Effect of Fluorosilicone Rubber on Mechanical Properties, Dielectric Breakdown Strength and Hydrophobicity of Methyl Vinyl Silicone Rubber
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation
2.3. Characterization and Measurements
3. Results and Discussion
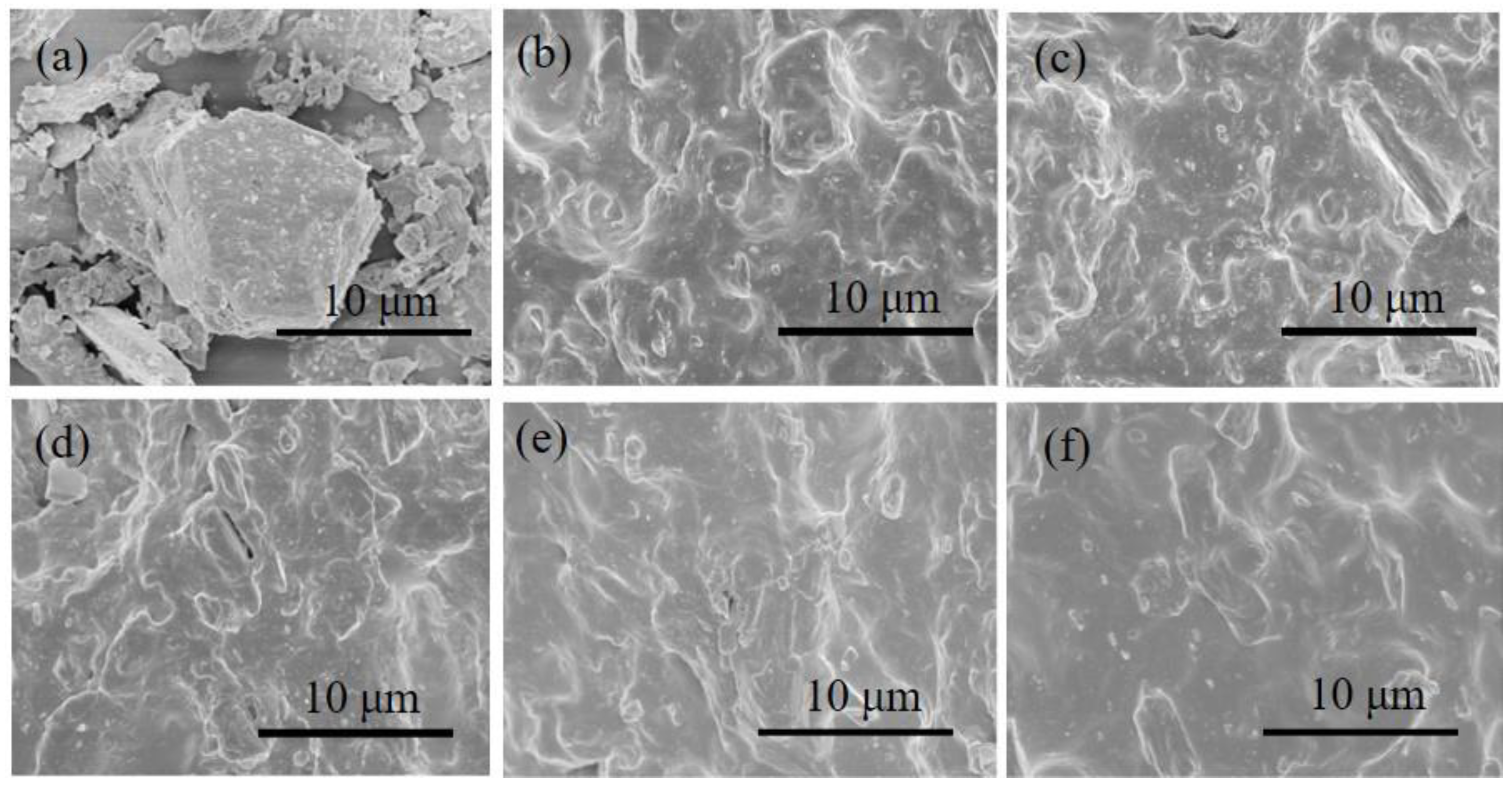
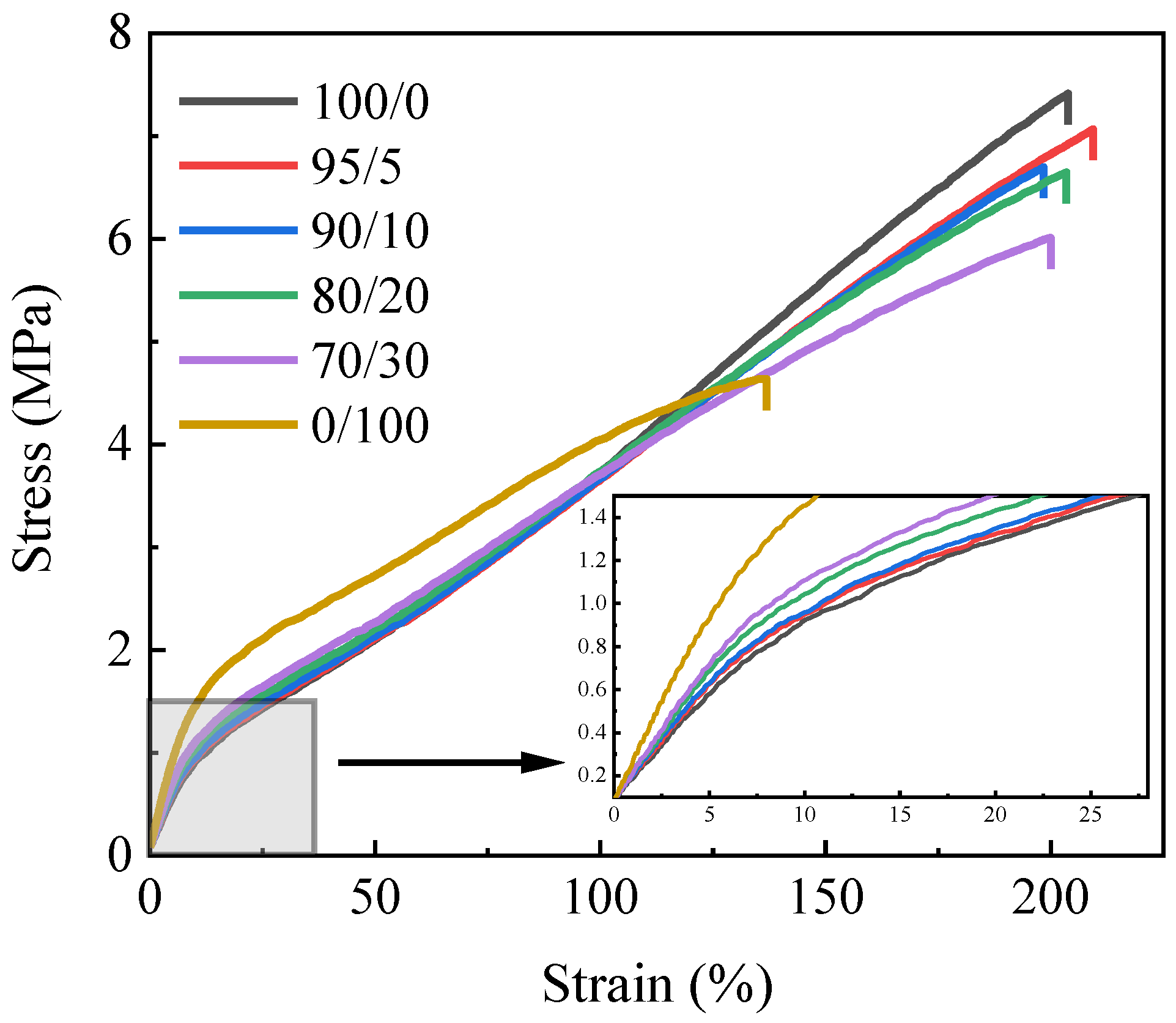
3.1. Mechanical Properties
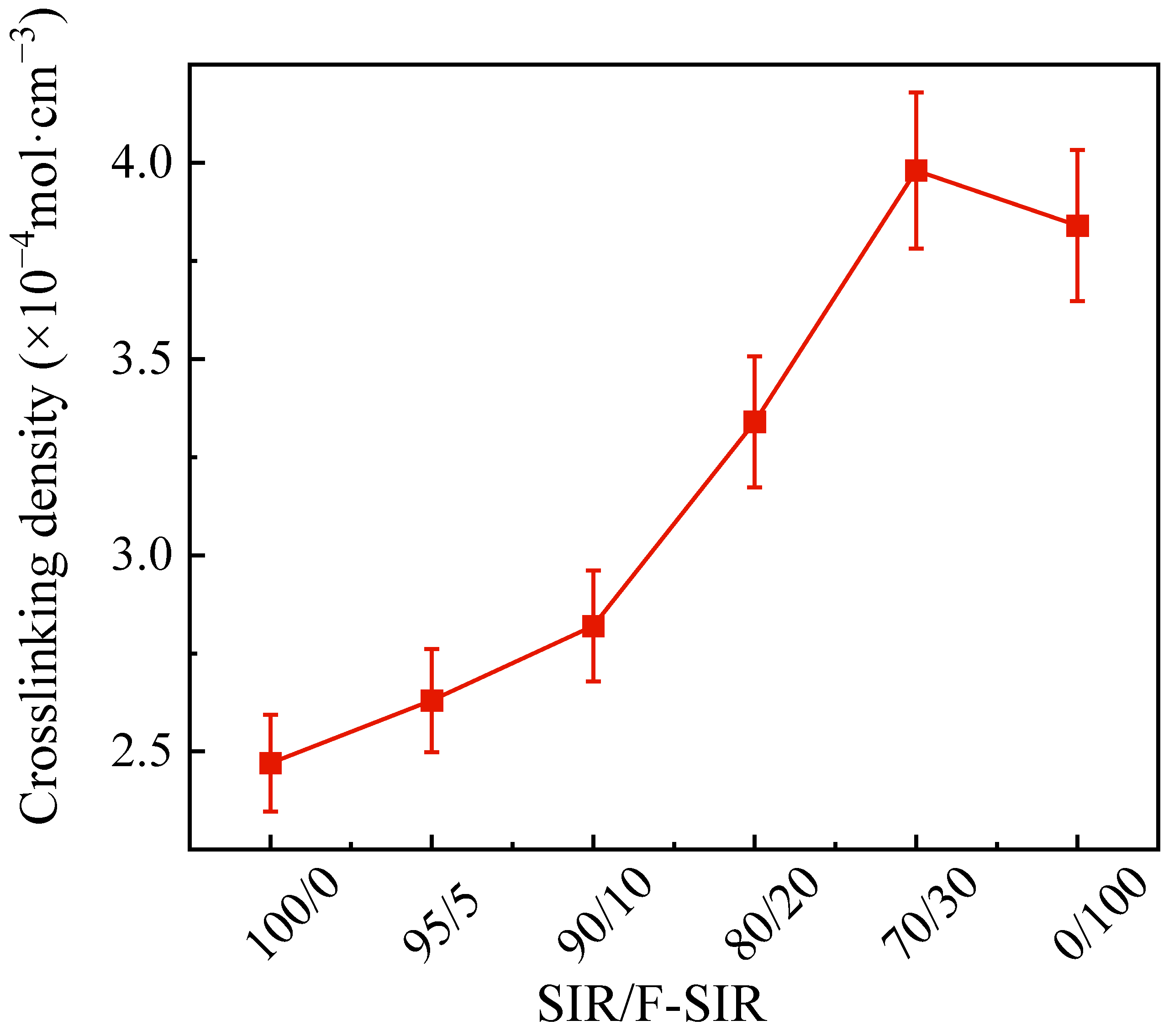
3.2. Crosslinking Density
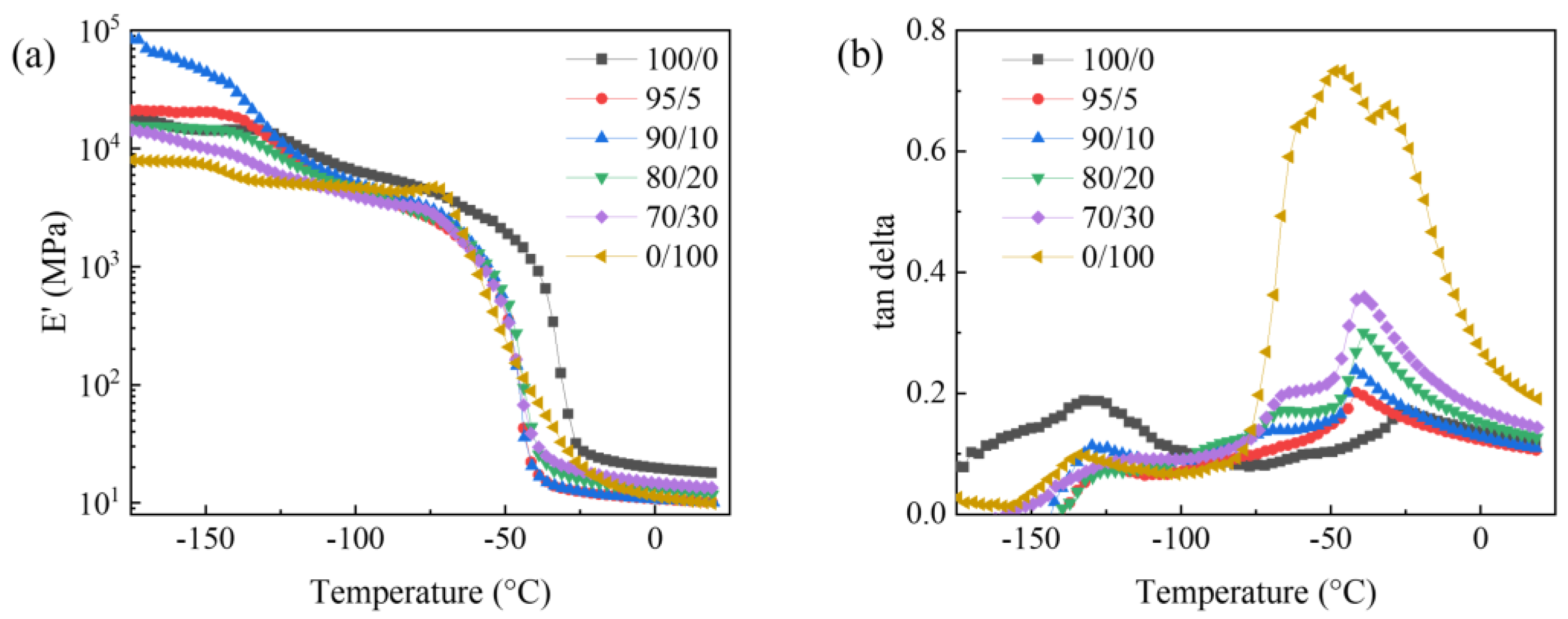
3.3. Dynamic Mechanical Properties
3.4. Breakdown Strength
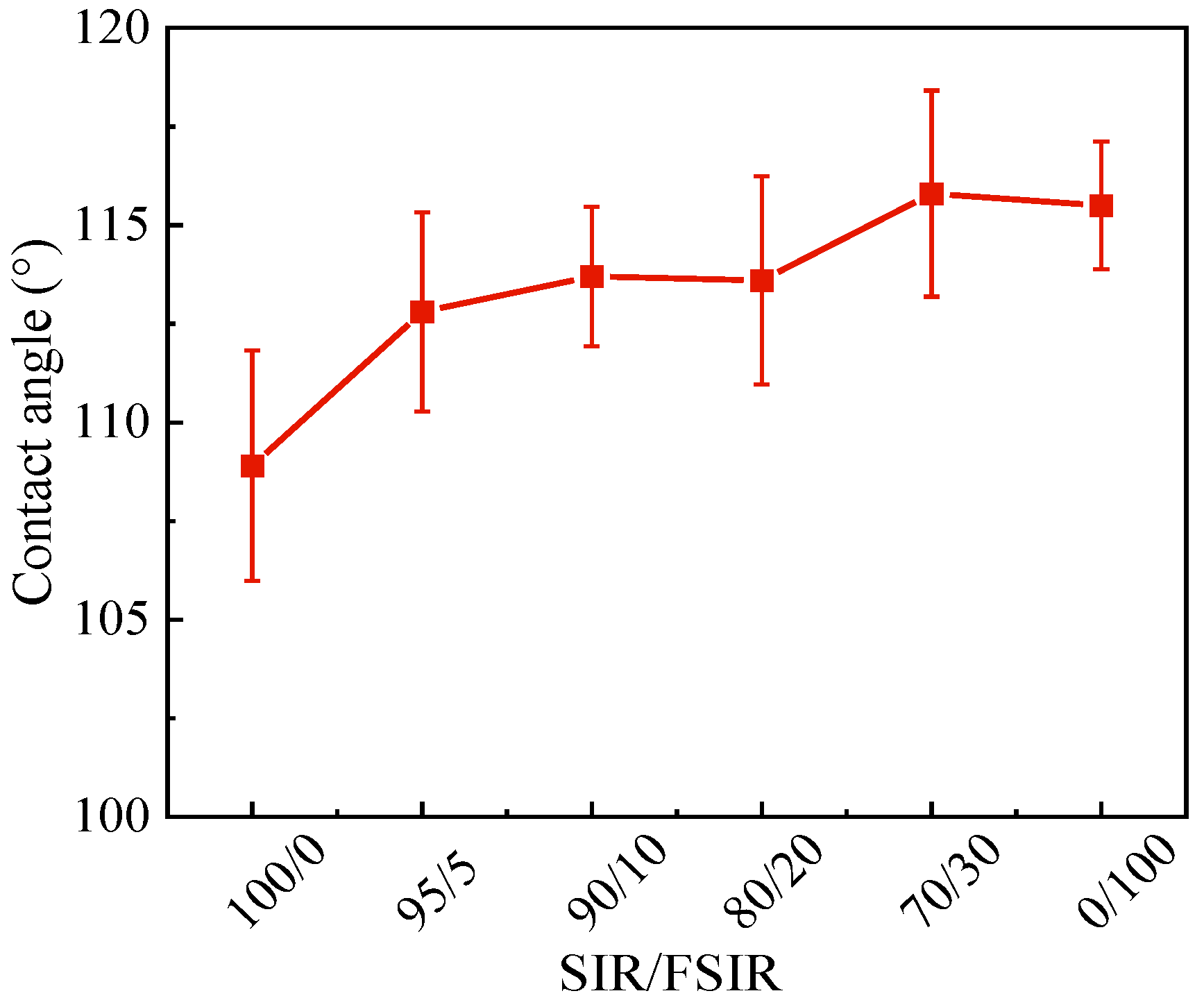
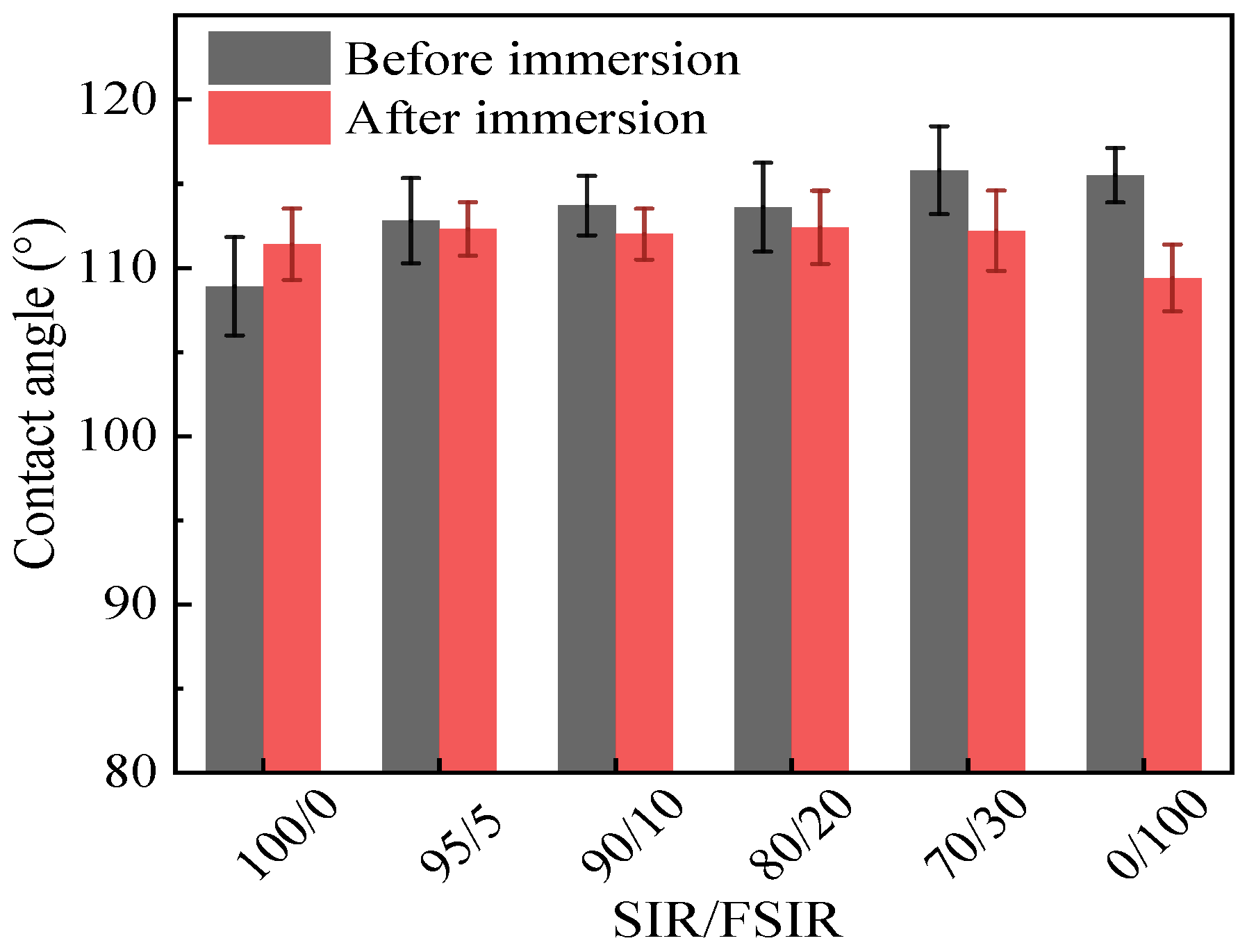
3.5. Static Contact Angle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| SIR | Silicone rubber |
| FSIR | Fluorosilicone rubber |
| HSO | Hydroxy silicone oil |
| ATH | Aluminum hydroxide |
| VTMS | Vinyltrimethoxysilane |
| DBPH | 2,5-dimethyl-2,5-di (tert-butylperoxy) hexane |
| SEM | Scanning electron microscope |
| DMA | Dynamic mechanical properties |
| SCA | Static contact angle |
References
- Liao, Y.; Weng, Y.; Wang, J.; Zhou, H.; Lin, J.; He, S. Silicone Rubber Composites with High Breakdown Strength and Low Dielectric Loss Based on Polydopamine Coated Mica. Polymers 2019, 11, 2030. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Hu, J.; Zhang, C.; Wang, J.; Chen, L.; Bian, X.; Lin, J.; Du, X. Performance improvement in nano-alumina filled silicone rubber composites by using vinyl tri-methoxysilane. Polym. Test. 2018, 67, 295–301. [Google Scholar] [CrossRef]
- Li, S.; Yu, S.; Feng, Y. Progress in and prospects for electrical insulating materials. High Volt. 2016, 1, 122–129. [Google Scholar] [CrossRef]
- Hall, J.F. History and bibliography of polymeric insulators for outdoor applications. IEEE Trans. Power Deliv. 1993, 8, 376–385. [Google Scholar] [CrossRef]
- Hillborgl, H.; Gedde, U.W. Hydrophobicity changes in silicone rubbers. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 703–717. [Google Scholar] [CrossRef]
- Polmanteer, K.E. Current perspectives on silicone rubber technology. Rubber Chem. Technol. 1981, 54, 1051–1080. [Google Scholar] [CrossRef]
- Reynders, J.P.; Jandrell, I.R.; Reynders, S.M. Review of Aging and Recovery of Silicone Rubber Insulation for Outdoor Use. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 620–631. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Hu, J.; Zhou, H.; Nguyen, H.; Luo, C.; Lin, J. Silicone rubber composites incorporating graphitic carbon nitride and modified by vinyl tri-methoxysilane. Polym. Test. 2019, 79, 106005. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Zhang, C.; Yang, W.; Lin, J.; Bian, X.; He, S. Performance of silicone rubber composites using boron nitride to replace alumina tri-hydrate. High Volt. 2020, 6, 480–486. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Farzaneh, M.; Li, Y.; Zhang, J.; Shu, L.; Jiang, X.; Sima, W.; Sun, C. Electrical performance of ice-covered insulators at high altitudes. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 870–880. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Gao, Y. Failure analysis of decay-like fracture of composite insulator. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2503–2511. [Google Scholar] [CrossRef]
- Amin, M.; Akbar, M.; Amin, S. Hydrophobicity of silicone rubber used for outdoor insulation (an overview). Rev. Adv. Mater. Sci. 2007, 16, 10–26. [Google Scholar]
- Chen, Y.; Wang, K.; Zhang, C.; Yang, W.; Qiao, B.; Yin, L. The Effect of Various Fillers on the Properties of Methyl Vinyl Silicone Rubber. Polymers 2023, 15, 1584. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, R.; Wang, S.; Fu, Y. Plasma treatment to improve the hydrophobicity of contaminated silicone rubber—The role of LMW siloxanes. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 416–422. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Huang, B.; Xia, L.; Kong, F.; Zhang, C.; Shao, T. Rapid hydrophobicity recovery of contaminated silicone rubber using low-power microwave plasma in ambient air. Chem. Eng. J. 2023, 465, 142921. [Google Scholar] [CrossRef]
- Zhu, M.; Song, H.; Li, J.; Xue, J.; Yu, Q.; Chen, J.; Zhang, G. Superhydrophobic and high-flashover-strength coating for HVDC insulating system. Chem. Eng. J. 2021, 404, 126476. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B.; Bezdomnikov, A.A.; Chulkova, E.V.; Emelyanenko, K.A. Reinforced Superhydrophobic Coating on Silicone Rubber for Longstanding Anti-Icing Performance in Severe Conditions. ACS Appl. Mater. Interfaces 2017, 9, 24210–24219. [Google Scholar] [CrossRef]
- Mendoza, A.I.; Moriana, R.; Hillborg, H.; Strömberg, E. Super-hydrophobic zinc oxide/silicone rubber nanocomposite surfaces. Surf. Interfaces 2019, 14, 146–157. [Google Scholar] [CrossRef]
- Nazir, M.T.; Khalid, A.; Wang, C.; Baena, J.-C.; Phung, B.T.; Akram, S.; Wong, K.L.; Yeoh, G.H. Enhanced fire retardancy with excellent electrical breakdown voltage, mechanical and hydrophobicity of silicone rubber/aluminium trihydroxide composites by milled glass fibres and graphene nanoplatelets. Surf. Interfaces 2022, 35, 102494. [Google Scholar] [CrossRef]
- Zhu, Y. Influence of corona discharge on hydrophobicity of silicone rubber used for outdoor insulation. Polym. Test. 2019, 74, 14–20. [Google Scholar] [CrossRef]
- Khan, H.; Mahmood, A.; Ullah, I.; Amin, M.; Nazir, M.T. Hydrophobic, dielectric and water immersion performance of 9000 h multi-stresses aged silicone rubber composites for high voltage outdoor insulation. Eng. Fail. Anal. 2021, 122, 105223. [Google Scholar] [CrossRef]
- Sheng, K.; Dong, X.; Chen, Z.; Zhou, Z.; Gu, Y.; Huang, J. Increasing the surface hydrophobicity of silicone rubber by electron beam irradiation in the presence of a glycerol layer. Appl. Surf. Sci. 2022, 591, 153097. [Google Scholar] [CrossRef]
- Du, B.; Li, Z. Hydrophobicity surface charge and DC flashover characteristics of direct-fluorinated RTV silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 934–940. [Google Scholar] [CrossRef]
- Qi, M.; Jia, X.; Wang, G.; Xu, Z.; Zhang, Y.; He, Q. Research on high temperature friction properties of PTFE/Fluorosilicone rubber/silicone rubber. Polym. Test. 2020, 91, 106817. [Google Scholar] [CrossRef]
- Zhao, R.; Yin, Z.; Zou, W.; Yang, H.; Yan, J.; Zheng, W.; Li, H. Preparation of High-Strength and Excellent Compatibility Fluorine/Silicone Rubber Composites under the Synergistic Effect of Fillers. ACS Omega 2023, 8, 3905–3916. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, J.; Chen, P.; Wei, D.; Guan, Y.; Lu, X.; Xiao, H. The effect of ceria nanoparticles on improving heat resistant properties of fluorosilicone rubber. J. Appl. Polym. Sci. 2016, 133, 44117. [Google Scholar] [CrossRef]
- Sun, C.; Shi, K.; Wang, H.; Chen, B.; Liu, J. Reliability Study of Fluorosilicone Rubber Composite Insulators under Strong Radiation and Low Temperature. In Proceedings of the 2023 the 5th Asia Energy and Electrical Engineering Symposium, Chengdu, China, 23–26 March 2023; pp. 367–371. [Google Scholar]
- Wei, Y.; Yang, J.; Li, G.; Zhou, X.; Hao, C.; Lei, Q.; Li, S. Influence of Molecular Chain Side Group on the Electrical Properties of Silicone Rubber and Mechanism Analysis. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1465–1473. [Google Scholar] [CrossRef]
- Metivier, T.; Cassagnau, P. Compatibilization of silicone/fluorosilicone blends by dynamic crosslinking and fumed silica addition. Polymer 2018, 147, 20–29. [Google Scholar] [CrossRef]
- Khanra, S.; Ganguly, D.; Ghorai, S.K.; Goswami, D.; Chattopadhyay, S. The synergistic effect of fluorosilicone and silica towards the compatibilization of silicone rubber and fluoroelastomer based high performance blend. J. Polym. Res. 2020, 27, 96. [Google Scholar] [CrossRef]
- Valentın, J.L.; Carretero-Gonzalez, J.; Mora-Barrantes, I.; Chasse, W.; Saalwachter, K. Uncertainties in the Determination of Cross-Link Density by Equilibrium Swelling Experiments in Natural Rubber. Macromolecules 2008, 41, 4717–4729. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Zheng, J. Effect and mechanism of iron oxide modified carbon nanotubes on thermal oxidative stability of silicone rubber. Compos. Sci. Technol. 2014, 99, 1–7. [Google Scholar] [CrossRef]
- Fabiani, D.; Simoni, L. Discussion on application of the Weibull distribution to electrical breakdown of insulating materials. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 11–16. [Google Scholar] [CrossRef]

| Ingredients | 100/0 | 95/5 | 90/10 | 80/20 | 70/30 | 0/100 |
|---|---|---|---|---|---|---|
| SIR | 100 | 95 | 90 | 80 | 70 | 0 |
| FSIR | 0 | 5 | 10 | 20 | 30 | 100 |
| Silica | 30 | 30 | 30 | 30 | 30 | 30 |
| ATH | 100 | 100 | 100 | 100 | 100 | 100 |
| Fe2O3 | 4 | 4 | 4 | 4 | 4 | 4 |
| HSO | 5 | 5 | 5 | 5 | 5 | 5 |
| VTMS | 2 | 2 | 2 | 2 | 2 | 2 |
| DBPH | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Properties | 100/0 | 95/5 | 90/10 | 80/20 | 70/30 | 0/100 |
|---|---|---|---|---|---|---|
| Shore A hardness | 70 | 71 | 70 | 72 | 74 | 77 |
| Tensile strength (MPa) | 7.3 | 6.9 | 6.6 | 6.5 | 5.9 | 4.5 |
| Elongation at break (%) | 204 | 210 | 199 | 204 | 200 | 138 |
| Stress at 100% strain (MPa) | 3.7 | 3.6 | 3.5 | 3.7 | 3.6 | 4.0 |
| Elasticity modulus (MPa) | 13.3 | 14.1 | 14.1 | 15.0 | 16.3 | 21.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Lin, Y.; Li, Z.; Yang, Y.; Lin, J.; He, S. Effect of Fluorosilicone Rubber on Mechanical Properties, Dielectric Breakdown Strength and Hydrophobicity of Methyl Vinyl Silicone Rubber. Polymers 2023, 15, 3448. https://doi.org/10.3390/polym15163448
Wang Z, Lin Y, Li Z, Yang Y, Lin J, He S. Effect of Fluorosilicone Rubber on Mechanical Properties, Dielectric Breakdown Strength and Hydrophobicity of Methyl Vinyl Silicone Rubber. Polymers. 2023; 15(16):3448. https://doi.org/10.3390/polym15163448
Chicago/Turabian StyleWang, Zhaoyang, Yankai Lin, Zhanxu Li, Yumeng Yang, Jun Lin, and Shaojian He. 2023. "Effect of Fluorosilicone Rubber on Mechanical Properties, Dielectric Breakdown Strength and Hydrophobicity of Methyl Vinyl Silicone Rubber" Polymers 15, no. 16: 3448. https://doi.org/10.3390/polym15163448
APA StyleWang, Z., Lin, Y., Li, Z., Yang, Y., Lin, J., & He, S. (2023). Effect of Fluorosilicone Rubber on Mechanical Properties, Dielectric Breakdown Strength and Hydrophobicity of Methyl Vinyl Silicone Rubber. Polymers, 15(16), 3448. https://doi.org/10.3390/polym15163448








