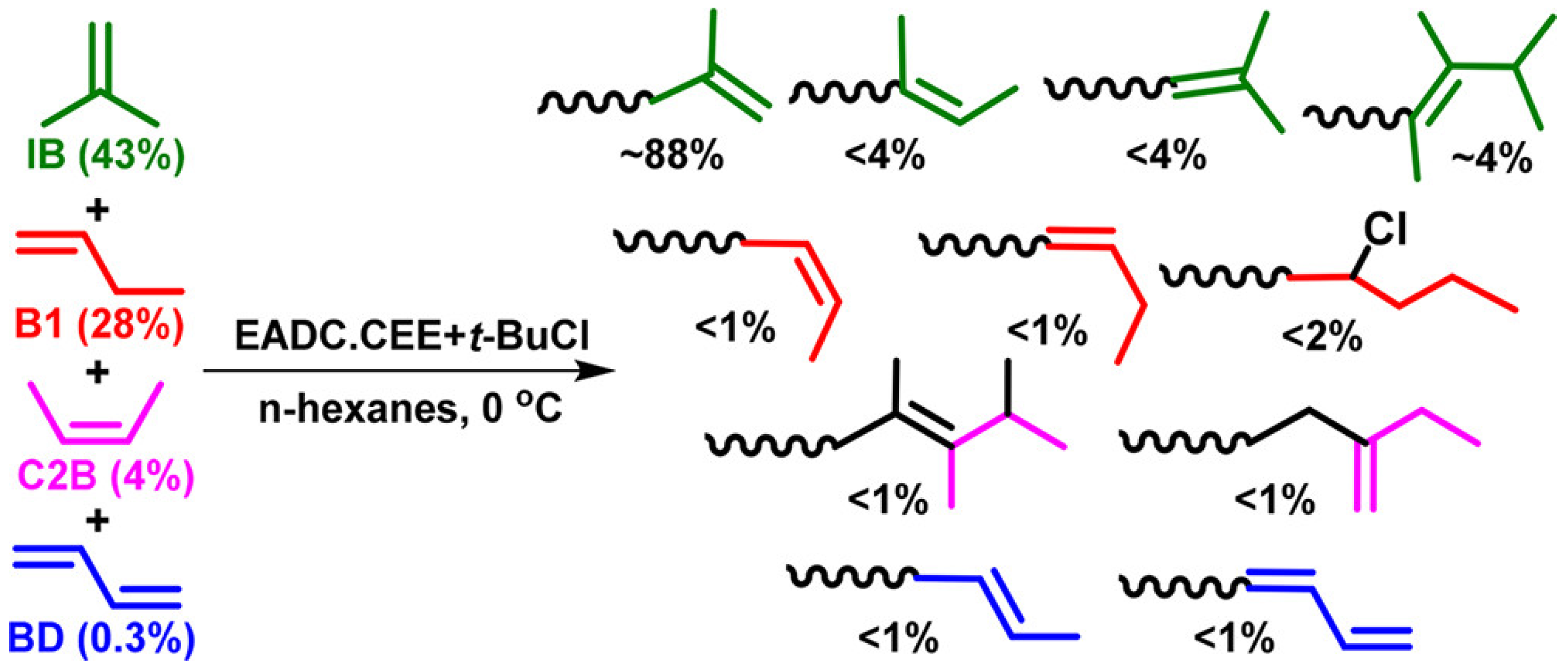
4.2. Second-Generation Catalysts: Alkyl Metal Chlorides/Ethers
4.2.1. Alkylaluminum Chlorides/R2O and Related Systems
4.2.2. Alkoxyaluminum Chloride/R2O
4.3. Third-Generation Catalysts: Heterogeneous Systems
4.3.1. AlCl3-Based Ionic Liquids
4.3.2. Other Ionic Liquids
4.3.3. Liquid Coordination Complexes
4.3.4. Silica-Supported Catalysts
5. Other Catalysts and Methods
5.1. Bis(Arene) Metal Cationic Complexes
5.2. Perfluorophenyl Metal Derivatives
5.3. Metal Trifluoromethylsulfonates
5.4. Visible Light-Induced Polymerization
5.5. Synthesis of PIBs with Higher Content of Trisubstituted Olefinic End-Groups
6. Further Transformations of HRPIB (PIB-Cl) and Applications of the Products
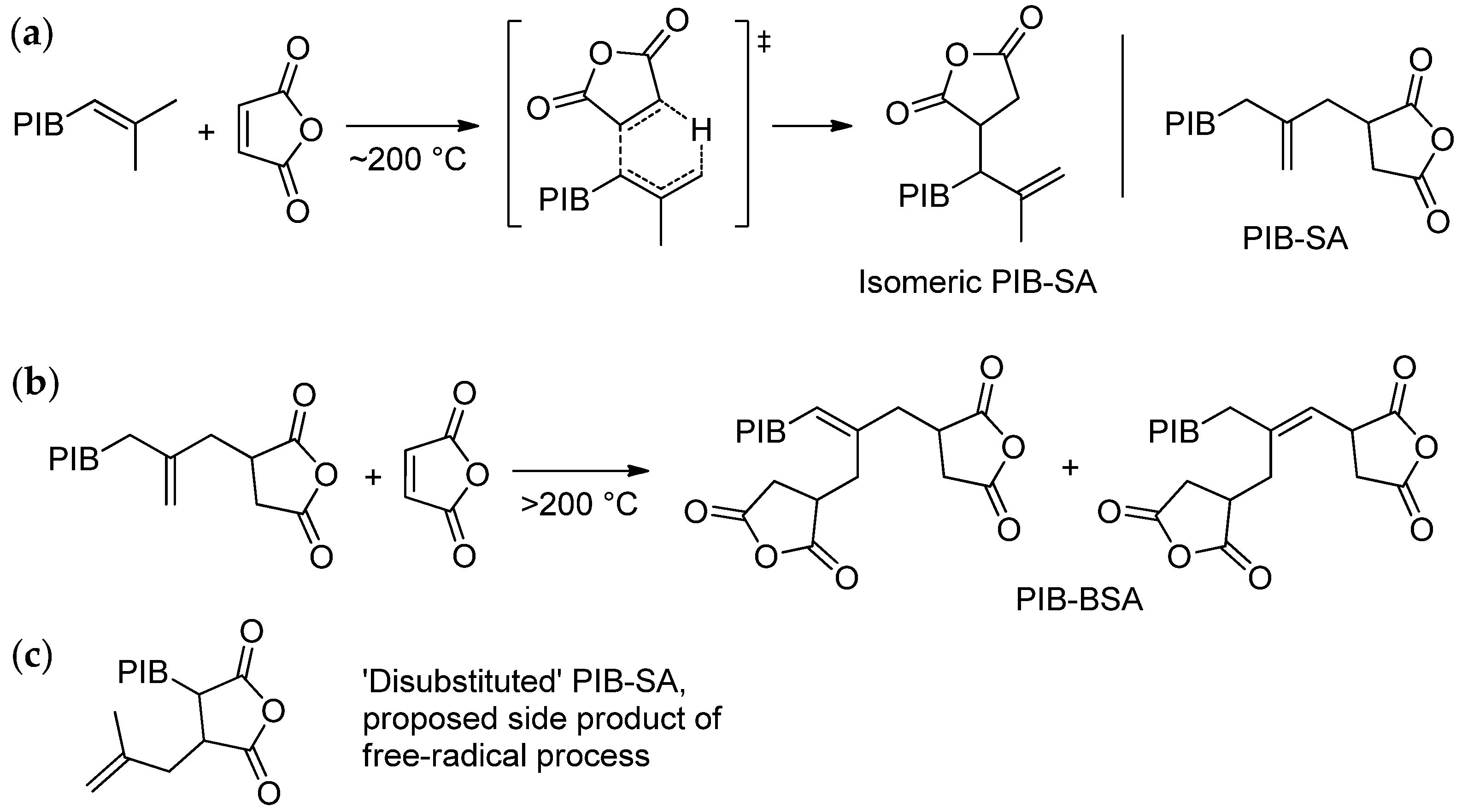
6.1. Recent Studies on the Synthesis of PIB-SA and PIB-SI
6.1.1. Synthesis and Structure of PIB-SA
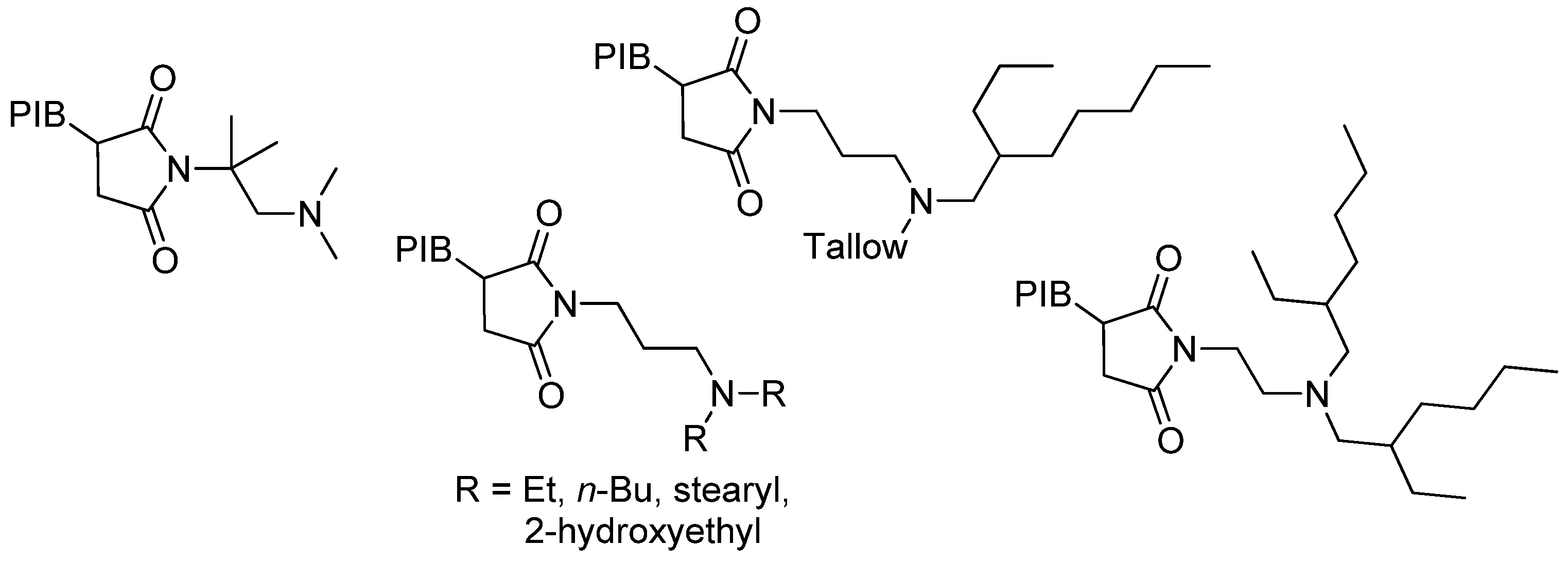
6.1.2. PIB-SI and Related Compounds
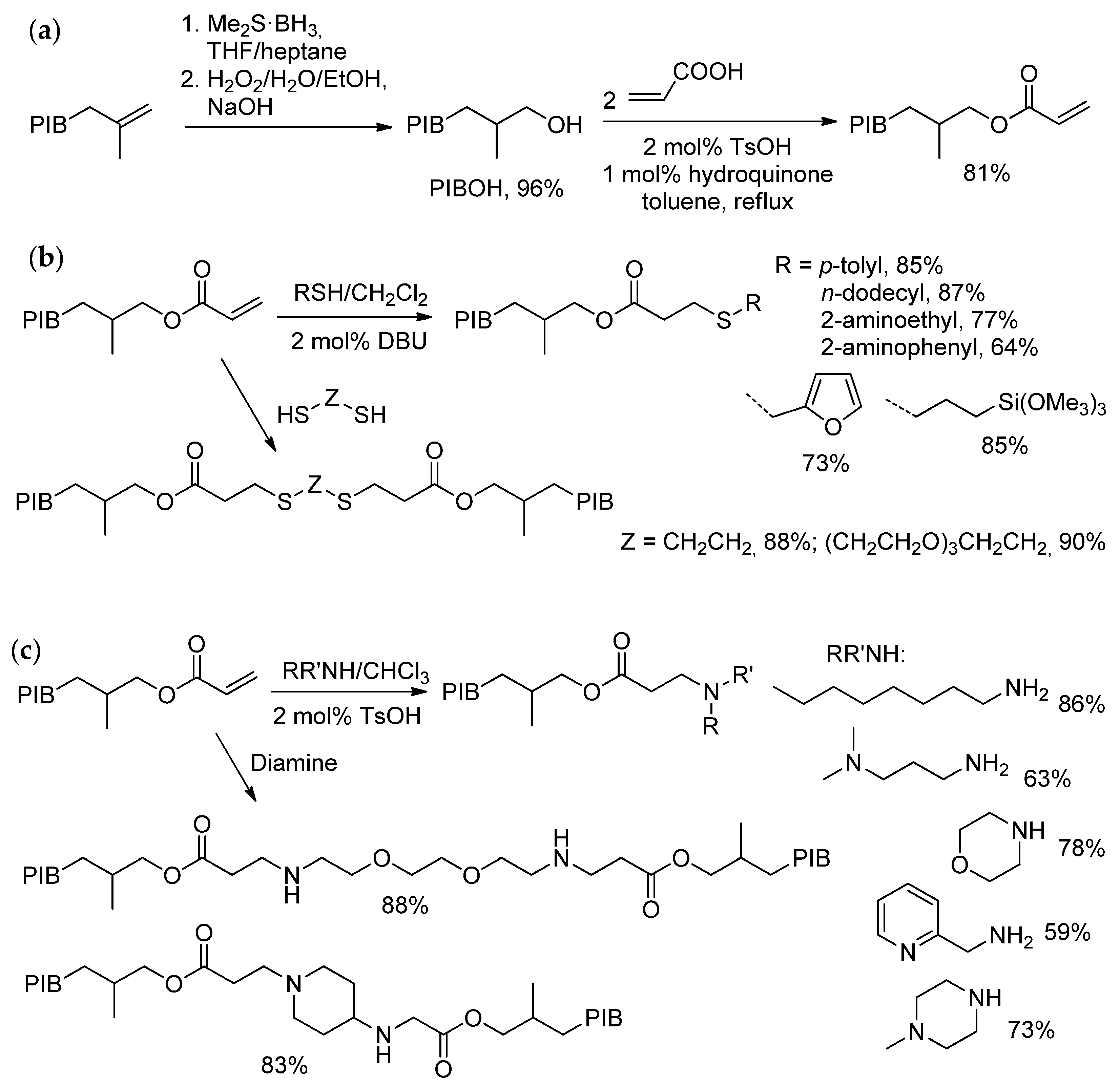
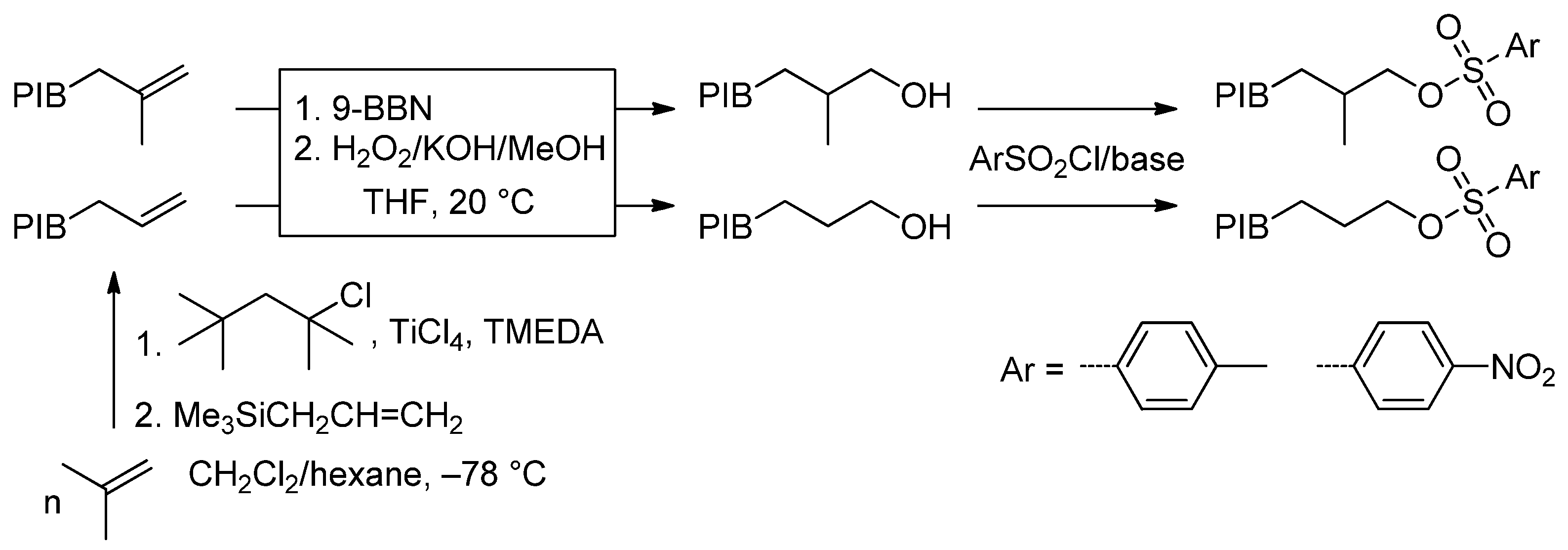
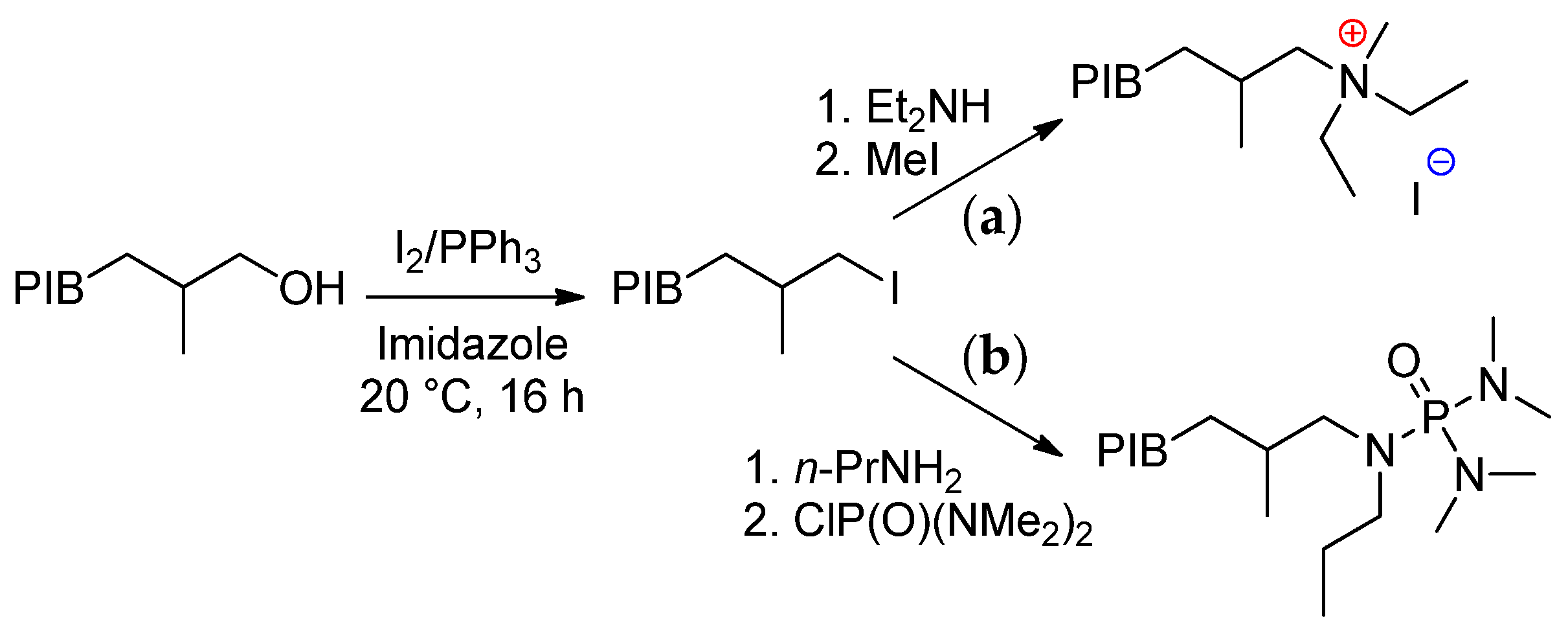
6.2. Recent Studies on Other Methods of HRPIB Functionalization
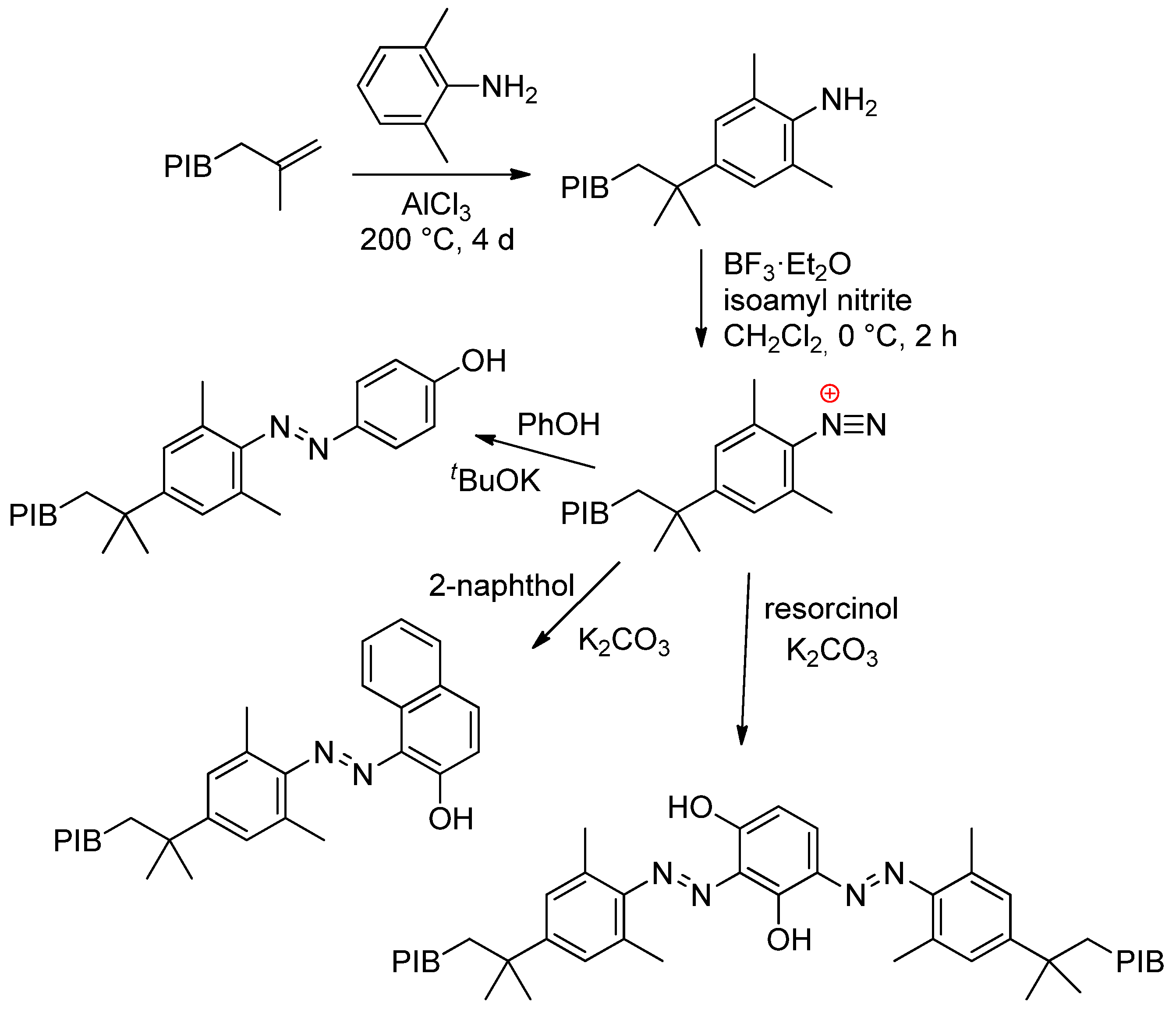
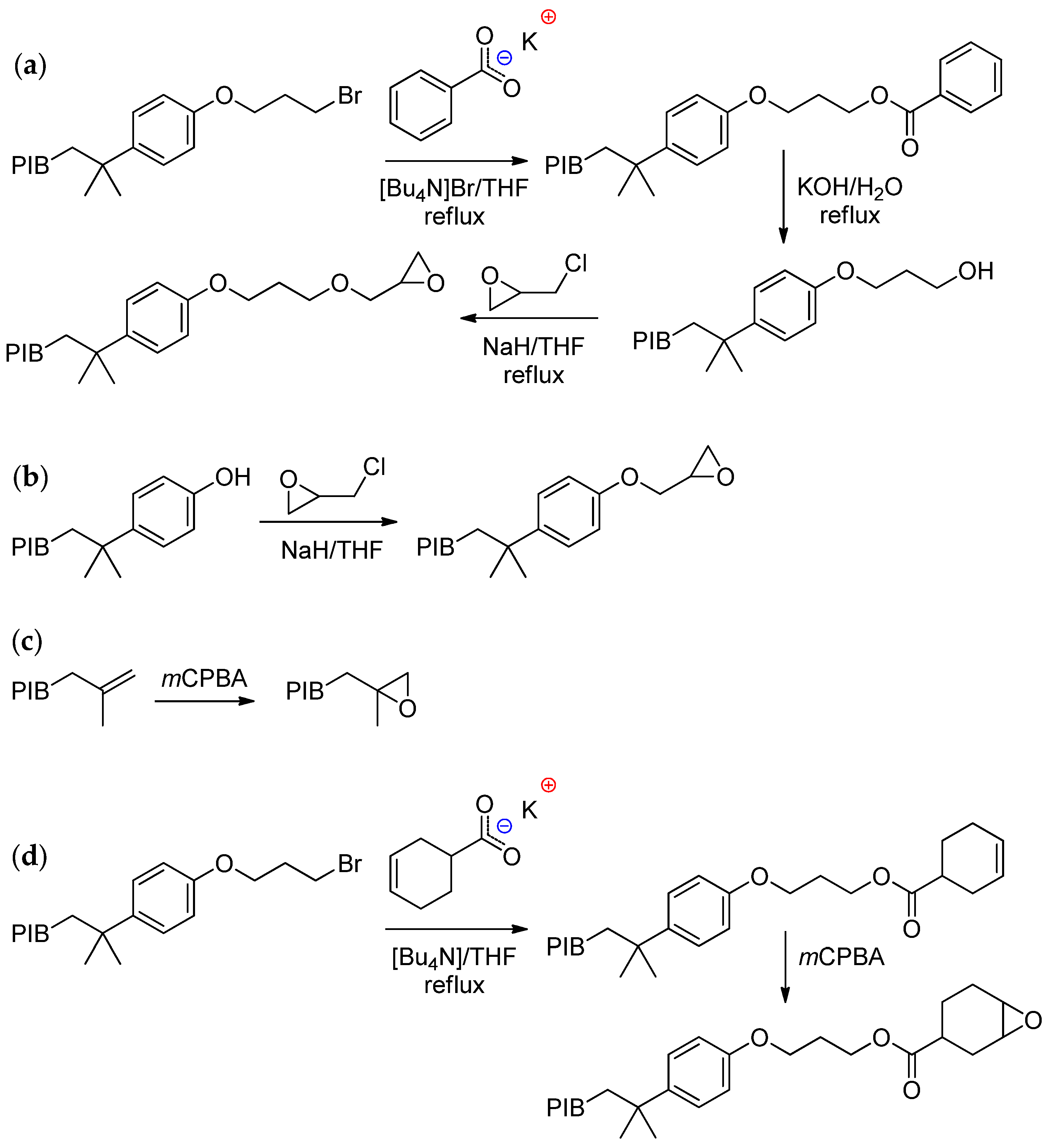
6.3. PIB-Based Copolymers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, M.F., Jr.; Shaffer, T.D.; Tsou, A.H. Commercial Isobutylene Polymers. In Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 1–25. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Kostjuk, S.V. Homogeneous and heterogeneous catalysts for the synthesis of highly reactive polyisobutylene: Discovery, development and perspectives. J. Macromol. Sci. A 2021, 58, 725–735. [Google Scholar] [CrossRef]
- Sharma, R.K.; Mohanty, S.; Gupta, V. Advances in butyl rubber synthesis via cationic polymerization: An overview. Polym. Int. 2021, 70, 1165–1175. [Google Scholar] [CrossRef]
- Global Polyisobutylene (Conventional/Highly Reactive) Markets, 2021–2028: Growing Demand for Polyisobutylene in the Food Industry and Growing Demand for PIB from Developed and Developing Economies—ResearchAndMarkets.com. Available online: https://www.businesswire.com/news/home/20220419006001/en/Global-Polyisobutylene-ConventionalHighly-Reactive-Markets-2021-2028-Growing-Demand-for-Polyisobutylene-in-the-Food-Industry-Growing-Demand-for-PIB-from-Developed-and-Developing-Economies---ResearchAndMarkets.com (accessed on 4 August 2023).
- Polisobutylene (PIB) Market Research Information. Available online: https://www.marketresearchfuture.com/reports/polyisobutylene-market-5911 (accessed on 4 August 2023).
- BASF Increases Production Capacity for Medium-Molecular Weight Polyisobutenes in Ludwigshafen, Germany. Available online: https://www.basf.com/global/en/media/news-releases/2023/08/p-23-277.html (accessed on 4 August 2023).
- OPPANOL® One Product—Many Opportunities. Available online: https://automotive-transportation.basf.com/global/en/fuel-and-lubricants/fuel-and-lubricant-solutions/polyisobutenes--pib-/oppanol_more-than-just-polyisobutene.html (accessed on 4 August 2023).
- Stuart, F.A.; Anderson, R.G.; Drummond, A.Y. Alkenyl Succinimides of Tetraethylene Pentamine. US Patent 3202678, 24 August 1965. [Google Scholar]
- Duncan, D.A.; Blythe, G.A.; Moreton, D. Additive Composition for Middle Distillate Fuels and Middle Distillate Fuel Compositions Containing Same. WO Patent 2002006428, 24 January 2002. [Google Scholar]
- Parmar, D.; Jones, J.L.; Saccomando, D.J.; Proust, N.; Mosier, P.E. Hindered Amine Terminated Succinimide Dispersants and Lubricating Compositions Containing Same. US Patent 11421174, 23 August 2022. [Google Scholar]
- Rudyak, K.B.; Polyanskii, K.B.; Vereshchagina, N.V.; Zemtsov, D.B.; Panov, D.M.; Yumasheva, T.M. Depressant and Dispersant Additives for Diesel Fuel. Components, Brands, New Technologies and Developments. Chem. Technol. Fuels Oils 2022, 58, 741–748. [Google Scholar] [CrossRef]
- Mach, H.; Rath, P. Highly reactive polyisobutene as a component of a new generation of lubricant and fuel additives. Lubr. Sci. 1999, 11, 175–185. [Google Scholar] [CrossRef]
- Rath, H.P.; Hoffmann, F.; Reuter, P.; Mach, H. Preparation of Polyisobutylene. US Patent 5191044, 2 March 1993. [Google Scholar]
- Rath, H.P.; Kanne, U. Method for Producing Low-Molecular, Highly Reactive Polyisobutylene. US Patent 6407186, 18 June 2002. [Google Scholar]
- Li, Y.; Cokoja, M.; Kühn, F.E. Inorganic/organometallic catalysts and initiators involving weakly coordinating anions for isobutene polymerisation. Coord. Chem. Rev. 2011, 255, 1541–1557. [Google Scholar] [CrossRef]
- Rense, R.J. Lubricant. US Patent 3215707, 2 November 1965. [Google Scholar]
- Rivera-Tirado, E.; Aaserud, D.J.; Wesdemiotis, C. Characterization of polyisobutylene succinic anhydride chemistries using mass spectrometry. J. Appl. Polym. Sci. 2012, 124, 2682–2690. [Google Scholar] [CrossRef]
- Huang, C.; Loper, J.T. Process for the Preparation of Polyalkenyl Succininc Anhydride. US Patent 8728995, 20 May 2014. [Google Scholar]
- Environmental Protection Agency (EPA). Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press: Washington, DC, USA, 2014; Volume 17. [CrossRef]
- Goethals, E.J.; Du Prez, F. Carbocationic polymerizations. Prog. Polym. Sci. 2007, 32, 220–246. [Google Scholar] [CrossRef]
- Alves, J.B.; Vasconcelos, M.K.; Mangia, L.H.R.; Tatagiba, M.; Fidalgo, J.; Campos, D.; Invernici, P.L.; Rebouças, M.V.; Andrade, M.H.S.; Pinto, J.C. A Bibliometric Survey on Polyisobutylene Manufacture. Processes 2021, 9, 1315. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Yeong, H.Y.; Voit, B. Cationic polymerization of isobutylene at room temperature. J. Polym. Sci. A Polym. Chem. 2013, 51, 471–486. [Google Scholar] [CrossRef]
- Rajasekhar, T.; Singh, G.; Kapur, G.S.; Ramakumar, S.S.V. Recent advances in catalytic chain transfer polymerization of isobutylene: A review. RSC Adv. 2020, 10, 18180–18191. [Google Scholar] [CrossRef]
- Bochmann, M. Highly Electrophilic Organometallics for Carbocationic Polymerizations: From Anion Engineering to New Polymer Materials. Acc. Chem. Res. 2010, 43, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Diebl, B.E.; Li, Y.; Cokoya, M.; Kühn, F.E.; Radhakrishnan, N.; Zschoeche, S.; Komber, H.; Yeong, H.Y.; Voit, B.; Nuyken, O.; et al. Synthesis and Application of Molybdenum (III) Complexes Bearing Weakly Coordinating Anions as Catalysts of Isobutylene Polymerization. J. Polym. Sci. A Polym. Chem. 2010, 48, 3775–3786. [Google Scholar] [CrossRef]
- Yang, K.; Li, T.; Tan, R.; Shen, K.; Li, Y. Cationic Half-Sandwich Scandium Complex for the Synthesis of Polyisobutylene with High Molecular Weight Under Mild Conditions. J. Polym. Sci. A Polym. Chem. 2018, 56, 467–470. [Google Scholar] [CrossRef]
- Qian, L.; Mathers, R.T.; Damodaran, K.; Godugu, B.; Lewis, S.P. Greener, cleaner polymerization of isobutene. Green Mater. 2013, 1, 161–175. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Li, S.; Cui, D. Isobutene (co)polymerization initiated by rare-earth metal cationic catalysts. Polymer 2020, 187, 122105. [Google Scholar] [CrossRef]
- Simison, K.L.; Stokes, C.D.; Harrison, J.J.; Storey, R.F. End-Quenching of Quasiliving Carbocationic Isobutylene Polymerization with Hindered Bases: Quantitative Formation of exo-Olefin-Terminated Polyisobutylene. Macromolecules 2006, 39, 2481–2487. [Google Scholar] [CrossRef]
- Morgan, D.L.; Harrison, J.J.; Stokes, C.D.; Storey, R.F. Kinetics and Mechanism of End-Quenching of Quasiliving Polyisobutylene with Sterically Hindered Bases. Macromolecules 2011, 44, 2438–2443. [Google Scholar] [CrossRef]
- Dimitrov, P.; Emert, J.; Hua, J.; Keki, S.; Faust, R. Mechanism of Isomerization in the Cationic Polymerization of Isobutylene. Macromolecules 2011, 44, 1831–1840. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Yan, P.; Zhang, Y.; Xu, R. Polyisobutylene with High exo-Olefin Content via β-H Elimination in the Cationic Polymerization of Isobutylene with H2O/FeCl3/Dialkyl Ether Initiating System. Macromolecules 2011, 44, 1866–1875. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.-X.; Zhang, Y.; Yan, P.-F.; Xu, R.-W. A cost-effective process for highly reactive polyisobutylenes via cationic polymerization coinitiated by AlCl3. Polymer 2010, 51, 5960–5969. [Google Scholar] [CrossRef]
- Guo, A.-R.; Yang, X.-J.; Yan, P.-F.; Wu, Y.-X. Synthesis of highly reactive polyisobutylenes with exo-olefin terminals via controlled cationic polymerization with H2O/FeCl3/iPrOH initiating system in nonpolar hydrocarbon media. J. Polym. Sci. A Polym. Chem. 2013, 51, 4200–4212. [Google Scholar] [CrossRef]
- Grubisic, Z.; Rempp, P.; Benoit, H. A universal calibration for gel permeation chromatography. J. Polym. Sci. B Polym. Lett. 1967, 5, 753–759. [Google Scholar] [CrossRef]
- Puskas, J.E.; Hutchinson, R. GPC Calibration for the Molecular Weight Measurement of Butyl Rubbers. Rubber Chem. Technol. 1993, 66, 742–748. [Google Scholar] [CrossRef]
- Puskas, J.E.; Chen, Y.; Kulbaba, K.; Kaszas, G. Comparison of the molecular weight and size measurement of polyisobutylenes by size exclusion chromatography/multi-angle laser light scattering and viscometry. J. Polym. Sci. A Polym. Chem. 2006, 44, 1777–1783. [Google Scholar] [CrossRef]
- Gocher, C.P.; Pandita, N.; Choudhury, R.P.; Bhakthavatsalam, V. Understanding microstructural heterogeneity in low and high molecular weight fractions of polydisperse polyisobutylene by SEC and NMR for its reactivity. J. Polym. Res. 2022, 29, 449. [Google Scholar] [CrossRef]
- Merna, J.; Vlček, P.; Volkis, V.; Michl, J. Li+ Catalysis and Other New Methodologies for the Radical Polymerization of Less Activated Olefins. Chem. Rev. 2016, 116, 771–785. [Google Scholar] [CrossRef]
- Volkis, V.; Shoemaker, R.K.; Michl, J. Highly Branched Polyisobutylene by Radical Polymerization under Li[CB11(CH3)12] Catalysis. Macromolecules 2012, 45, 9250–9257. [Google Scholar] [CrossRef]
- Hero, D.; Kali, G. Free- and reversible deactivation radical (co)polymerization of isobutylene in water under environmentally benign conditions. Eur. Polym. J. 2021, 147, 110336. [Google Scholar] [CrossRef]
- Kostjuk, S.V. Recent progress in the Lewis acid co-initiated cationic polymerization of isobutylene and 1,3-dienes. RSC Adv. 2015, 5, 13125–13144. [Google Scholar] [CrossRef]
- Penczek, S. On the role of added lewis bases in the living cationic vinyl polymerization. Makromol. Chem. Rapid Commun. 1992, 13, 147–150. [Google Scholar] [CrossRef]
- Ummadisetty, S.; Storey, R.F. Quantitative Synthesis of exo-Olefin-Terminated Polyisobutylene: Ether Quenching and Evaluation of Various Quenching Methods. Macromolecules 2013, 46, 2049–2059. [Google Scholar] [CrossRef]
- Saunders, M.; Kates, M.R. Rates of degenerate 1,2-hydride and 1,2-methide shifts from the carbon-13 nuclear magnetic resonance spectra of tertiary alkyl cations. J. Am. Chem. Soc. 1978, 100, 7082–7083. [Google Scholar] [CrossRef]
- Vrček, V.; Vinkovič Vrček, I.; Siehl, H.-U. Quantum Chemical Study of Solvent and Substituent Effects on the 1,5-Hydride Shift in 2,6-Dimethyl-2-heptyl Cations. J. Phys. Chem. A 2006, 110, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Vasilenko, I.V.; Nikishev, P.A.; Shiman, D.I.; Kostjuk, S.V. Cationic polymerization of isobutylene in toluene: Toward well-defined exo-olefin terminated medium molecular weight polyisobutylenes under mild conditions. Polym. Chem. 2017, 8, 1417–1425. [Google Scholar] [CrossRef]
- Shim, S.H.; Choi, J.; Lee, S.; Lee, J.H. Statistical kinetic modeling procedure for cationic polymerization and its application to polyisobutylene. AIChE J. 2023, 69, e18036. [Google Scholar] [CrossRef]
- De, P.; Faust, R. Relative Reactivity of C4 Olefins toward the Polyisobutylene Cation. Macromolecules 2006, 39, 6861–6870. [Google Scholar] [CrossRef]
- Mayr, H. CC Bond Formation by Addition of Carbenium Ions to Alkenes: Kinetics and Mechanism. Angew. Chem. Int. Ed. 1990, 29, 1371–1384. [Google Scholar] [CrossRef]
- Watson, C.B.; Tan, D.; Bergbreiter, D.E. Enthalpy-Driven Polyisobutylene Depolymerization. Macromolecules 2019, 52, 3042–3048. [Google Scholar] [CrossRef]
- Jones, G.R.; Wang, H.S.; Parkatzidis, K.; Whitfield, R.; Truong, N.P.; Anastasaki, A. Reversed Controlled Polymerization (RCP): Depolymerization from Well-Defined Polymers to Monomers. J. Am. Chem. Soc. 2023, 145, 9898–9915. [Google Scholar] [CrossRef]
- Puskas, J.E.; Kaszas, G. Polyisobutylene-Based Thermoplastic Elastomers: A Review. Rubber Chem. Technol. 1996, 69, 462–475. [Google Scholar] [CrossRef]
- Hanefeld, P.; Walter, H.-M.; Kiefer, M.; Mach, H.; Wettling, T.; Sigl, M.; Schubert, M.; Stephan, J.; Papp, R.; Poplow, F. Verfahren zur Herstellung von Polyisobutylen mit Einem Gehalt an Endständigen Doppelbindungen von Mehr als 50% aus Einem Technischen 1-buten-, 2-buten- und Isobuten-Haltigen c4-Kohlenwasserstoffstrom. WO Patent 2007096296, 22 January 2009. [Google Scholar]
- Wettling, T.; Hirsch, S.; Brym, M.; Weis, M. Process for Continuously Preparing Polyisobutylene. US Patent 2013178679, 11 July 2013. [Google Scholar]
- Wettling, T.; Hirsch, S.; Brym, M.; Weis, M. High-Reactivity Polyisobutylene Having a High Content of Vinylidene Double Bonds in the Side Chains. US Patent 2016145362, 26 May 2016. [Google Scholar]
- Kim, M.S.; Park, S.M.; Min, K.T. Device and Method for Re-Circulating Raw Material Used when Manufacturing Polybutene. EP Patent 3029075, 8 June 2016. [Google Scholar]
- Medzhibovskii, A.S.; Dementev, A.V.; Kolokolnikov, A.S.; Savchenko, A.O.; Koblov, E.A. Method for producing low molecular weight highly reactive polyisobutylene. RU Patent 2790160, 14 February 2023. [Google Scholar]
- Shikh, S.K.; Lawson, R. Production of highly reactive low molecular weight PIB oligomers. US Patent 9453089, 27 September 2016. [Google Scholar]
- Babkin, V.A.; Andreev, D.S.; Ignatov, A.V.; Titov, E.S.; Rakhimov, A.I.; Schreibert, N.A. Activation energy and thermal effect of initiation reaction of cationic isobutylene polymerization in the presence of complex catalyst “boron trifluoride–water” in heptane. Fluor. Notes 2022, 2, 3–4. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, K.; Xu, L.; Guo, X.; Cheng, R.; Liu, B. Facile synthesis of medium molecular weight polyisobutylene with remarkable efficiency employing the complexed BF3·EtOH/TiCl4·H2O initiating system. Eur. Polym. J. 2019, 120, 109204. [Google Scholar] [CrossRef]
- Baxter, C.E. Processes for Making Polyisobutylene Compositions. US Patent 11214637, 4 January 2022. [Google Scholar]
- Vo, M.N.; Basdogan, Y.; Derksen, B.S.; Proust, N.; Cox, G.A.; Kowall, C.; Keith, J.A.; Johnson, J.K. Mechanism of Isobutylene Polymerization: Quantum Chemical Insight into AlCl3/H2O-Catalyzed Reactions. ACS Catal. 2018, 8, 8006–8013. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, Y.; Wang, K.; Luo, G. Flow synthesis of medium molecular weight polyisobutylene coinitiated by AlCl3. Eur. Polym. J. 2016, 80, 219–226. [Google Scholar] [CrossRef]
- Davidson, G.J.E.; Bourque, J.L. Branched Polyisobutylene Polymers. WO Patent 2023000095, 26 January 2023. [Google Scholar]
- Nugay, T.; Deodhar, T.; Nugay, N.; Kennedy, J.P. Low-cost bifunctional initiators for bidirectional living cationic polymerization of olefins. III. Centrally functionalized polyisobutylenes. J. Polym. Sci. A Polym. Chem. 2018, 56, 1140–1145. [Google Scholar] [CrossRef]
- Faust, R.; Kennedy, J.P. Living carbocationic polymerization. IV. Living polymerization of isobutylene. J. Polym. Sci. A Polym. Chem. 1987, 25, 1847–1869. [Google Scholar] [CrossRef]
- Wu, K.; Wu, Y.; Huang, S.; Chen, Z.; Wang, H.; Shang, Y.; Li, S. Synthesis and characterization of hydroxyl-terminated butadiene-end-capped polyisobutylene and its use as a diol for polyurethane preparation. RSC Adv. 2020, 10, 9601–9609. [Google Scholar] [CrossRef] [PubMed]
- Nugay, T.; Keszler, B.L.; Deodhar, T.; Nugay, N.; Kennedy, J.P. Low cost bifunctional initiators for bidirectional living cationic polymerization of olefins. I. isobutylene. J. Polym. Sci. A Polym. Chem. 2017, 55, 3716–3724. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Frolov, A.V.; Kostjuk, S.V. Cationic Polymerization of Isobutylene Using AlCl3OBu2 as a Coinitiator: Synthesis of Highly Reactive Polyisobutylene. Macromolecules 2010, 43, 5503–5507. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Shiman, D.I.; Kostjuk, S.V. Highly reactive polyisobutylenes via AlCl3OBu2-coinitiated cationic polymerization of isobutylene: Effect of solvent polarity, temperature, and initiator. J. Polym. Sci. A Polym. Chem. 2012, 50, 750–758. [Google Scholar] [CrossRef]
- Dimitrov, P.; Emert, J.; Faust, R. Polymerization of Isobutylene by AlCl3/Ether Complexes in Nonpolar Solvent. Macromolecules 2012, 45, 3318–3325. [Google Scholar] [CrossRef]
- Shiman, D.I.; Vasilenko, I.V.; Kostjuk, S.V. Cationic polymerization of isobutylene by AlCl3/ether complexes in non-polar solvents: Effect of ether structure on the selectivity of β-H elimination. Polymer 2013, 54, 2235–2242. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Vasilenko, I.V.; Shiman, D.I.; Frolov, A.N.; Gaponik, L.V. Highly Reactive Polyisobutylenes via Cationic Polymerization of Isobutylene by AlCl3/Ether Complexes in Non-Polar Media: Scope and Limitations. Macromol. Symp. 2015, 349, 94–103. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, Y.C.; Wang, K.; Luo, G.S. Fast flow synthesis of highly reactive polyisobutylene co-initiated by an AlCl3/isopropyl ether complex. RSC Adv. 2016, 6, 9827–9834. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, Y.; Wang, K.; Luo, G. Cationic polymerization of isobutylene catalysed by AlCl3 with multiple nucleophilic reagents. RSC Adv. 2016, 6, 97983–97989. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, K.; Lu, Y. Effects of Ether on the Cationic Polymerization of Isobutylene Catalyzed by AlCl3. ACS Omega 2018, 3, 2033–2039. [Google Scholar] [CrossRef]
- Shiman, D.I.; Berezianko, I.A.; Vasilenko, I.V.; Kostjuk, S.V. Cationic Polymerization of Isobutylene and C4 Mixed Feed Using Complexes of Lewis Acids with Ethers: A Comparative Study. Chin. J. Polym. Sci. 2019, 37, 891–897. [Google Scholar] [CrossRef]
- Xie, D.; Zhu, S.; Lu, Y. Tailoring the AlCl3/iPr2O/Et2O initiation system for highly reactive polyisobutylene synthesis in pure n-hexane. RSC Adv. 2020, 10, 5183–5190. [Google Scholar] [CrossRef]
- Tota, R.; Singh, G.; Rani, R.; Mondal, S.; Kapur, G.S.; Ramakumar, S.S.V. Polymerisationskatalysatorsystem und Verfahren zur Herstellung von Hochreaktivem Polyisobutylen. EP Patent 3943518, 26 January 2022. [Google Scholar]
- Tota, R.; Singh, G.; Rani, R.; Mondal, S.; Kapur, G.S.; Ramakumar, S.S.V. Polymerization Catalyst System and Process to Produce Highly Reactive Polyisobutylene. US Patent 2022025076, 27 January 2022. [Google Scholar]
- Xie, J.; Miranda, M.S.L.; Gallaschun, C.; Andrade, M.; Panza, D.; Reboucas, M.; Caulkins, R.; Brasselle, A.; Kalfus, J. Heterogeneous Catalyst for Highly-Reactive Polyisobutylene. US Patent 2023167208, 1 June 2023. [Google Scholar]
- Jin, Y.; Chen, L.; Guo, X.; Xu, L.; Zhu, Z.; Liu, Z.; Cheng, R.; Liu, B. A Complexed Initiating System AlCl3·Phenetole/TiCl4·H2O with Dominant Synergistic Effect for Efficient Synthesis of High Molecular Weight Polyisobutylene. Polymers 2019, 11, 2121. [Google Scholar] [CrossRef]
- Deng, S.; Tian, H.; Sun, D.; Liu, S.; Zhao, Q. Method for initiating cationic polymerization of isobutylene by AlCl3. J. Polym. Res. 2020, 27, 55. [Google Scholar] [CrossRef]
- Hou, W.; Wang, W.; Xiang, Y.; Li, Y.; Chu, G.; Zou, H.; Sun, B. Polymerization of Isobutylene in a Rotating Packed Bed Reactor: Experimental and Modeling Studies. Appl. Sci. 2021, 11, 10194. [Google Scholar] [CrossRef]
- Kumar, R.; Dimitrov, P.; Bartelson, K.J.; Emert, J.; Faust, R. Polymerization of Isobutylene by GaCl3 or FeCl3/Ether Complexes in Nonpolar Solvents. Macromolecules 2012, 45, 8598–8603. [Google Scholar] [CrossRef]
- Kumar, R.; De, P.; Zheng, B.; Huang, K.-W.; Emert, J.; Faust, R. Synthesis of highly reactive polyisobutylene with FeCl3/ether complexes in hexane; kinetic and mechanistic studies. Polym. Chem. 2015, 6, 322–329. [Google Scholar] [CrossRef]
- Bartelson, K.J.; De, P.; Kumar, R.; Emert, J.; Faust, R. Cationic polymerization of isobutylene by FeCl3/ether complexes in hexanes: An investigation of the steric and electronic effects of ethers. Polymer 2013, 54, 4858–4863. [Google Scholar] [CrossRef]
- Yang, X.; Guo, A.; Xu, H.; Wu, Y. Direct synthesis of highly reactive polyisobutylenes via cationic polymerization of isobutylene co-initiated with TiCl4 in nonpolar hydrocarbon media. J. Appl. Polym. Sci. 2015, 32, 42232. [Google Scholar] [CrossRef]
- Bohdan, M.; Shiman, D.I.; Nikishau, P.A.; Vasilenko, I.V.; Kostjuk, S.V. Quasiliving carbocationic polymerization of isobutylene using FeCl3 as an efficient and water-tolerant Lewis acid: Synthesis of well-defined telechelic polyisobutylenes. Polym. Chem. 2022, 13, 6010–6021. [Google Scholar] [CrossRef]
- Kumar, R.; Zheng, B.; Huang, K.-W.; Emert, J.; Faust, R. Synthesis of Highly Reactive Polyisobutylene Catalyzed by EtAlCl2/Bis(2-chloroethyl) Ether Soluble Complex in Hexanes. Macromolecules 2014, 47, 1959–1965. [Google Scholar] [CrossRef]
- Banerjee, S.; Emert, J.; Wright, P.; Skourlis, T.; Severt, R.; Faust, R. Polymerization of isobutylene catalyzed by EtAlCl2/bis(2-chloroethyl) ether complex in steel vessels. Polym. Chem. 2015, 6, 4902–4910. [Google Scholar] [CrossRef]
- Banerjee, S.; Jha, B.N.; De, P.; Emert, J.; Faust, R. Kinetic and Mechanistic Studies of the Polymerization of Isobutylene Catalyzed by EtAlCl2/Bis(2-chloroethyl) Ether Complex in Hexanes. Macromolecules 2015, 48, 5474–5480. [Google Scholar] [CrossRef]
- Rajasekhar, T.; Haldar, U.; Emert, J.; Dimitrov, P.; Severt, R.; Faust, R. Catalytic chain transfer polymerization of isobutylene: The role of nucleophilic impurities. J. Polym. Sci. A Polym. Chem. 2017, 55, 3697–3704. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, Y.; Faust, R. Micromixing enhanced synthesis of HRPIBs catalyzed by EADC/bis(2-chloroethyl)ether complex. RSC Adv. 2017, 7, 27629–27636. [Google Scholar] [CrossRef]
- Rajasekhar, T.; Emert, J.; Faust, R. Synthesis of highly reactive polyisobutylene by catalytic chain transfer in hexanes at elevated temperatures; determination of the kinetic parameters. Polym. Chem. 2017, 8, 2852–2859. [Google Scholar] [CrossRef]
- Rajasekhar, T.; Haldar, U.; De, P.; Emert, J.; Faust, R. Cationic Copolymerization and Multicomponent Polymerization of Isobutylene with C4 Olefins. Macromolecules 2017, 50, 8325–8333. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Shiman, D.I.; Kostjuk, S.V. Alkylaluminum dichloride–ether complexes which are fully soluble in hydrocarbons as catalysts for the synthesis of exo-olefin terminated polyisobutylene at room temperature. Polym. Chem. 2014, 5, 3855–3866. [Google Scholar] [CrossRef]
- Shiman, D.I.; Vasilenko, I.V.; Kostjuk, S.V. Cationic polymerization of isobutylene by complexes of alkylaluminum dichlorides with diisopropyl ether: An activating effect of water. J. Polym. Sci. A Polym. Chem. 2014, 52, 2386–2393. [Google Scholar] [CrossRef]
- Shiman, D.I.; Vasilenko, I.V.; Kostjuk, S.V. Cationic polymerization of isobutylene catalyzed by iBuAlCl2 in the presence of ethers: Effect of catalyst pre-activation and mixture of two ethers. Polymer 2016, 99, 633–641. [Google Scholar] [CrossRef]
- Roc, R.C.; Muehlbach, K.; Wettling, T.; Kostjuk, S.V.; Vasilenko, I.; Shiman, D. Process for Preparing High-Reactivity Isobutene Homo- or Copolymers. US Patent 10968294, 6 April 2021. [Google Scholar]
- Zaikov, G.E.; Artsis, M.I.; Andreev, D.S.; Ignatov, A.V. Selectivity of Ethylaluminium Dichloride–Proton Donor Complex Catalysts from the C4 Fraction in Initiating the Oligomerization of Isobutylene. Russ. J. Phys. Chem. B 2022, 16, 606–614. [Google Scholar] [CrossRef]
- Dimitrov, P.; Severt, R.J.; Hobin, P.J.; Nesti, K. Method for Forming Highly Reactive Olefin Functional Polymers. US Patent 2020369799, 26 November 2020. [Google Scholar]
- Dimitrov, P.; Severt, R.J.; Skourlis, T.; Weber, J.; Emert, J. Polymerization Initiating System and Method to Produce Highly Reactive Olefin Functional Polymers. US Patent 10047174, 14 August 2018. [Google Scholar]
- Rajasekhar, T.; Emert, J.; Wolf, L.M.; Faust, R. Controlled Catalytic Chain Transfer Polymerization of Isobutylene in the Presence of tert-Butanol as Exo-Enhancer. Macromolecules 2018, 51, 3041–3049. [Google Scholar] [CrossRef]
- Shiman, D.I.; Vasilenko, I.V.; Kostjuk, S.V. Alkoxy aluminum chlorides in the cationic polymerization of isobutylene: A co-initiator, carbocation stabilizer and chain-transfer agent. Polym. Chem. 2019, 10, 5998–6002. [Google Scholar] [CrossRef]
- Roc, R.C.; Muehlbach, K.; Wettling, T.; Kostjuk, S.V.; Vasilenko, I.; Shiman, D. Process for Preparing High-Reactivity Isobutene Homo- or Copolymers. US Patent 11359034, 14 June 2022. [Google Scholar]
- Vasilenko, I.V.; Berezianko, I.A.; Shiman, D.I.; Kostjuk, S.V. New catalysts for the synthesis of highly reactive polyisobutylene: Chloroaluminate imidazole-based ionic liquids in the presence of diisopropyl ether. Polym. Chem. 2016, 7, 5615–5619. [Google Scholar] [CrossRef]
- Yousefi, S.; Bahri-Laleh, N.; Nekoomanesh, M.; Emami, M.; Sadjadi, S.; Mirmohammadi, S.A.; Tomasini, M.; Bardají, E.; Poater, A. An efficient initiator system containing AlCl3 and supported ionic-liquid for the synthesis of conventional grade polyisobutylene in mild conditions. J. Mol. Liq. 2022, 367, 120381. [Google Scholar] [CrossRef]
- Kahkeshi, Z.I.; Bahri-Laleh, N.; Sadjadi, S.; Haghighi, M.N. An environmentally benign approach for the synthesis of low molar mass polybutenes from mixed C4 monomers using AlCl3/ionic-liquid initiating systems. Mol. Catal. 2023, 547, 113332. [Google Scholar] [CrossRef]
- Gupta, V.; Mishra, A.; Singh, R.; Sapre, A. Process for Preparing High Reactive Polyisobutylene. WO Patent 2020084557, 30 April 2020. [Google Scholar]
- Berezianko, I.A.; Vasilenko, I.V.; Kostjuk, S.V. Acidic imidazole-based ionic liquids in the presence of diisopropyl ether as catalysts for the synthesis of highly reactive polyisobutylene: Effect of ionic liquid nature, catalyst aging, and sonication. Polymer 2018, 145, 382–390. [Google Scholar] [CrossRef]
- Berezianko, I.A.; Vasilenko, I.V.; Kostjuk, S.V. Cationic polymerization of isobutylene co-initiated by chloroferrate imidazole-based ionic liquid: The advantageous effect of initiator and aromatic compounds. Eur. Polym. J. 2019, 121, 109307. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, J.; Li, S.; Zhang, M.; Yang, D.; Wang, H.; Shang, Y.; Guo, W.; Yand, P. Synthesis of highly reactive polyisobutylenes via cationic polymerization in ionic liquids: Characteristics and mechanism. Polym. Chem. 2019, 10, 201–208. [Google Scholar] [CrossRef]
- Berezianko, I.A.; Vasilenko, I.V.; Kostjuk, S.V. Silica gel supported ionic liquids as effective and reusable catalysts for the synthesis of highly reactive polyisobutylene in non-polar media. Polym. Chem. 2022, 13, 6625–6636. [Google Scholar] [CrossRef]
- Berezianko, I.A.; Nikishau, P.A.; Vasilenko, I.V.; Kostjuk, S.V. Liquid coordination complexes as a new class of catalysts for the synthesis of highly reactive polyisobutylene. Polymer 2021, 226, 123825. [Google Scholar] [CrossRef]
- Lichtenthaler, M.R.; Higelin, A.; Kraft, A.; Hughes, S.; Steffani, A.; Plattner, D.A.; Slattery, J.M.; Krossing, I. Univalent Gallium Salts of Weakly Coordinating Anions: Effective Initiators/Catalysts for the Synthesis of Highly Reactive Polyisobutylene. Organometallics 2013, 32, 6725–6735. [Google Scholar] [CrossRef]
- Lichtenthaler, M.R.; Maurer, S.; Mangan, R.J.; Stahl, F.; Mönkemeyer, F.; Hamann, J.; Krossing, I. Univalent Gallium Complexes of Simple and ansa-Arene Ligands: Effects on the Polymerization of Isobutylene. Chem. Eur. J. 2015, 21, 157–165. [Google Scholar] [CrossRef]
- Petersen, T.O.; Simone, D.; Krossing, I. From Ion-Like Ethylzinc Aluminates to [EtZn(arene)2]+[Al(ORF)4]− Salts. Chem. Eur. J. 2016, 22, 15847–15855. [Google Scholar] [CrossRef]
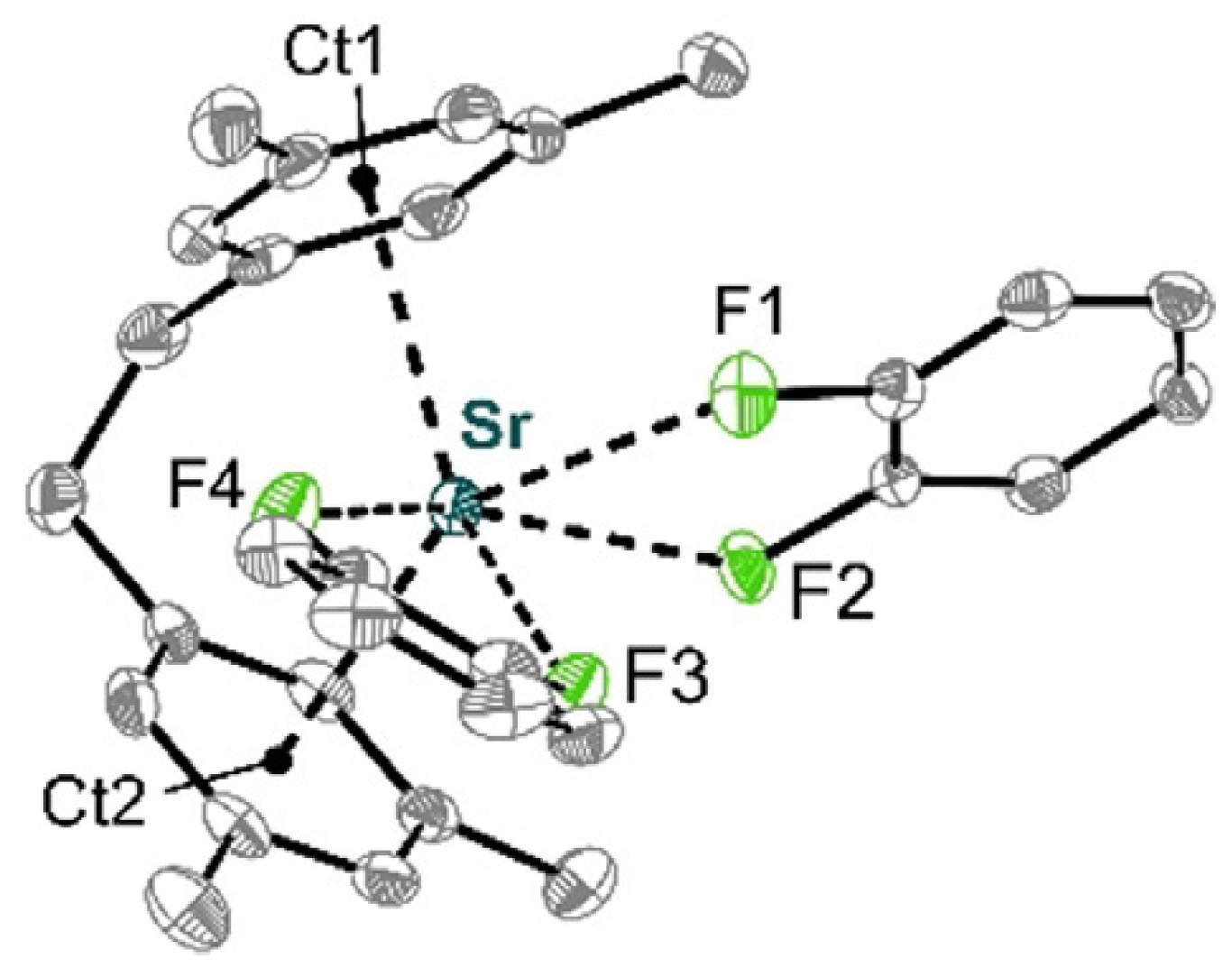
- Dabringhaus, P.; Schorpp, M.; Scherer, H.; Krossing, I. A Highly Lewis Acidic Strontium ansa-Arene Complex for Lewis Acid Catalysis and Isobutylene Polymerization. Angew. Chem. Int. Ed. 2020, 59, 22023–22027. [Google Scholar] [CrossRef] [PubMed]
- Garratt, S.; Guerrero, A.; Hughes, D.L.; Bochmann, M. Arylzinc Complexes as New Initiator Systems for the Production of Isobutene Copolymers with High Isoprene Content. Angew. Chem. Int. Ed. 2004, 43, 2166–2169. [Google Scholar] [CrossRef] [PubMed]
- Mathers, R.T.; Lewis, S.P. Aqueous cationic olefin polymerization using tris(pentafluorophenyl)gallium and aluminum. J. Polym. Sci. A Polym. Chem. 2012, 50, 1325–1332. [Google Scholar] [CrossRef]
- Hand, N.; Mathers, R.T.; Damodaran, K.; Lewis, S.P. Bulk and Solution Polymerization of Isobutene Using Tris(pentafluorophenyl)gallium and Tris(pentafluorophenyl)aluminum. Ind. Eng. Chem. Res. 2014, 53, 2718–2725. [Google Scholar] [CrossRef]
- Cai, L.; Liu, B.; Liu, X.; Cui, D. Synthesis of high molecular weight polyisobutylene with Al(C6F5)3-based initiating systems under mild conditions. Polymer 2022, 241, 124539. [Google Scholar] [CrossRef]
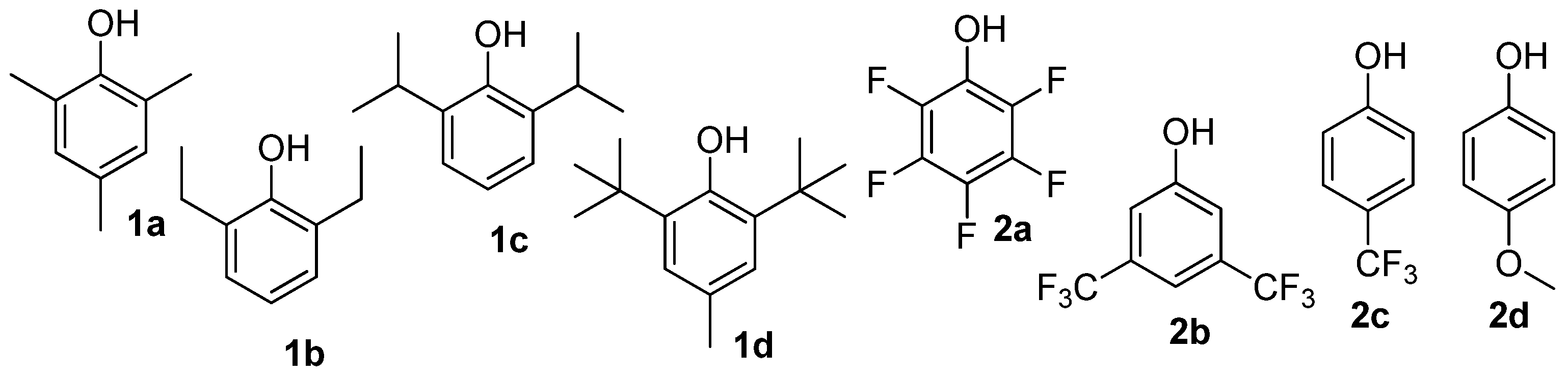
- Cai, L.; Wang, Q.-Y.; Liu, X.-L.; Cui, D.-M. Phenols/Al(C6F5)3 Initiation Systems for Cationic Polymerizations of Isobutylene. Chin. J. Polym. Sci. 2023, 41, 713–719. [Google Scholar] [CrossRef]
- Lewis, S.P.; Damodaran, K. Highly exo-olefinic polyisobutene synthesized using a recyclable acid. J. Polym. Sci. 2023, 61, 685–699. [Google Scholar] [CrossRef]
- Aydogan, C.; Yilmaz, G.; Shegiwal, A.; Haddleton, D.M.; Yagci, Y. Photoinduced Controlled/Living Polymerizations. Angew. Chem. Int. Ed. 2022, 61, e202117377. [Google Scholar] [CrossRef]
- Sifri, R.J.; Ma, Y.; Fors, B.P. Photoredox Catalysis in Photocontrolled Cationic Polymerizations of Vinyl Ethers. Acc. Chem. Res. 2022, 55, 1960–1971. [Google Scholar] [CrossRef]
- Hulnik, M.; Trofimuk, M.D.; Celiker, M.T.; Yagci, Y.; Kostjuk, S. Visible Light-Induced Cationic Polymerization of Isobutylene: Mechanistic Insight and Synthesis of End-Functional Polyisobutylene. In Proceedings of the Advanced Polymers via Macromolecular Engineering Conference, Paris, France, 23–27 April 2023. [Google Scholar]
- Lederhose, P.; Pierre, B.; Wettling, T.; Brym, M. Polyisobutene with High Content of Certain Double Bond Isomers. WO Patent 2022258417, 15 December 2022. [Google Scholar]
- Balzano, F.; Pucci, A.; Rausa, R.; Uccello-Barretta, G. Alder-ene addition of maleic anhydride to polyisobutene: Nuclear magnetic resonance evidence for an unconventional mechanism. Polym. Int. 2012, 61, 1256–1262. [Google Scholar] [CrossRef]
- Streets, J.; Proust, N.; Parmar, D.; Walker, G.; Licence, P.; Woodward, S. Rate of Formation of Industrial Lubricant Additive Precursors from Maleic Anhydride and Polyisobutylene. Org. Process Res. Dev. 2022, 26, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Pucci, A.; Barsocchi, C.; Rausa, R.; D’Elia, L.; Ciardelli, F. Alder ene functionalization of polyisobutene oligomer and styrene-butadiene-styrene triblock copolymer. Polymer 2005, 46, 1497–1505. [Google Scholar] [CrossRef]
- van der Merwe, M.M.; Landman, M.; van Rooyen, P.H.; Jordaan, J.H.L.; Otto, D.P. Structural Assignment of Commercial Polyisobutylene Succinic Anhydride-based Surfactants. J. Surfact. Deterg. 2016, 20, 193–205. [Google Scholar] [CrossRef]
- Frasca, F.; Duhamel, J. End Group Analysis of Polyisobutylene Succinic Anhydride (PIBSA) Carried Out with Pyrene Excimer Fluorescence. Ind. Eng. Chem. Res. 2022, 61, 14747–14759. [Google Scholar] [CrossRef]
- Frasca, F.; Duhamel, J. Characterization of Polyisobutylene Succinic Anhydride (PIBSA) and Its PIBSI Products from the Reaction of PIBSA with Hexamethylene Diamine. Polymers 2023, 15, 2350. [Google Scholar] [CrossRef]
- Morales-Rivera, C.A.; Proust, N.; Burrington, J.; Mpourmpakis, G. Computational Screening of Lewis Acid Catalysts for the Ene Reaction between Maleic Anhydride and Polyisobutylene. Ind. Eng. Chem. Res. 2021, 60, 154–161. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Ivchenko, P. DFT Modeling of the Alternating Radical Copolymerization and Alder-Ene Reaction between Maleic Anhydride and Olefins. Polymers 2020, 12, 744. [Google Scholar] [CrossRef]
- Tiekink, E.H.; Vermeeren, P.; Bickelhaupt, F.M.; Hamlin, T.A. How Lewis Acids Catalyze Ene Reactions. Eur. J. Org. Chem. 2021, 2021, 5275–5283. [Google Scholar] [CrossRef]
- Morales-Rivera, C.A.; Cormack, G.; Burrington, J.; Proust, N.; Mpourmpakis, G. Understanding and Optimizing the Behavior of Al- and Ru-Based Catalysts for the Synthesis of Polyisobutenyl Succinic Anhydrides. Ind. Eng. Chem. Res. 2022, 61, 14462–14471. [Google Scholar] [CrossRef]
- Reid, J.; Broom, N.J.; Cross, A. Methods and Uses Relating to Fuel Compositions. WO Patent 2023111550, 22 June 2023. [Google Scholar]
- Reid, J.; Roberts, M.; Ross, A.; Simms, M.J. Fuel Compositions. WO Patent 2023111551, 22 June 2023. [Google Scholar]
- Pike, P.W.; Parmar, D.; Proust, N.; Guo, B.; Johnson, J.R.; Short, A.L.; Williamson, A.M.; Armbrecht, J.; Newby, P.; Wollenberg, K.F. Processes for Producing Reaction Products of Polyisobutylene and an Ethylenically Unsaturated Acylating Agent. WO Patent 2023014928, 9 February 2023. [Google Scholar]
- Amiri, S.; Mokhtari, S. Synthesis and characterization of modified polyisobutylene-based dispersants from polyisobutylene succinimides. Polym. Bull. 2022, 79, 1069–1079. [Google Scholar] [CrossRef]
- Vo, M.N.; Call, M.; Kowall, C.; Johnson, J.K. Effect of Chain Length on the Dipole Moment of Polyisobutylene Succinate Anhydride. Ind. Eng. Chem. Res. 2022, 61, 2359–2365. [Google Scholar] [CrossRef]
- Pirouz, S.; Wang, Y.; Chong, J.M.; Duhamel, J. Characterization of the Chemical Composition of Polyisobutylene-Based Oil-Soluble Dispersants by Fluorescence. J. Phys. Chem. B 2014, 118, 3899–3911. [Google Scholar] [CrossRef] [PubMed]
- Pirouz, S.; Wang, Y.; Chong, J.M.; Duhamel, J. Chemical Modification of Polyisobutylene Succinimide Dispersants and Characterization of Their Associative Properties. J. Phys. Chem. B 2015, 119, 12202–12211. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.J.; Naveiro, R.; Soto, A.J.; Talavante, P.; Lee, S.-H.K.; Arrayas, R.G.; Franco, M.; Mauleón, P.; Ordóñez, H.L.; López, G.R.; et al. Design of New Dispersants Using Machine Learning and Visual Analytics. Polymers 2023, 15, 1324. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahman, A.M.N.A.; Yoong, Z.K.; Abdullah, M.K.; Ariffin, A.; Shafiq, M.D. Impact of Oil Viscosity on Carbon Stabilization Using Polyisobutylene Succinimide (PIBSI) Dispersant. Phys. Chem. Res. 2024, 12, 85–93. [Google Scholar]
- Chen, S.; Song, N.; Zhang, S.; Zhang, Y.; Yu, L.; Zhang, P. Synergistic tribological effect between polyisobutylene succinimide-modified molybdenum oxide nanoparticle and zinc dialkyldithiophosphate for reducing friction and wear of diamond-like carbon coating under boundary lubrication. Friction 2023, in press. [Google Scholar] [CrossRef]
- Parada, C.M.; Storey, R.F. Functionalization of polyisobutylene and polyisobutylene oligomers via the Ritter reaction. J. Polym. Sci. A Polym. Chem. 2018, 56, 840–852. [Google Scholar] [CrossRef]
- Kulai, I.; Karpus, A.; Bergbreiter, D.E.; Al-Hashimi, M.; Bazzi, H.S. Organocatalytic Michael Addition as a Method for Polyisobutylene Chain-End Functionalization. Macromol. Rapid Commun. 2020, 41, 2000382. [Google Scholar] [CrossRef]
- Pásztói, B.; Trötschler, T.M.; Szabó, Á.; Szarka, G.; Kerscher, B.; Mülhaupt, R.; Iván, B. Synthesis of Tosyl- and Nosyl-Ended Polyisobutylenes with High Extent of Functionalities: The Effect of Reaction Conditions. Polymers 2020, 12, 2504. [Google Scholar] [CrossRef]
- Samunual, P.; Bergbreiter, D.E. SN2 Reactions in Hydrocarbon Solvents Using Ammonium-Terminated Polyisobutylene Oligomers as Phase-Solubilizing Agents and Catalysts. J. Org. Chem. 2018, 83, 11101–11107. [Google Scholar] [CrossRef]
- Fu, Y.-H.; Bergbreiter, D.E. Recyclable Polyisobutylene-Bound HMPA as an Organocatalyst in Recyclable Poly(α-olefin) Solvents. ChemCatChem 2020, 12, 6050–6058. [Google Scholar] [CrossRef]
- Rosenfeld, N.; Quinn, E.; Malinski, T.J.; Bergbreiter, D.E. Sequestration of Phenols from Water into Poly(α-olefins) Facilitated by Hydrogen Bonding Polyisobutylene Additives. ACS EST Water 2022, 2, 1391–1401. [Google Scholar] [CrossRef]
- Czarny, K.; Krawczyk, B.; Szczukocki, D. Toxic effects of bisphenol A and its analogues on cyanobacteria Anabaena variabilis and Microcystis aeruginosa. Chemosphere 2021, 263, 128299. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Akhtar, T.; Hameed, N.; Sheikh, N. In vivo assessment of bisphenol A induced histopathological alterations and inflammatory gene expression in lungs of male Wistar rats. Hum. Experim. Toxicol. 2021, 40, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.B.; Kuechle, A.; Bergbreiter, D.E. Fully recyclable Brønsted acid catalyst systems. Green Chem. 2021, 23, 1266–1273. [Google Scholar] [CrossRef]
- Harrell, M.L.; Malinski, T.; Torres-López, C.; Gonzalez, K.; Suriboot, J.; Bergbreiter, D.E. Alternatives for Conventional Alkane Solvents. J. Am. Chem. Soc. 2016, 138, 14650–14657. [Google Scholar] [CrossRef]
- Shiman, D.I.; Sayevich, V.; Meerbach, C.; Nikishau, P.A.; Vasilenko, I.V.; Gaponik, N.; Kostjuk, S.V.; Lesnyak, V. Robust Polymer Matrix Based on Isobutylene (Co)polymers for Efficient Encapsulation of Colloidal Semiconductor Nanocrystals. ACS Appl. Nano Mater. 2019, 2, 956–963. [Google Scholar] [CrossRef]
- Misske, A.; Thomas, A.; Fleckenstein, C.; Fleischhaker, F.; Csihony, S.; Gerst, M.; Moebius, S.; Licht, U.; Schwarz, A.; Neuhaus, E. Macromonomers Containing Polyisobutene Groups, and Homopolymers or Copolymers Thereof. US Patent 11174333, 16 November 2021. [Google Scholar]
- Yang, B.; Storley, R.F. Synthesis and characterization of polyisobutylene telechelic prepolymers with epoxide functionality. React. Funct. Polym. 2020, 150, 104563. [Google Scholar] [CrossRef]
- Prudnikau, A.; Shiman, D.I.; Ksendzov, E.; Harwell, J.; Bolotina, E.A.; Nikishau, P.A.; Kostjuk, S.V.; Samuel, I.D.W.; Lesnyak, V. Design of cross-linked polyisobutylene matrix for efficient encapsulation of quantum dots. Nanoscale Adv. 2021, 3, 1443–1454. [Google Scholar] [CrossRef]
- Sukirno, I.; Pebriani, S.; Febriantini, D.; Nugraha, A.S.; Purnomo, B. Optimization and characterization of facile synthesis of pentaethylenehexamine-terminated polyisobutylene. Rasayan J. Chem. 2021, 2021, 40–46. [Google Scholar] [CrossRef]
- Sukirno, I.; Farhandika, L. Synthesis and characterization of polyisobutylene-amine from polyisobutylene and tetraethylenepentamine and its performance as deposit control additive in one cylinder gasoline engine. AIP Conf. Proc. 2020, 2255, 060016. [Google Scholar] [CrossRef]
- Pinchuk, L. The use of polyisobutylene-based polymers in ophthalmology. Bioact. Mater. 2022, 10, 185–194. [Google Scholar] [CrossRef]
- Deodhar, T.; Nugay, N.; Nugay, T.; Patel, R.; Moggridge, G.; Kennedy, J.P. Synthesis of High-Molecular-Weight and Strength Polyisobutylene-Based Polyurethane and Its Use for the Development of a Synthetic Heart Valve. Macromol. Rapid Commun. 2023, 44, 2200147. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, K.A.; Chen, X.; Jolly, M.; Lyu, S.P.; Thor, P.L.; Untereker, D.F.; Wang, Z. Modified Polyisobutylene-Based Polymers, Methods of Making, and Medical Devices. US Patent 11548974, 10 January 2023. [Google Scholar]
- Shen, D.; Shi, B.; Zhou, P.; Li, D.; Wang, G. Temperature-Dependent Ring-Opening Polymerization-Induced Self-Assembly Using Crystallizable Polylactones as Core-Forming Blocks. Macromolecules 2023, 56, 4814–4822. [Google Scholar] [CrossRef]
- Dey, A.; Mete, S.; Banerjee, S.; Haldar, U.; Rajasekhar, T.; Srikanth, K.; Faust, R.; De, P. Crystallinity of side-chain fatty acid containing block copolymers with polyisobutylene segment. Eur. Polym. J. 2023, 187, 111879. [Google Scholar] [CrossRef]
- Dey, A.; Haldar, U.; Tota, R.; Faust, R.; De, P. PIB-based block copolymer with a segment having alternating sequence of leucine and alanine side-chain pendants. J. Macromol. Sci. A Pure Appl. Chem. 2023, 60, 161–170. [Google Scholar] [CrossRef]
- Dey, A.; Haldar, U.; Rajasekhar, T.; Faust, R.; De, P. Charge variable PIB-based block copolymers as selective transmembrane ion transporters. Polym. Chem. 2023, 14, 2581–2587. [Google Scholar] [CrossRef]
- Dey, A.; Haldar, U.; Rajasekhar, T.; Ghosh, P.; Faust, R.; De, P. Polyisobutylene-based glycopolymers as potent inhibitors for in vitro insulin aggregation. J. Mater. Chem. B 2022, 10, 9446–9456. [Google Scholar] [CrossRef]
- Yamamoto, S.; Matsuda, M.; Lin, C.-Y.; Enomoto, K.; Lin, Y.-C.; Chen, W.-C.; Higashihara, T. Intrinsically Stretchable Block Copolymer Composed of Polyisobutene and Naphthalenediimide–Bithiophene-Based π-Conjugated Polymer Segments for Field-Effect Transistors. ACS Appl. Polym. Mater. 2022, 4, 8942–8951. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, Z.; Wang, L.; Zhang, X.; Fu, Y.; Xu, G.; Liu, G. Synthesis and Performance of a Series of Polyisobutylene-Substituted Succinic Acid Ester Dispersants for Reducing Thermal Oxidation Deposition of Jet Fuel. Energy Fuels 2020, 34, 5634–5640. [Google Scholar] [CrossRef]
- Kummer, R.; Franz, D.; Rath, H.P. Polybutyl- and Polysobutylamines, Their Preparation, and Fuel Compositions Containing These. US Patent 4832702, 23 May 1989. [Google Scholar]
- Garcia Castro, I.; Koschabek, R.; Misske, A.; Fleckenstein, C. Viscosity Index Improver for Lubricants Based on Polyisobutylene Acrylamide Comb Copolymer. WO Patent 2023104579, 15 June 2023. [Google Scholar]
- Garcia Castro, I.; Koschabek, R.; Misske, A.; Fleckenstein, C. Viscosity Index Improver for Lubricants Based on Polyisobutylenephenyl Acrylate Comb Copolymers. WO Patent 2023104584, 15 June 2023. [Google Scholar]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bagrov, V.V.; Churakov, A.V.; Minyaev, M.E.; Kiselev, A.V.; Salakhov, I.I.; Ivchenko, P.V. A competetive way to low-viscosity PAO base stocks via heterocene-catalyzed oligomerization of dec-1-ene. Mol. Catal. 2022, 529, 112542. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bagrov, V.V.; Kiselev, A.V.; Minyaev, M.E.; Samurganova, T.I.; Ivchenko, P.V. Heterocene Catalysts and Reaction Temperature Gradient in Dec-1-ene Oligomerization for the Production of Low Viscosity PAO Base Stocks. Ind. Eng. Chem. Res. 2023, 62, 6347–6353. [Google Scholar] [CrossRef]
- Yin, Y.-N.; Ding, R.-Q.; Ouyang, D.-C.; Zhang, Q.; Zhu, R. Highly chemoselective synthesis of hindered amides via cobalt-catalyzed intermolecular oxidative hydroamidation. Nat. Commun. 2021, 12, 2552. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, T.; Ma, M.; Shi, Z. SOMOphilic Alkynylation of Unreactive Alkenes Enabled by Iron-Catalyzed Hydrogen Atom Transfer. Molecules 2022, 27, 33. [Google Scholar] [CrossRef]
- Nafant’ev, I.E.; Sevostyanova, N.T.; Batashev, S.A.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Ivchenko, P.V. Synthesis of methyl β-alkylcarboxylates by Pd/diphosphine-catalyzed methoxycarbonylation of methylenealkanes RCH2CH2C(R)=CH2. Appl. Catal. A Gen. 2019, 581, 123–132. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Sang, Y.-Q.; Wang, C.-Q.; Dai, P.; Xue, X.-S.; Piper, J.L.; Peng, Z.-H.; Ma, J.-A.; Zhang, F.-G.; Wu, J. Difluoromethylation of Unactivated Alkenes Using Freon-22 through Tertiary Amine-Borane-Triggered Halogen Atom Transfer. J. Am. Chem. Soc. 2022, 144, 14288–14296. [Google Scholar] [CrossRef] [PubMed]
- Frye, N.L.; Daniliuc, C.G.; Studer, A. Radical 1-Fluorosulfonyl-2-alkynylation of Unactivated Alkenes. Angew. Chem. Int. Ed. 2022, 61, e202115593. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Guan, R.; Shanmugam, M.; Bennett, E.L.; Robertson, C.M.; Brookfield, A.; McInnes, E.J.L.; Xiao, J. Oxidative Cleavage of Alkenes by O2 with a Non-Heme Manganese Catalyst. J. Am. Chem. Soc. 2021, 143, 10005–10013. [Google Scholar] [CrossRef]

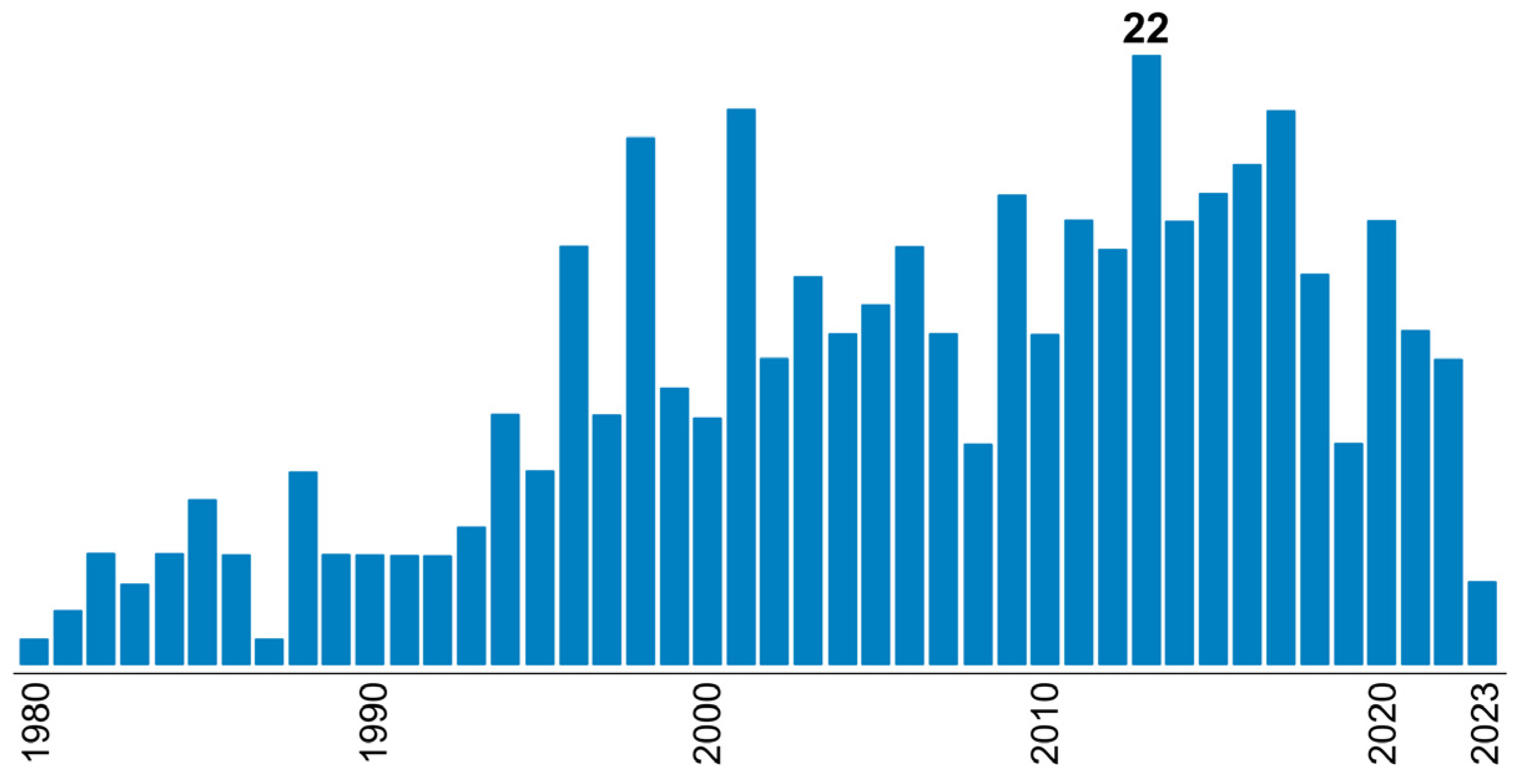
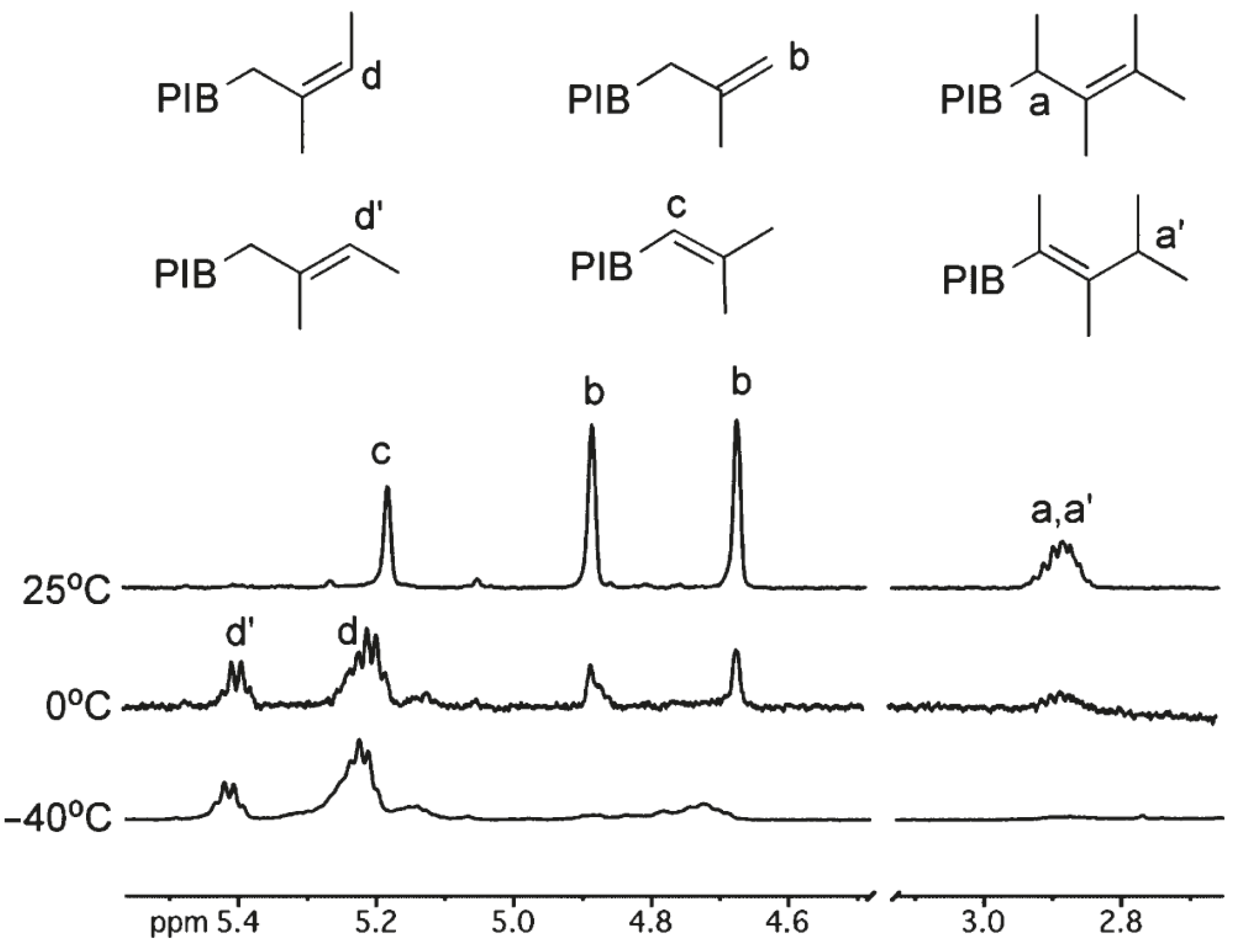


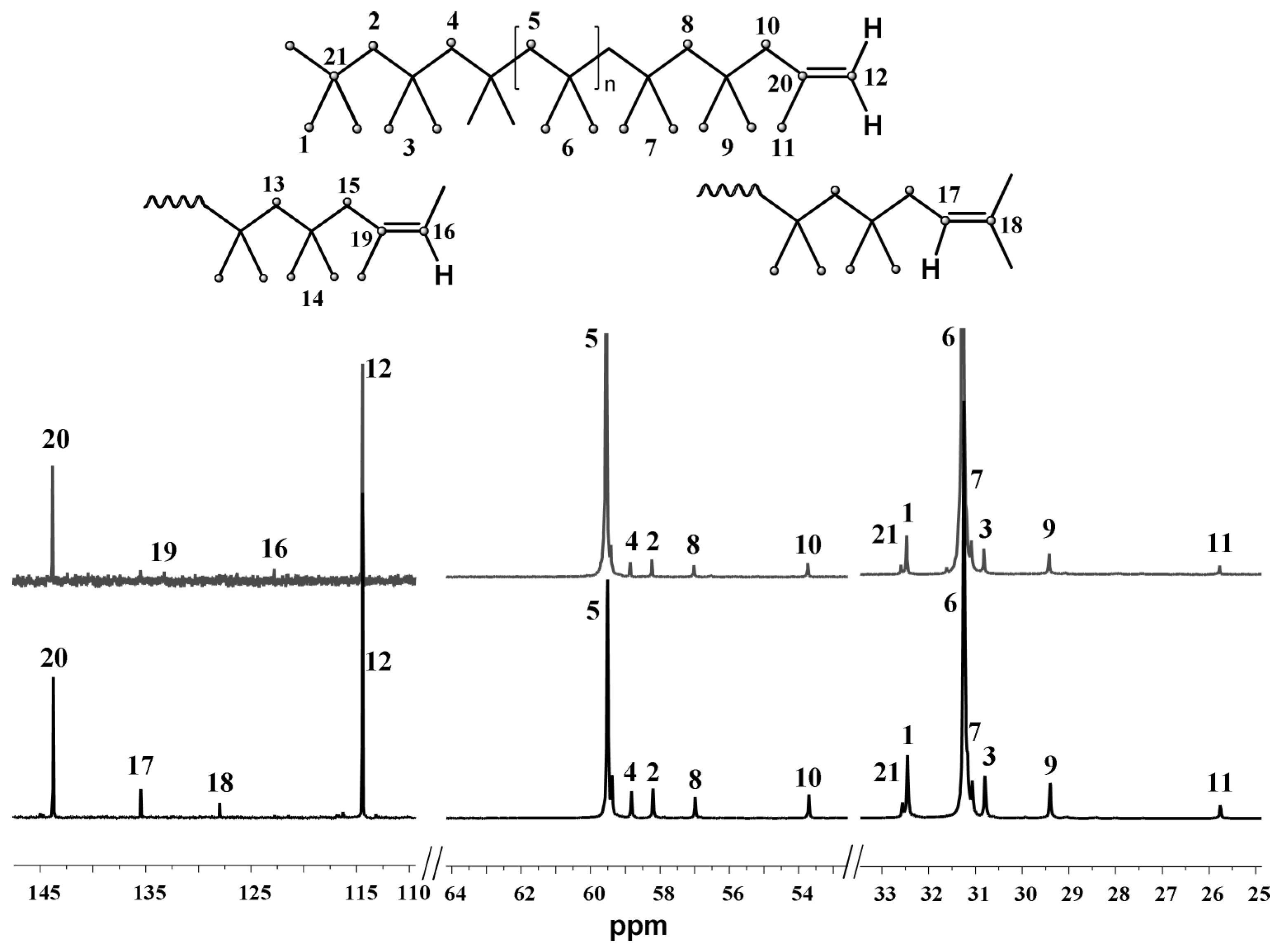
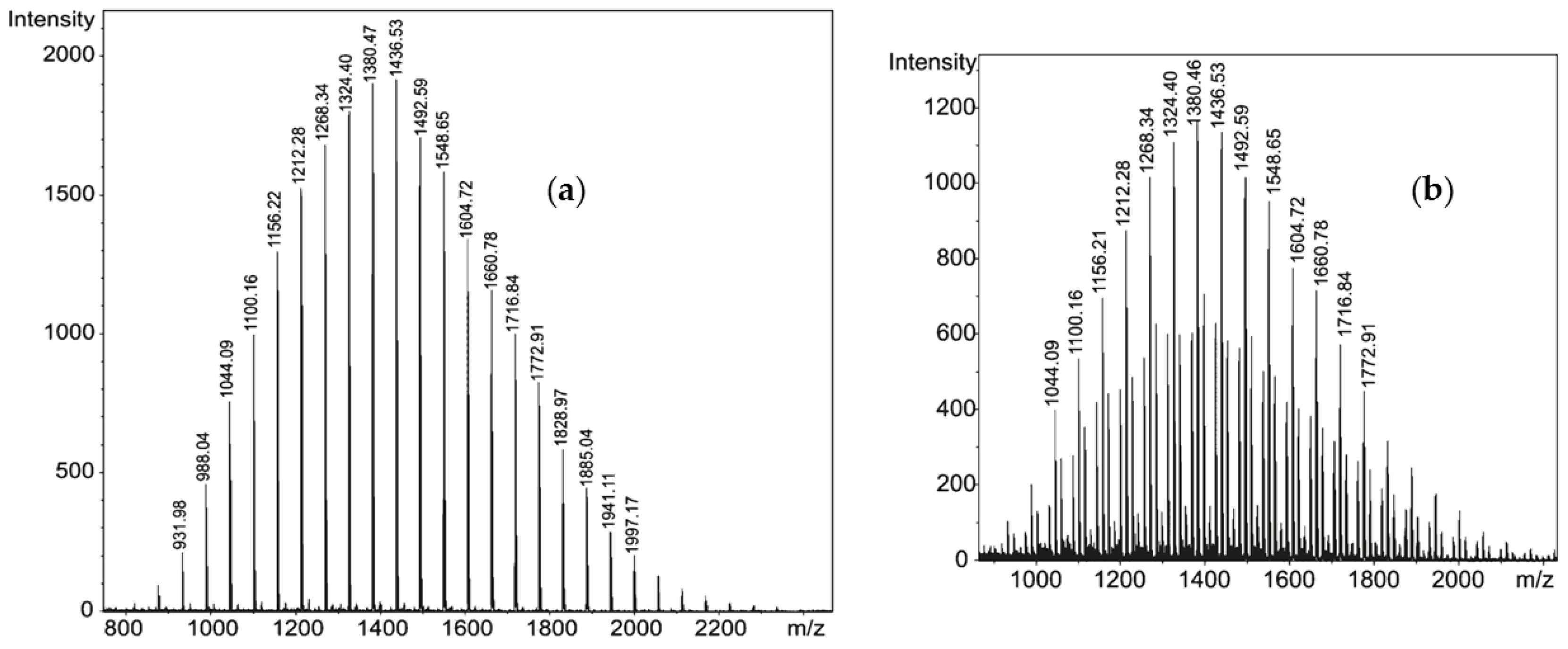








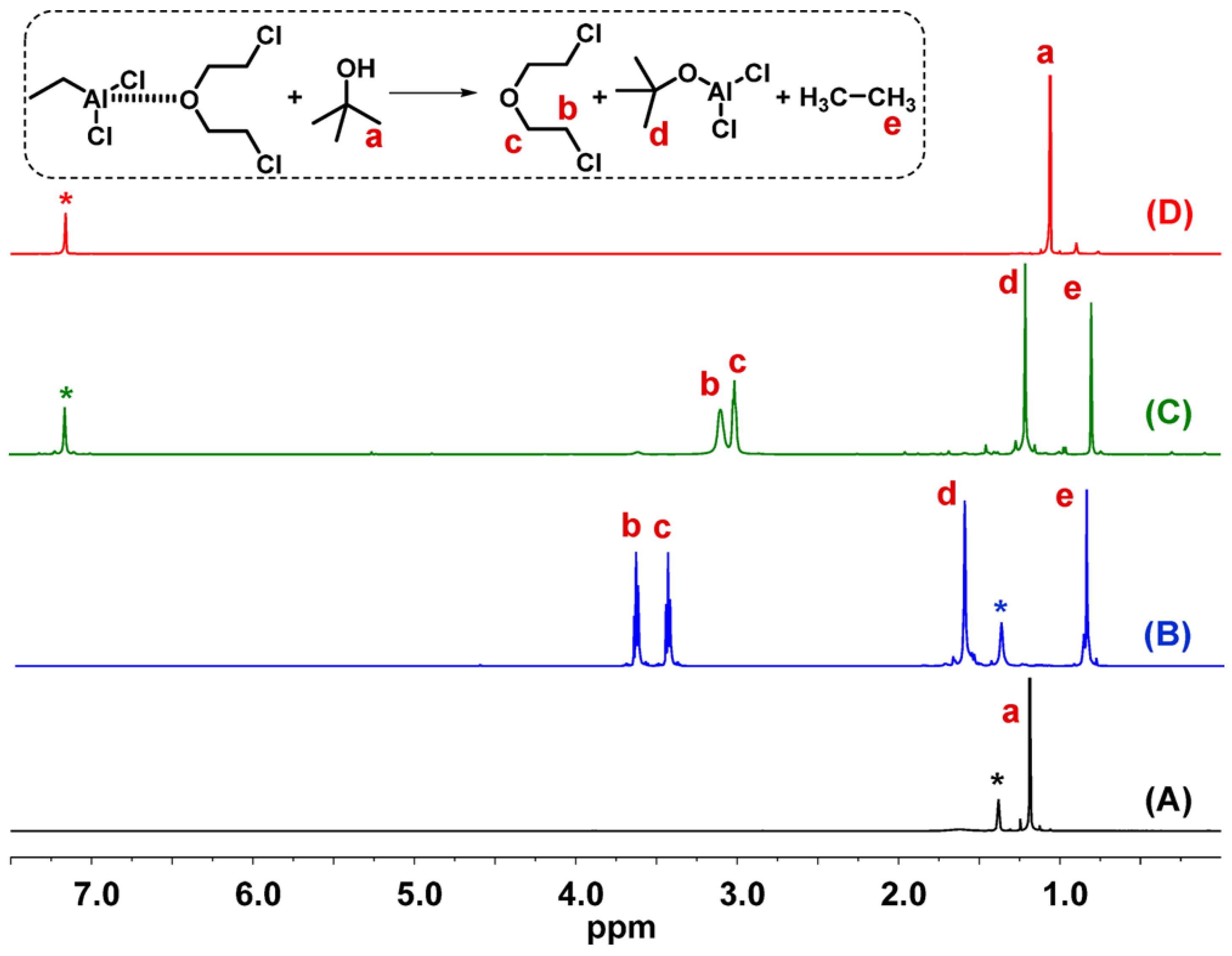

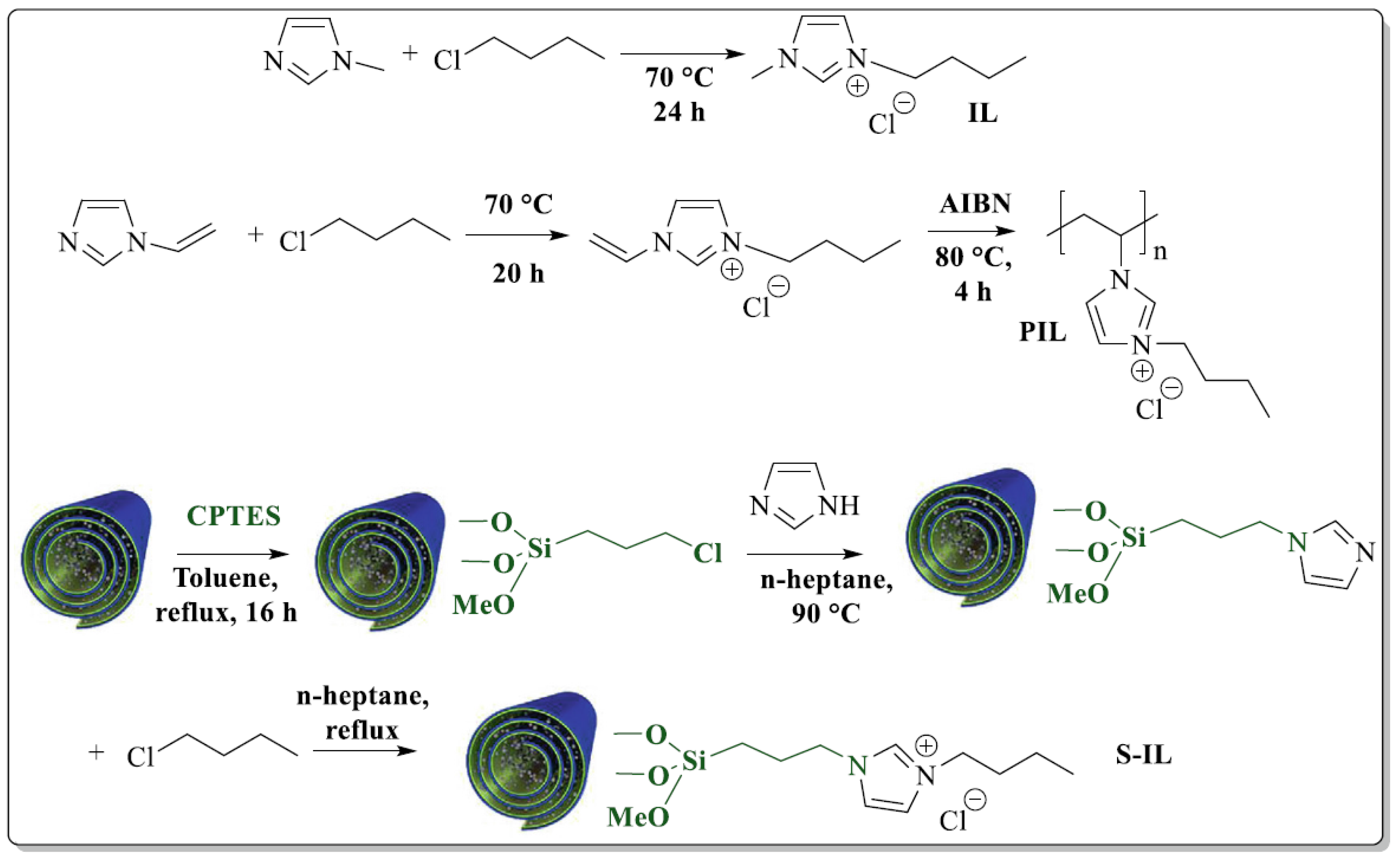
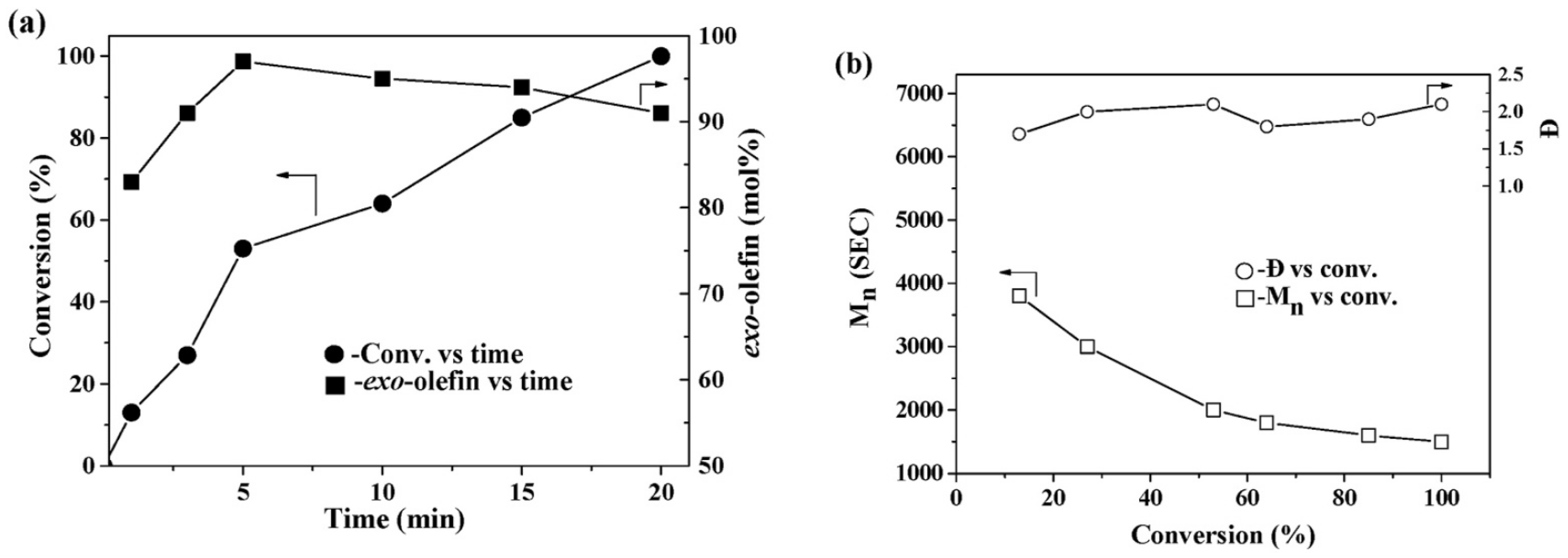

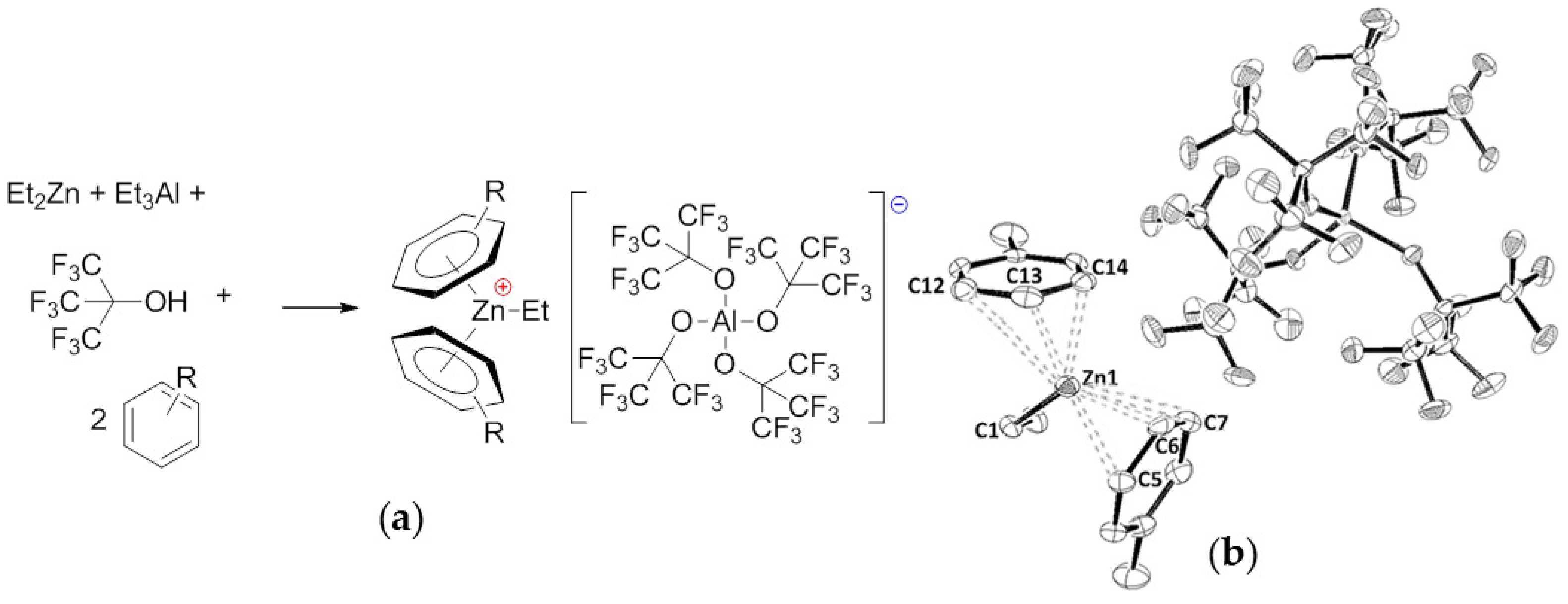


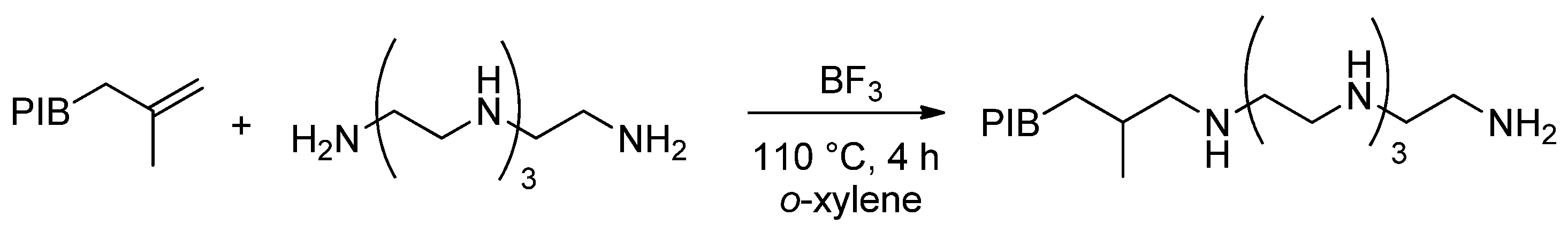
| T, °C | Olefin End-Group Distribution, % | |||
|---|---|---|---|---|
| tri-Substituted | exo- | endo- | tetra-Substituted | |
| −40 | 77 | 8 | 4 | 11 |
| 0 | 61 | 18 | 9 | 12 |
| 20 | 0 | 40 | 20 | 40 |
| Entry | [AlCl3], M | [iPr2O], M | [Bu2O], M | Conv., % 1 | Mn, kDa 2 | exo-, % |
|---|---|---|---|---|---|---|
| 1 | 0.01 | 0.01 | – | 98 | 1.3 | 56 |
| 2 | 0.01 | – | 0.01 | 28 | 2.8 | 77 |
| 3 | 0.01 | 0.005 | 0.005 | 98 | 1.5 | 78 |
| 4 | 0.01 | 0.0025 | 0.0075 | 97 | 1.5 | 66 |
| 5 | 0.01 | 0.0075 | 0.0025 | 62 | 2.1 | 79 |
| 6 | 0.01 | 0.001 | 0.009 | 30 | 2.8 | 76 |
| 7 | 0.01 | 0.009 | 0.001 | 98 | 1.4 | 58 |
| 8 | 0.01 | 0.006 | 0.006 | 98 | 1.0 | 84 |
| 9 | 0.01 | 0.0075 | 0.0075 | 72 | 0.8 | 85 |
| 10 | 0.01 | 0.01 | 0.01 | 14 | 3.2 | 85 |
| 11 | 0.005 | 0.0025 | 0.0025 | 74 | 2.6 | 77 |
| 12 3 | 0.01 | 0.005 | 0.005 | 98 | 1.4 | 77 |
| 13 4 | 0.01 | 0.005 | 0.005 | 95 | 1.5 | 79 |
| Entry | [AlCl3], M | [EtAlCl2], M | [iPr2O], M | [Bu2O], M | Conv., % 1 | Mn, kDa 2 | exo-, % |
|---|---|---|---|---|---|---|---|
| 1 | 0.01 | – | 0.01 | – | 98 | 1.3 | 56 |
| 2 | – | 0.01 | 0.01 | – | 7 | 4.2 | 82 |
| 3 | 0.005 | 0.005 | 0.01 | – | 98 | 1.4 | 84 |
| 4 | 0.0025 | 0.0075 | 0.01 | – | 85 | 1.8 | 80 |
| 5 | 0.0075 | 0.0025 | 0.01 | – | 98 | 1.4 | 64 |
| 6 | 0.001 | 0.009 | 0.01 | – | 10 | 3.8 | 83 |
| 7 | 0.009 | 0.001 | 0.01 | – | 98 | 1.3 | 55 |
| 8 | 0.01 | 0.01 | 0.02 | – | 98 | 1.0 | 81 |
| 9 | 0.0025 | 0.0025 | 0.005 | – | 70 | 2.8 | 80 |
| 10 | 0.01 | – | – | 0.01 | 28 | 2.8 | 77 |
| 11 | – | 0.01 | – | 0.01 | <5 | 4.2 | 88 |
| 12 | 0.005 | 0.005 | – | 0.01 | 20 | 3.4 | 82 |
| 13 3 | 0.005 | 0.005 | 0.01 | – | 98 | 1.3 | 83 |
| Entry | Init. | Conv., % | MnSEC, kDa | MnNMR, kDa | ÐM | End Group Distribution (mol%) | |||
|---|---|---|---|---|---|---|---|---|---|
| exo- | endo- + tri- | tetra- | PIB-Cl | ||||||
| 1 | HexOAlCl2 | 93 | 8.20 | 6.96 | 2.9 | 58 | 18 | 22 | 2 |
| 2 | BuOAlCl2 | 100 | 5.62 | 5.89 | 3.7 | 46 | 26 | 23 | 5 |
| 3 | iPrOAlCl2 | 99 | 4.27 | 3.71 | 5.4 | 32 | 33 | 35 | 0 |
| 4 | PhOAlCl2 | 84 | 0.50 | 0.43 | 9.4 | 14 | 37 | 48 | 1 |
| 5 | (BuO)0.8AlCl2.2 | 100 | 0.81 | 0.46 | 21.3 | 0 | 66 | 34 | 0 |
| 6 | (BuO)1.2AlCl1.8 | 44 | 18.10 | 15.20 | 2.0 | 71 | 16 | 12 | 1 |
| 7 2 | (BuO)1.2AlCl1.8 | 81 | 12.44 | 13.20 | 2.6 | 83 | 14 | 0 | 3 |
| Entry | T, °C | Ether | Ether/IL, mol | Conv., % | MnSEC, kDa | MnNMR, kDa | ÐM | End-Group Distribution (mol%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| exo- | endo- + tri- | tetra- | PIB-Cl | ||||||||
| 1 | 0 | – | – | 100 | 1.50 | 1.36 | 6.8 | 2 | 74 | 24 | 0 |
| 2 | 0 | iPr2O | 0.5 | 51 | 3.94 | 3.34 | 2.0 | 92 | 4 | 3 | 1 |
| 3 | 0 | Bu2O | 0.5 | 12 | 9.35 | 5.17 | 1.6 | 86 | 6 | 5 | 3 |
| 4 | 0 | tBuOMe | 0.5 | 90 | 2.70 | 1.51 | 4.0 | 3 | 69 | 26 | 2 |
| 5 2 | 0 | iPr2O | 1.0 | 28 | 4.90 | 3.24 | 1.9 | 95 | 2 | 1 | 2 |
| 6 | 0 | iPr2O | 0.4 | 57 | 7.75 | 4.46 | 1.7 | 84 | 6 | 8 | 2 |
| 7 3 | 0 | iPr2O | 0.35 | 62 | 6.50 | 4.51 | 1.9 | 89 | 4 | 4 | 3 |
| 8 | 0 | iPr2O | 0.3 | 71 | 12.90 | 9.57 | 1.8 | 47 | 30 | 19 | 4 |
| 9 | −20 | iPr2O | 0.5 | 54 | 9.20 | 4.63 | 3.3 | 36 | 45 | 17 | 2 |
| 10 | −10 | iPr2O | 0.5 | 13 | 5.76 | 6.52 | 2.9 | 67 | 15 | 15 | 3 |
| 11 | 10 | iPr2O | 0.5 | 59 | 5.60 | 4.65 | 2.0 | 90 | 6 | 3 | 1 |
| 12 4 | 10 | iPr2O | 1.0 | 57 | 3.47 | 2.90 | 2.7 | 69 | 11 | 17 | 3 |
| 13 5,6 | 10 | iPr2O | 0.9 | 55 | 1.40 | 1.06 | 3.6 | 84 | 5 | 7 | <1 |
| Entry | Phenol | Yield, % | MnSEC, 2 kDa | MnNMR, 3 kDa | ÐM | End Group Distribution (mol%) | |||
|---|---|---|---|---|---|---|---|---|---|
| exo- | endo- + tri- | tetra- | Coupled | ||||||
| 1 | 1a | 12.7 | 469 | 269 | 1.59 | 65.4 | 32.0 | 1.5 | 1.1 |
| 2 | 1b | 59.5 | 126 | 205 | 1.63 | 61.4 | 31.0 | 5.5 | 2.1 |
| 3 | 1c | 67.5 | 98 | 166 | 1.70 | 59.5 | 13.2 | 25.9 | 1.4 |
| 4 | 1d | 93.0 | 72 | 169 | 2.37 | 63.1 | 28.5 | 7.1 | 1.3 |
| 5 | 2a | 76.2 | 16 | 49 | 3.06 | 46.3 | 28.5 | 18.4 | 6.8 |
| 6 | 2b | 95.5 | 45 | 114 | 2.52 | 54.6 | 35.9 | 8.3 | 1.2 |
| 7 | 2c | 14.8 | 93 | 175 | 1.87 | 61.1 | 36.9 | 0.6 | 1.4 |
| 8 | 2d | 34.4 | 119 | 192 | 1.61 | 56.2 | 36.4 | 6.7 | 0.7 |
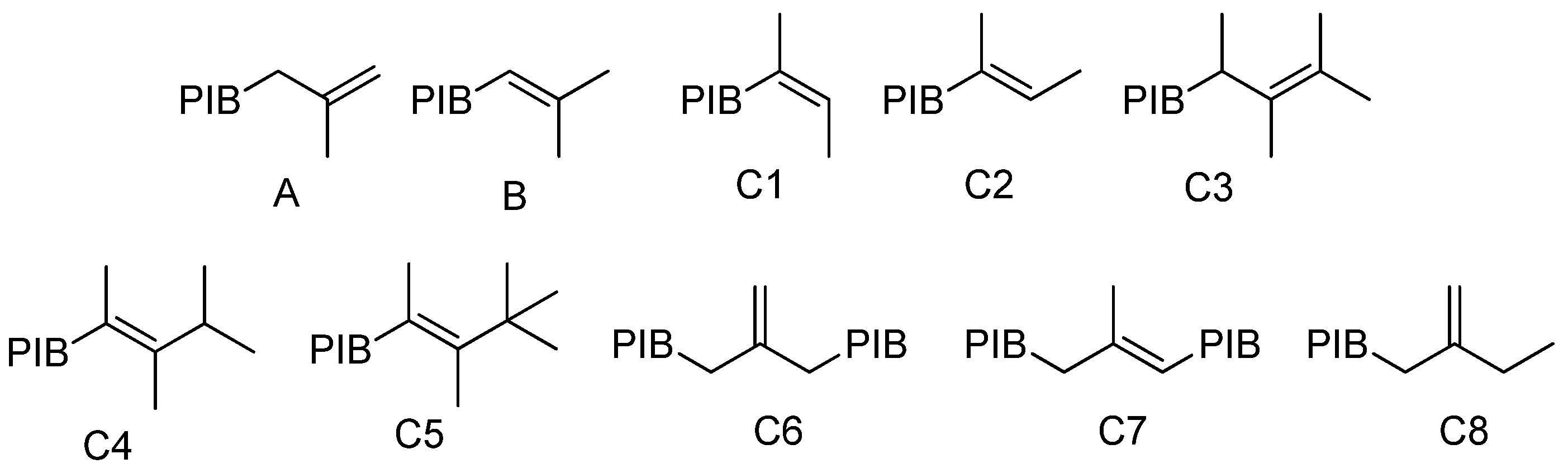
| Reaction Time, h | A, C6, C8 2 | B 2 | C1, C2, B, C7 2 | C3, C4, C5 2 | Mn, kDa |
|---|---|---|---|---|---|
| 0 | 87.1 | 8.7 | 11.4 | 1.5 | 1030 |
| 1 | 38.8 | 45.0 | 56.4 | 6.6 | 1100 |
| 2 | 33.3 | 44.8 | 56.8 | 9.9 | 1110 |
| 3 | 31.1 | 42.1 | 56.2 | 12.7 | 1120 |
| 4 | 28.7 | 40.1 | 54.7 | 16.6 | 1150 |
| 5 | 26.8 | 37.8 | 52.6 | 20.6 | 1080 |
| 6 | 25.0 | 34.6 | 51.0 | 24.0 | 1050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.E.; Korchagina, S.A.; Chinova, M.S.; Tavtorkin, A.N. Polyisobutylenes with Controlled Molecular Weight and Chain-End Structure: Synthesis and Actual Applications. Polymers 2023, 15, 3415. https://doi.org/10.3390/polym15163415
Nifant’ev IE, Korchagina SA, Chinova MS, Tavtorkin AN. Polyisobutylenes with Controlled Molecular Weight and Chain-End Structure: Synthesis and Actual Applications. Polymers. 2023; 15(16):3415. https://doi.org/10.3390/polym15163415
Chicago/Turabian StyleNifant’ev, Ilya E., Sofia A. Korchagina, Maria S. Chinova, and Alexander N. Tavtorkin. 2023. "Polyisobutylenes with Controlled Molecular Weight and Chain-End Structure: Synthesis and Actual Applications" Polymers 15, no. 16: 3415. https://doi.org/10.3390/polym15163415
APA StyleNifant’ev, I. E., Korchagina, S. A., Chinova, M. S., & Tavtorkin, A. N. (2023). Polyisobutylenes with Controlled Molecular Weight and Chain-End Structure: Synthesis and Actual Applications. Polymers, 15(16), 3415. https://doi.org/10.3390/polym15163415







