Preparation and Characterization of Dopamine-Modified Carbon Fiber Paper Composites for Gas Diffusion Layers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of PD-Modified CFs
2.3. Preparation of the Modified CF Paper
2.4. Characterization
3. Results and Discussion
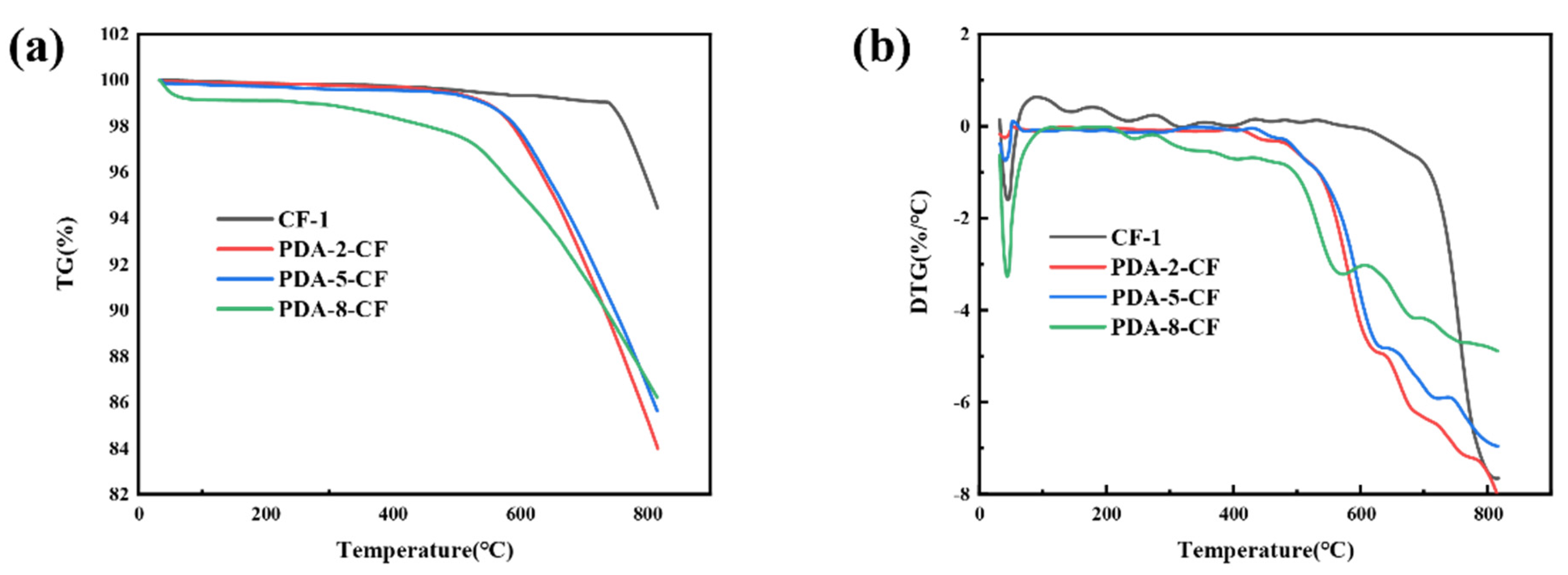
3.1. Surface Analysis of CF
3.2. Dispersibility of CFs
3.3. Performance Testing of CF Paper
3.3.1. Homogeneity of the CF Paper
3.3.2. Tightness and Tensile Strength Performance of the CF Paper
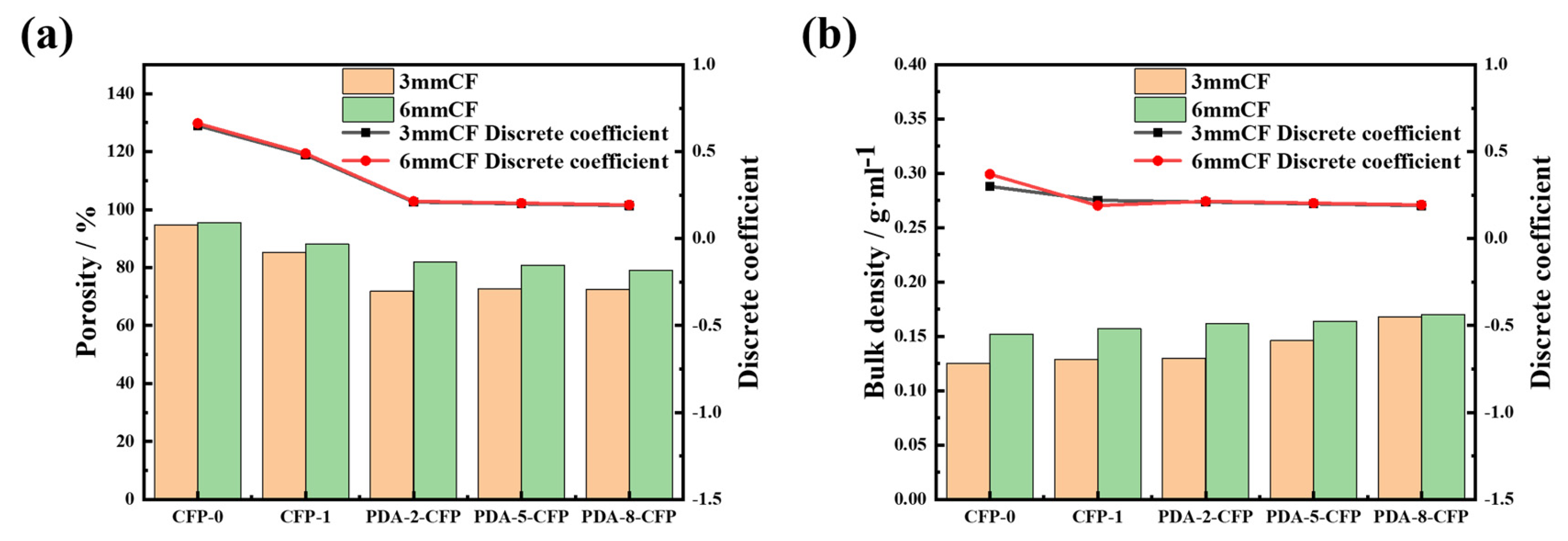
3.3.3. Porosity and Bulk Density of the CF Paper
3.3.4. Contingency and Stresses of the CF Paper
3.3.5. Resistivity and Permeability of the CF Paper
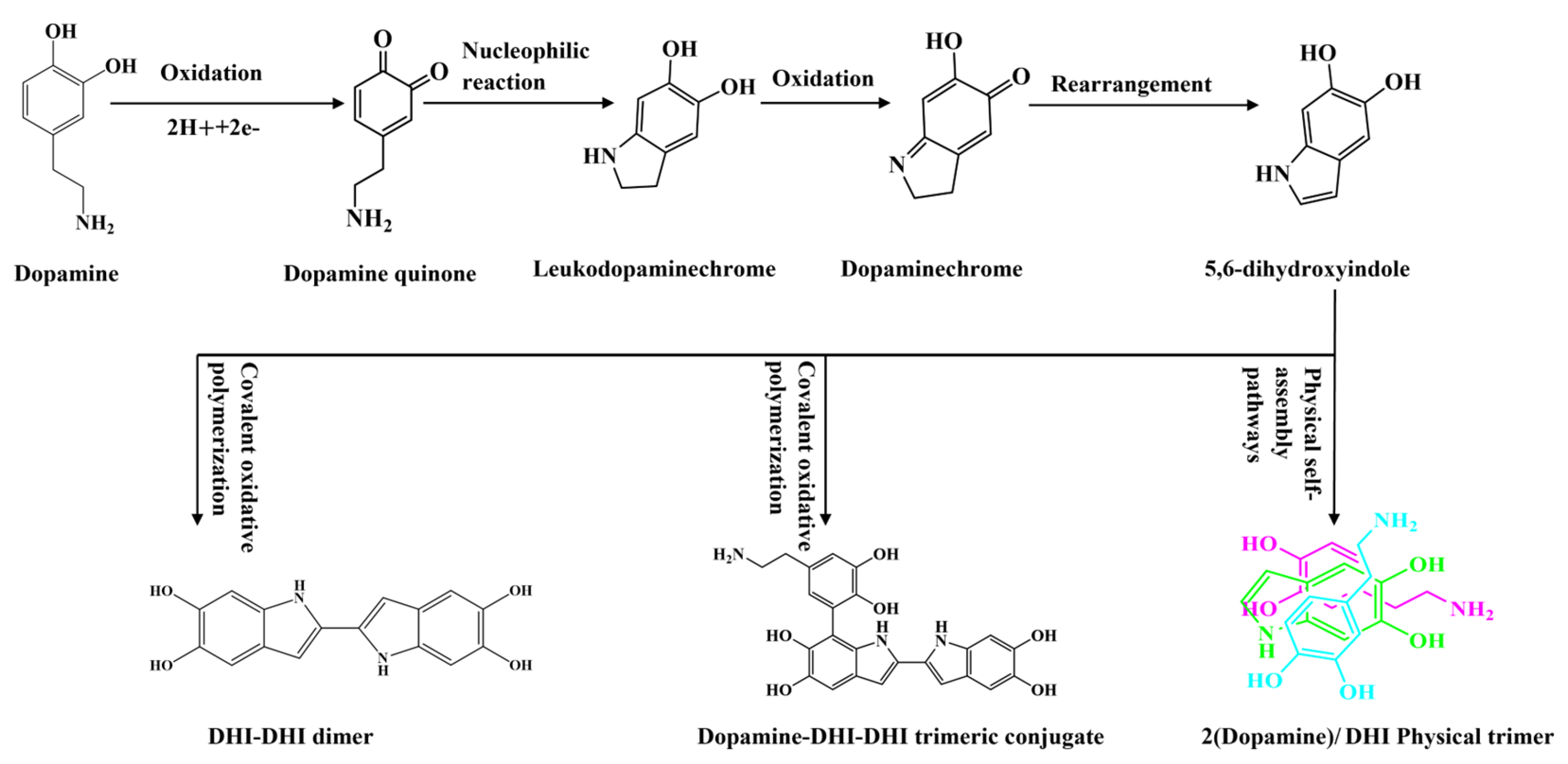
3.4. Mechanistic Analysis of Dopamine-Modified Carbon Fiber
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kallem, P.; Yanar, N.; Choi, H. Nanofiber-Based Proton Exchange Membranes: Development of Aligned Electrospun Nanofibers for Polymer Electrolyte Fuel Cell Applications. ACS Sustain. Chem. Eng. 2019, 7, 1808–1825. [Google Scholar] [CrossRef]
- Liu, C.Y.; Sung, C.C. A review of the performance and analysis of proton exchange membrane fuel cell membrane electrode assemblies. J. Power Sources 2012, 220, 348–353. [Google Scholar] [CrossRef]
- Ye, D.H.; Zhan, Z.G. A review on the sealing structures of membrane electrode assembly of proton exchange membrane fuel cells. J. Power Sources 2013, 231, 285–292. [Google Scholar] [CrossRef]
- Liang, P.; Qiu, D.K.; Peng, L.F.; Yi, P.Y.; Lai, X.M.; Ni, J. Structure failure of the sealing in the assembly process for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 10217–10227. [Google Scholar] [CrossRef]
- Sadeghifar, H. In-plane and through-plane local and average Nusselt numbers in fibrous porous materials with different fiber layer temperatures: Gas diffusion layers for fuel cells. J. Power Sources 2016, 325, 311–321. [Google Scholar] [CrossRef]
- Yang, W.F.; Zhu, L.J.; Wang, S.L.; Yin, Z.Q.; Xiao, L.S.; Shao, Q.S.; Jung, J.C.Y.; Sui, P.C. Investigation of fabrication of gas diffusion substrate for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2022, 47, 35423–35436. [Google Scholar] [CrossRef]
- Corujeira-Gallo, S.; Dong, H.S. Effect of microstructure on the plasma surface treatment of carbon fibres. J. Compos. Mater. 2017, 51, 3239–3256. [Google Scholar] [CrossRef]
- Guo, X.S.; Cheng, Y.X.; Fan, Z.; Feng, Z.H.; He, L.L.; Liu, R.G.; Xu, J. New insights into orientation distribution of high strength polyacrylonitrile-based carbon fibers with skin-core structure. Carbon 2016, 109, 444–452. [Google Scholar] [CrossRef]
- Jiang, J.J.; Yao, X.M.; Xu, C.M.; Su, Y.; Zhou, L.C.; Deng, C. Influence of electrochemical oxidation of carbon fiber on the mechanical properties of carbon fiber/graphene oxide/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2017, 95, 248–256. [Google Scholar] [CrossRef]
- Kruppke, I.; Scheffler, C.; Simon, F.; Hund, R.D.; Cherif, C. Surface Treatment of Carbon Fibers by Oxy-Fluorination. Materials 2019, 12, 565. [Google Scholar] [CrossRef]
- Xu, Z.W.; Huang, Y.D.; Song, Y.J.; Zhang, C.H.; Li, L. Surface characteristics of rare earth treated carbon fibers and interfacial properties of composites. J. Rare Earths 2007, 25, 462–468. [Google Scholar] [CrossRef]
- Xu, Z.W.; Huang, Y.D.; Zhang, C.H.; Chen, G. Influence of rare earth treatment on interfacial properties of carbon fiber/epoxy composites. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2007, 444, 170–177. [Google Scholar] [CrossRef]
- Moosburger-Will, J.; Lachner, E.; Loffler, M.; Kunzmann, C.; Greisel, M.; Ruhland, K.; Horn, S. Adhesion of carbon fibers to amine hardened epoxy resin: Influence of ammonia plasma functionalization of carbon fibers. Appl. Surf. Sci. 2018, 453, 141–152. [Google Scholar] [CrossRef]
- Zheng, L.B.; Wang, Y.X.; Qin, J.J.; Wang, X.H.; Lu, R.J.; Qu, C.; Wang, C.G. Scalable manufacturing of carbon nanotubes on continuous carbon fibers surface from chemical vapor deposition. Vacuum 2018, 152, 84–90. [Google Scholar] [CrossRef]
- Rohini, R.; Bose, S. Electrodeposited carbon fiber and epoxy based sandwich architectures suppress electromagnetic radiation by absorption. Compos. Part B Eng. 2019, 161, 578–585. [Google Scholar] [CrossRef]
- Naito, K. Effect of Hybrid Surface Modifications on Tensile Properties of Polyacrylonitrile- and Pitch-Based Carbon Fibers. J. Mater. Eng. Perform. 2016, 25, 2074–2083. [Google Scholar] [CrossRef]
- Yu, W.Q.; Zhang, H.B.; Lan, A.; Yang, S.H.; Zhang, J.J.; Wang, H.C.; Zhou, Z.; Zhou, Y.M.; Zhao, J.H.; Jiang, Z.H. Enhanced bioactivity and osteogenic property of carbon fiber reinforced polyetheretherketone composites modified with amino groups. Colloids Surf. B Biointerfaces 2020, 193, 111098. [Google Scholar] [CrossRef]
- Tian, X.L.; Han, S.; Zhuang, Q.X.; Bian, H.G.; Li, S.M.; Zhang, C.Q.; Wang, C.S.; Han, W.W. Surface Modification of Staple Carbon Fiber by Dopamine to Reinforce Natural Latex Composite. Polymers 2020, 12, 988. [Google Scholar] [CrossRef]
- Xing, Y.; Deng, S.Y.; Feng, S.L.; Wang, Q.Q.; Hou, Y.P. Selective oxidation of carbon to enhance both tensile strength and interfacial adhesion of carbon fiber. J. Adhes. 2020, 96, 873–882. [Google Scholar] [CrossRef]
- Huan, X.H.; Shi, K.; Yan, J.Q.; Lin, S.; Li, Y.J.; Jia, X.L.; Yang, X.P. High performance epoxy composites prepared using recycled short carbon fiber with enhanced dispersibility and interfacial bonding through polydopamine surface-modification. Compos. Part B Eng. 2020, 193, 107987. [Google Scholar] [CrossRef]
- Chen, T.P.; Liu, T.C.; Su, T.L.; Liang, J.F. Self-Polymerization of Dopamine in Acidic Environments without Oxygen. Langmuir 2017, 33, 5863–5871. [Google Scholar] [CrossRef] [PubMed]
- Della Vecchia, N.F.; Luchini, A.; Napolitano, A.; D’Errico, G.; Vitiello, G.; Szekely, N.; d’Ischia, M.; Paduano, L. Tris Buffer Modulates Polydopamine Growth, Aggregation, and Paramagnetic Properties. Langmuir 2014, 30, 9811–9818. [Google Scholar] [CrossRef]
- Szabo, L.; Imanishi, S.; Hirose, D.; Tsukegi, T.; Wada, N.; Takahashi, K. Mussel-Inspired Design of a Carbon Fiber-Cellulosic Polymer Interface toward Engineered Biobased Carbon Fiber-Reinforced Composites. ACS Omega 2020, 5, 27072–27082. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, C.; Venkatesh, K.; Hsu, L.F.; Arunachalam, P.; Yang, C.C.; Ramaraj, S.K.; Ramalingam, R.J.; Arokiyaraj, S.; Al-Lohedan, H.A. An improving aqueous dispersion of polydopamine functionalized vapor grown carbon fiber for the effective sensing electrode fabrication to chloramphenicol drug detection in food samples. Microchem. J. 2021, 170, 106675. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.C.; Qian, J.H.; Liu, Z.Z.; Yang, B.; Wang, X.L. Bio-inspired polydopamine functionalization of carbon fiber for improving the interfacial adhesion of polypropylene composites. RSC Adv. 2015, 5, 107652–107661. [Google Scholar] [CrossRef]
- Wang, T.Y.; Qiblawey, H.; Judd, S.; Benamor, A.; Nasser, M.S.; Mohammadian, A. Fabrication of high flux nanofiltration membrane via hydrogen bonding based co-deposition of polydopamine with poly(vinyl alcohol). J. Membr. Sci. 2018, 552, 222–233. [Google Scholar] [CrossRef]
- Wu, Q.; Razzak, A.; Bai, H.H.; Deng, H.; Ye, Z.Y.; Zhu, J.F. Dopamine concentration-dependent surface modification for gaining carbon fiber composites with enhanced interfacial adhesion. Compos. Commun. 2022, 29, 101047. [Google Scholar] [CrossRef]
- Chen, Z.H.; Du, K.K.; Yin, H.N.; Zhang, D.Y.; Gao, J.; Song, W.; Zhang, S.B. Surface modification of bamboo fiber with dopamine associated by laccase for poly(3-hydroxylbutyrate) biocomposites. J. Clean. Prod. 2023, 389, 135996. [Google Scholar] [CrossRef]
- Heo, Y.J.; Park, M.; Kang, W.S.; Rhee, K.Y.; Park, S.J. Preparation and characterization of carbon black/pitch-based carbon fiber paper composites for gas diffusion layers. Compos. Part B Eng. 2019, 159, 362–368. [Google Scholar] [CrossRef]
- Perdoch, W.; Cao, Z.R.; Florczak, P.; Markiewicz, R.; Jarek, M.; Olejnik, K.; Mazela, B. Influence of Nanocellulose Structure on Paper Reinforcement. Molecules 2022, 27, 4696. [Google Scholar] [CrossRef]
- Qiu, D.K.; Peng, L.F.; Yi, P.Y.; Lai, X.M. A micro contact model for electrical contact resistance prediction between roughness surface and carbon fiber paper. Int. J. Mech. Sci. 2017, 124, 37–47. [Google Scholar] [CrossRef]
- Zhou, P.P.; Zhu, P.H.; Chen, G.; Liu, Y.; Kuang, Y.D.; Liu, Y.Y.; Fang, Z.Q. A study on the transmission haze and mechanical properties of highly transparent paper with different fiber species. Cellulose 2018, 25, 2051–2061. [Google Scholar] [CrossRef]
- Philips, M.F.; Gruter, G.J.M.; Koper, M.T.M.; Schouten, K.J.P. Production of Gas Diffusion Layers with Tunable Characteristics. ACS Omega 2022, 7, 23041–23049. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.B.; Chen, L.; Liu, M.R.; Fang, T.; Hu, J.; Li, H.L. The quantitative analysis for the formation of carbon fiber paper and its influencing factors. J. Mater. Sci. 2020, 55, 6566–6580. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Jiang, C.; Cho, C.D. An experimental investigation of three-dimensional mechanical characteristics of gas diffusion layers in proton electrolyte membrane fuel cells. J. Solid. State Electrochem. 2019, 23, 2021–2030. [Google Scholar] [CrossRef]
- Xia, L.C.; Ni, M.; He, Q.J.; Xu, Q.D.; Cheng, C. Optimization of gas diffusion layer in high temperature PEMFC with the focuses on thickness and porosity. Appl. Energy 2021, 300, 117357. [Google Scholar] [CrossRef]
- Gao, Y.; Montana, A.; Chen, F.X. Evaluation of porosity and thickness on effective diffusivity in gas diffusion layer. J. Power Sources 2017, 342, 252–265. [Google Scholar] [CrossRef]
- Shi, S.S.; Guo, X.; Chen, B.Z.; Sun, Z. A logarithmic-type constitutive model for carbon fiber papers considering Hertz contact effect. J. Eng. Fiber Fabr. 2019, 14, 1558925019896438. [Google Scholar] [CrossRef]
- Liu, Y.W.; Wu, S.Y.; Qin, Y.Z.; Zhang, M.F.; Liu, X.; Zhang, J.F.; Yin, Y. Mass transport and performance of proton exchange membrane fuel cell considering the influence of porosity distribution of gas diffusion layer. Int. J. Green Energy 2022, 19, 1503–1511. [Google Scholar] [CrossRef]
- Tamayol, A.; Bahrami, M. In-Plane Gas Permeability of Proton Exchange Membrane Fuel Cell Gas Diffusion Layers. In Proceedings of the 3rd Joint US-European Fluids Engineering Division Summer Meeting, Montreal, QC, Canada, 1–5 August 2010; pp. 1241–1248. [Google Scholar]
- Lindsay, B.; Abel, M.L.; Watts, J.F. A study of electrochemically treated PAN based carbon fibres by IGC and XPS. Carbon 2007, 45, 2433–2444. [Google Scholar] [CrossRef]
- Xu, N.; Li, Y.Z.; Zheng, T.; Xiao, L.; Liu, Y.Y.; Chen, S.H.; Zhang, D.X. A mussel-inspired strategy for CNT/carbon fiber reinforced epoxy composite by hierarchical surface modification. Colloid. Surf. A Physicochem. Eng. Asp. 2022, 635, 128085. [Google Scholar] [CrossRef]
- Szabo, L.; Milotskyi, R.; Tsukegi, T.; Wada, N.; Takahashi, K. Quantitative analysis of native reactive functional groups on carbon fiber surface: An electrochemical approach. Appl. Surf. Sci. 2019, 494, 315–325. [Google Scholar] [CrossRef]
- Deng, J.Y.; Xu, L.; Liu, J.H.; Peng, J.H.; Han, Z.H.; Shen, Z.G.; Guo, S.H. Efficient method of recycling carbon fiber from the waste of carbon fiber reinforced polymer composites. Polym. Degrad. Stabil. 2020, 182, 109419. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, B.; Gao, X.P.; Qiao, K.; Zhang, Y.; Wang, B.M.; Fan, J.Y.; Yu, K.; Liu, C.H.; Li, C.S.; et al. Improved the interfacial characteristics of carbon fiber/polyamide 6 composites by synthesizing polydopamine rapidly on the carbon fiber surface with ultrasound-assisted. Compos. Sci. Technol. 2023, 234, 109950. [Google Scholar] [CrossRef]
- Yang, W.W.; Cai, W.Q.; Zhou, J.X.; Dang, C.X.; Peng, X.; Chen, Y.T.; Wei, X.C.; Bo, S.L.; Liang, S.L.; Luo, Z.J. Mussel-inspired MgAl-LDH/carbon fiber film modified by polydopamine for highly efficient removal of Pb2+. J. Environ. Chem. Eng. 2021, 9, 106634. [Google Scholar] [CrossRef]


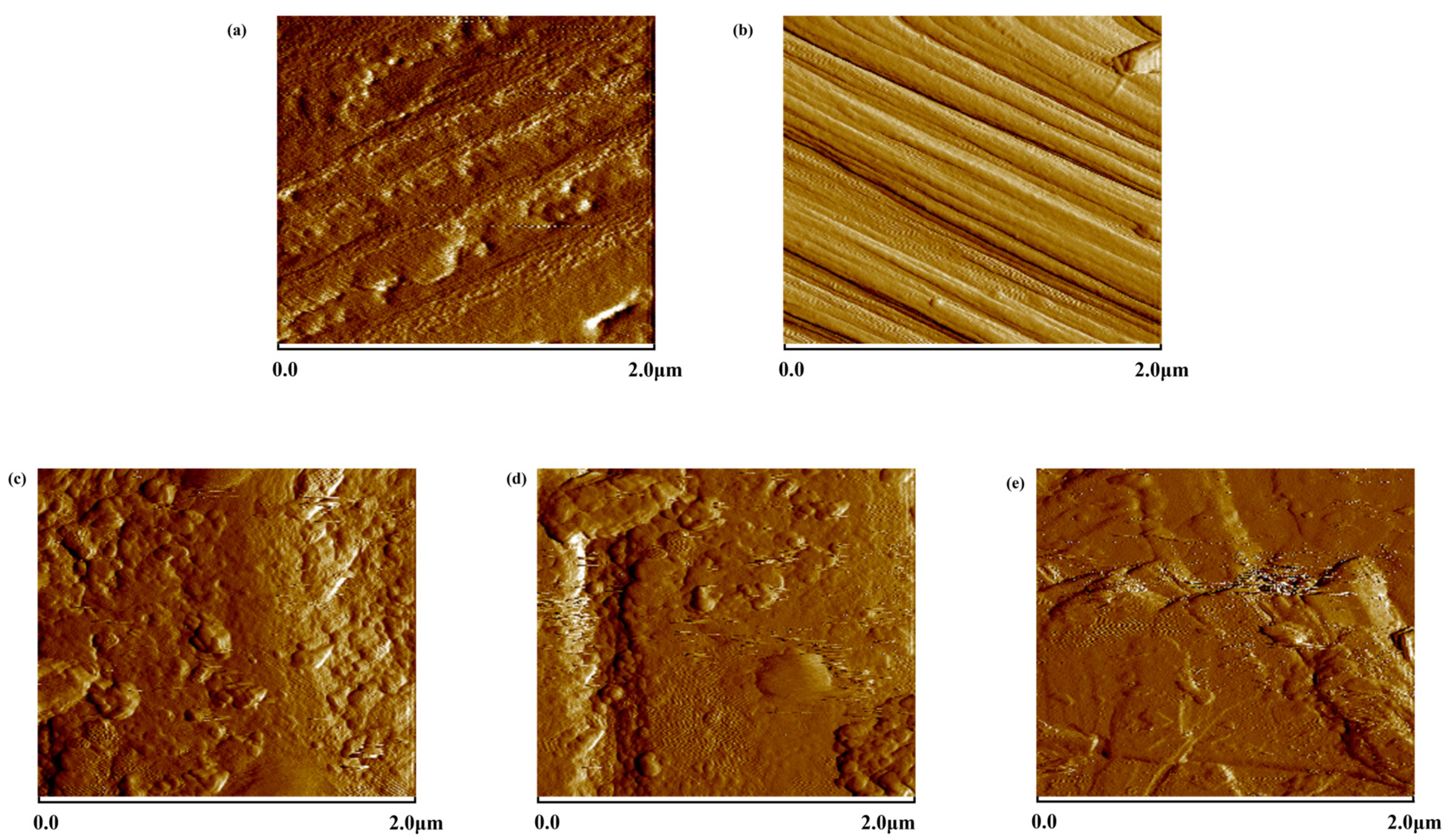
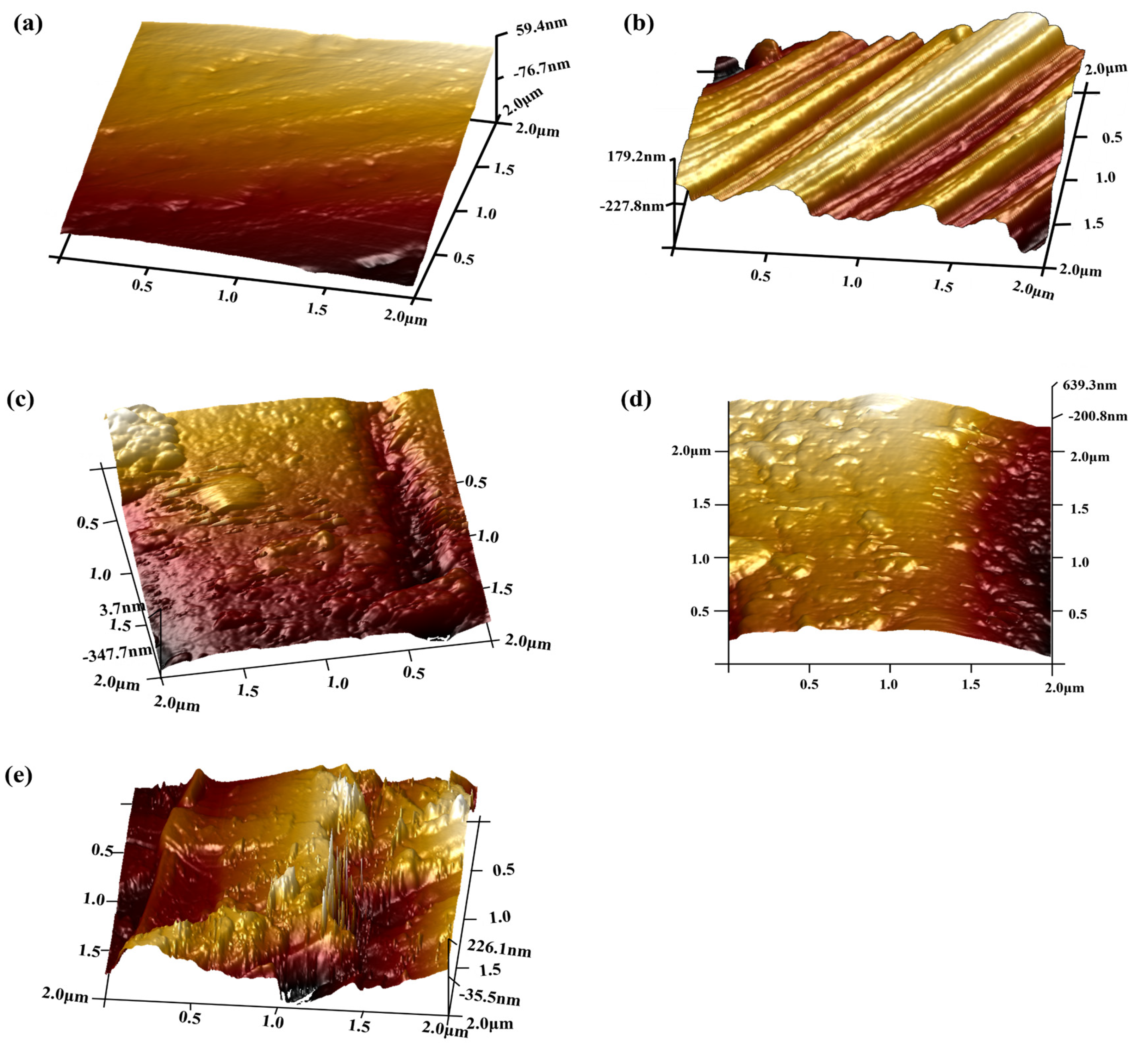
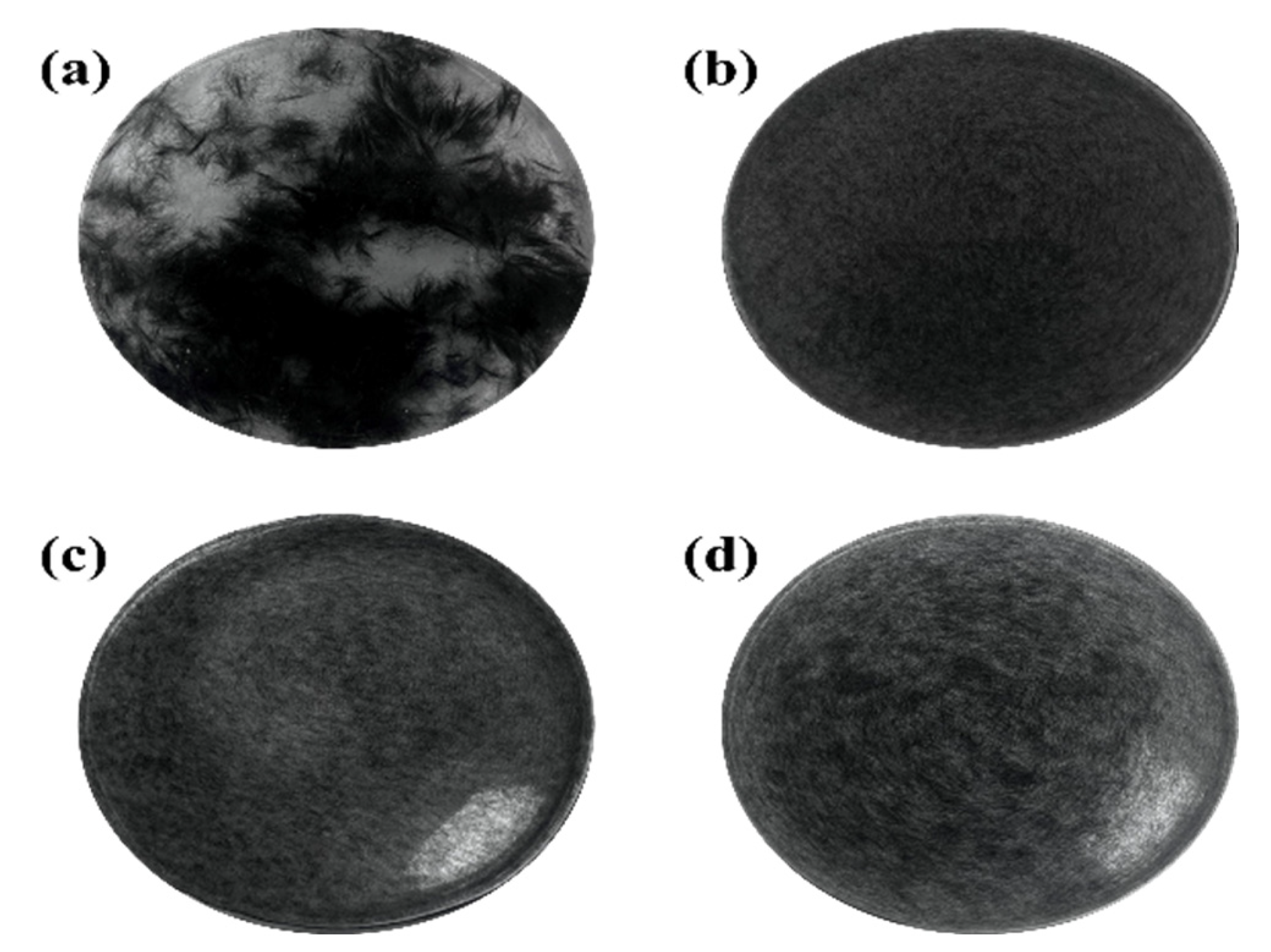







| Sample | Ra | Rq |
|---|---|---|
| CF-0 | 29.8 nm | 37.5 nm |
| CF-1 | 58.8 nm | 70.1 nm |
| PDA-2-CF | 39.5 nm | 53.4 nm |
| PDA-5-CF | 41.2 nm | 54.7 nm |
| PDA-8-CF | 53.9 nm | 68.3 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Chen, X.; Sun, X.; Zhao, C. Preparation and Characterization of Dopamine-Modified Carbon Fiber Paper Composites for Gas Diffusion Layers. Polymers 2023, 15, 3428. https://doi.org/10.3390/polym15163428
Ma J, Chen X, Sun X, Zhao C. Preparation and Characterization of Dopamine-Modified Carbon Fiber Paper Composites for Gas Diffusion Layers. Polymers. 2023; 15(16):3428. https://doi.org/10.3390/polym15163428
Chicago/Turabian StyleMa, Jiahua, Xiangyu Chen, Xiaoshuai Sun, and Chuanshan Zhao. 2023. "Preparation and Characterization of Dopamine-Modified Carbon Fiber Paper Composites for Gas Diffusion Layers" Polymers 15, no. 16: 3428. https://doi.org/10.3390/polym15163428
APA StyleMa, J., Chen, X., Sun, X., & Zhao, C. (2023). Preparation and Characterization of Dopamine-Modified Carbon Fiber Paper Composites for Gas Diffusion Layers. Polymers, 15(16), 3428. https://doi.org/10.3390/polym15163428






