Fabrication of Highly Conductive Porous Fe3O4@RGO/PEDOT:PSS Composite Films via Acid Post-Treatment and Their Applications as Electrochemical Supercapacitor and Thermoelectric Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
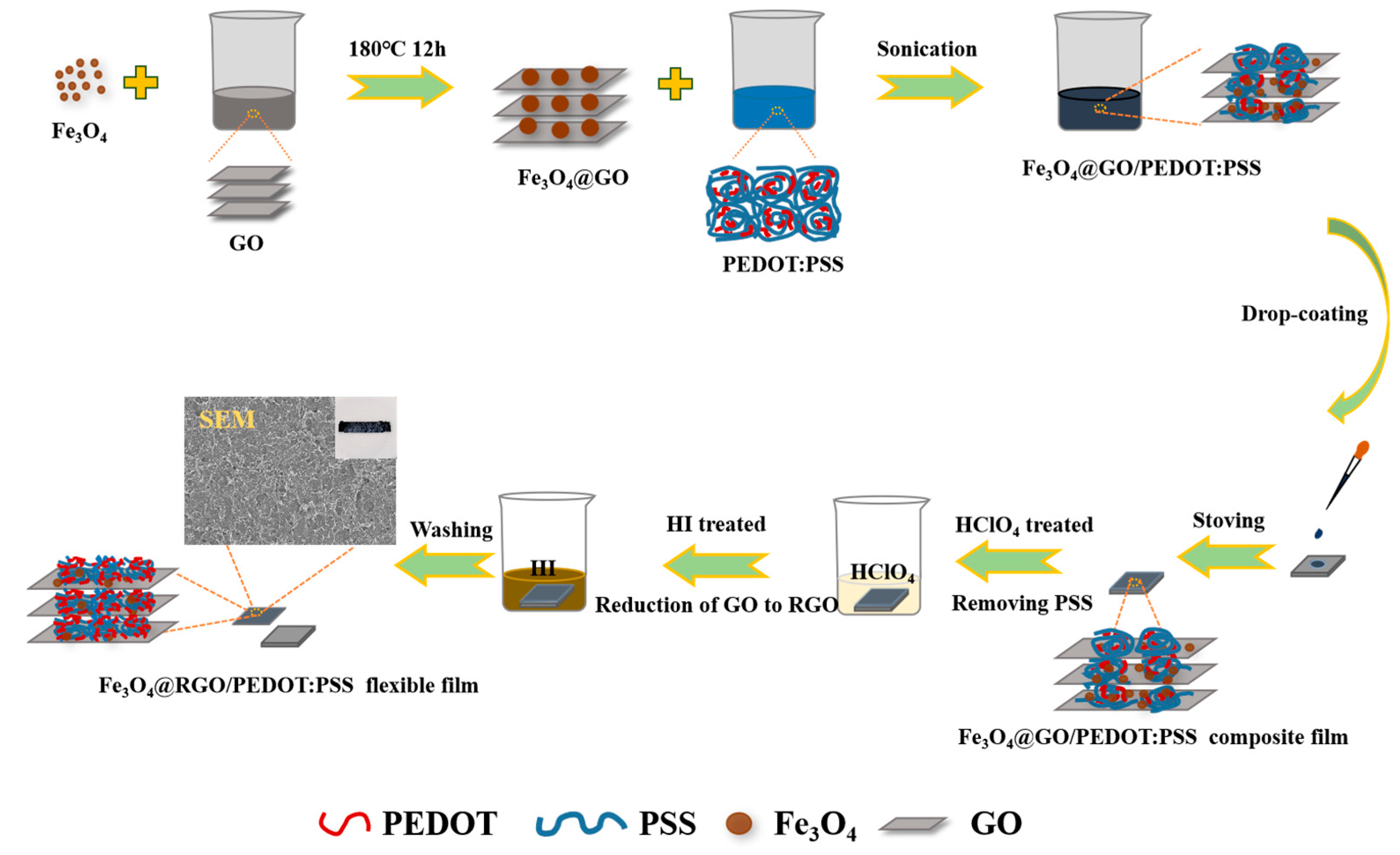
2.2. Preparation of Fe3O4@GO and Fe3O4@RGO
2.3. Preparation of Fe3O4@GO/PEDOT:PSS Composite Films
2.4. Preparation of Fe3O4@RGO/PEDOT:PSS Free-Standing Films
2.5. Characterization and Measurements
3. Results and Discussion
3.1. Fabrication of Fe3O4@RGO/PEDOT:PSS Free-Standing Film
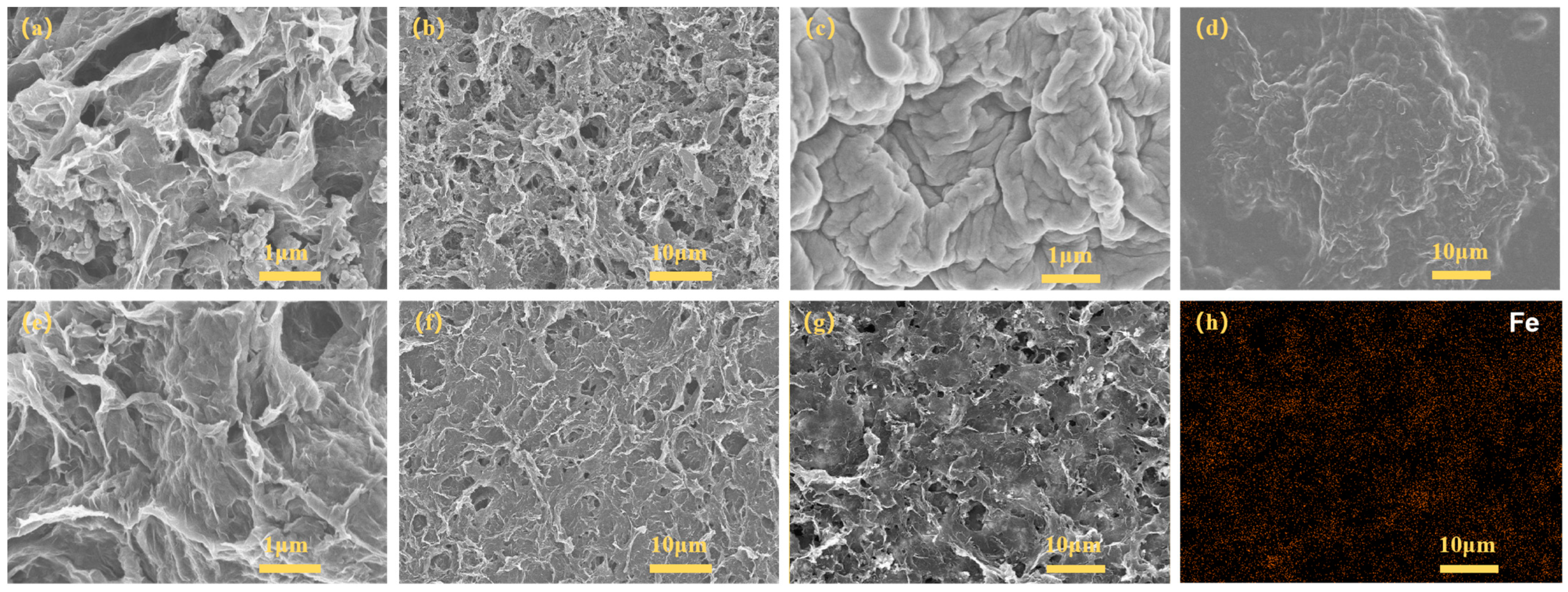
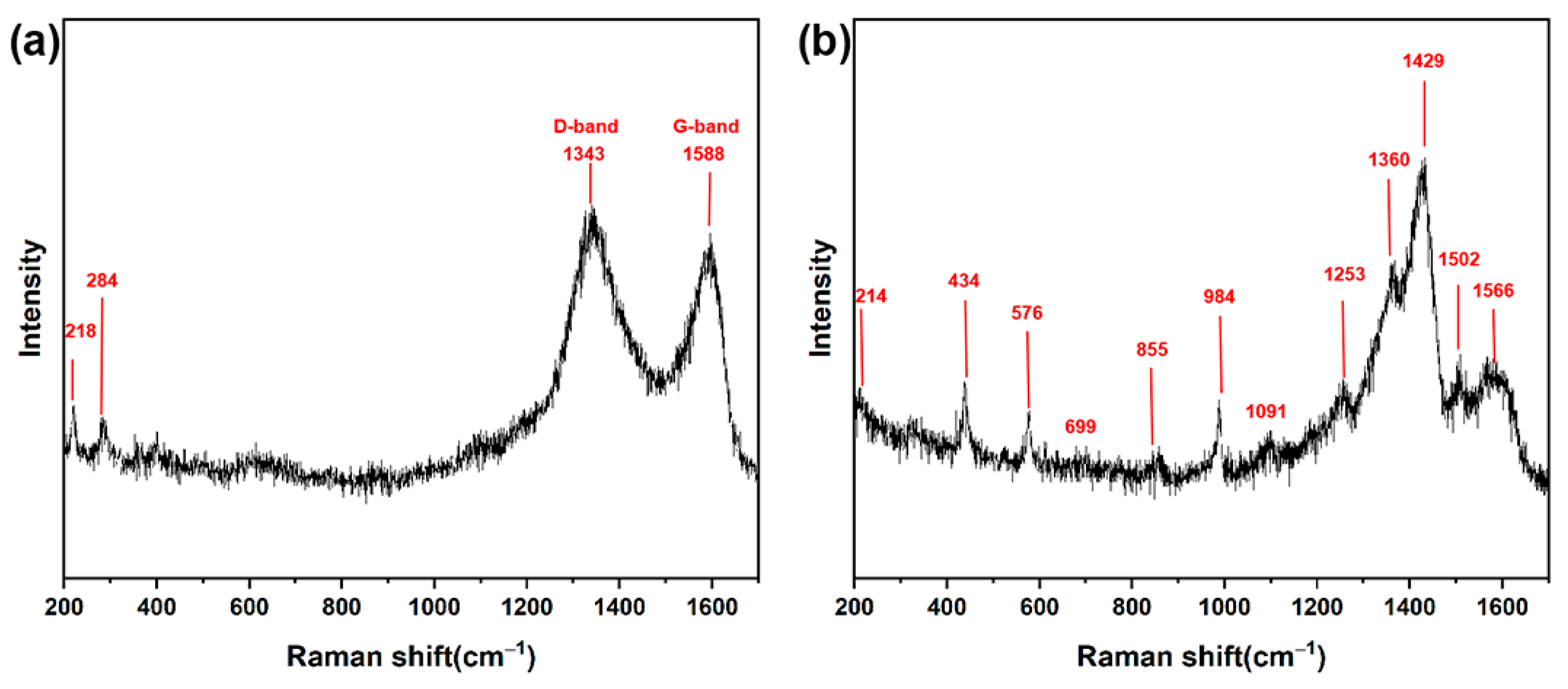
3.2. Structure Characterization and Analysis
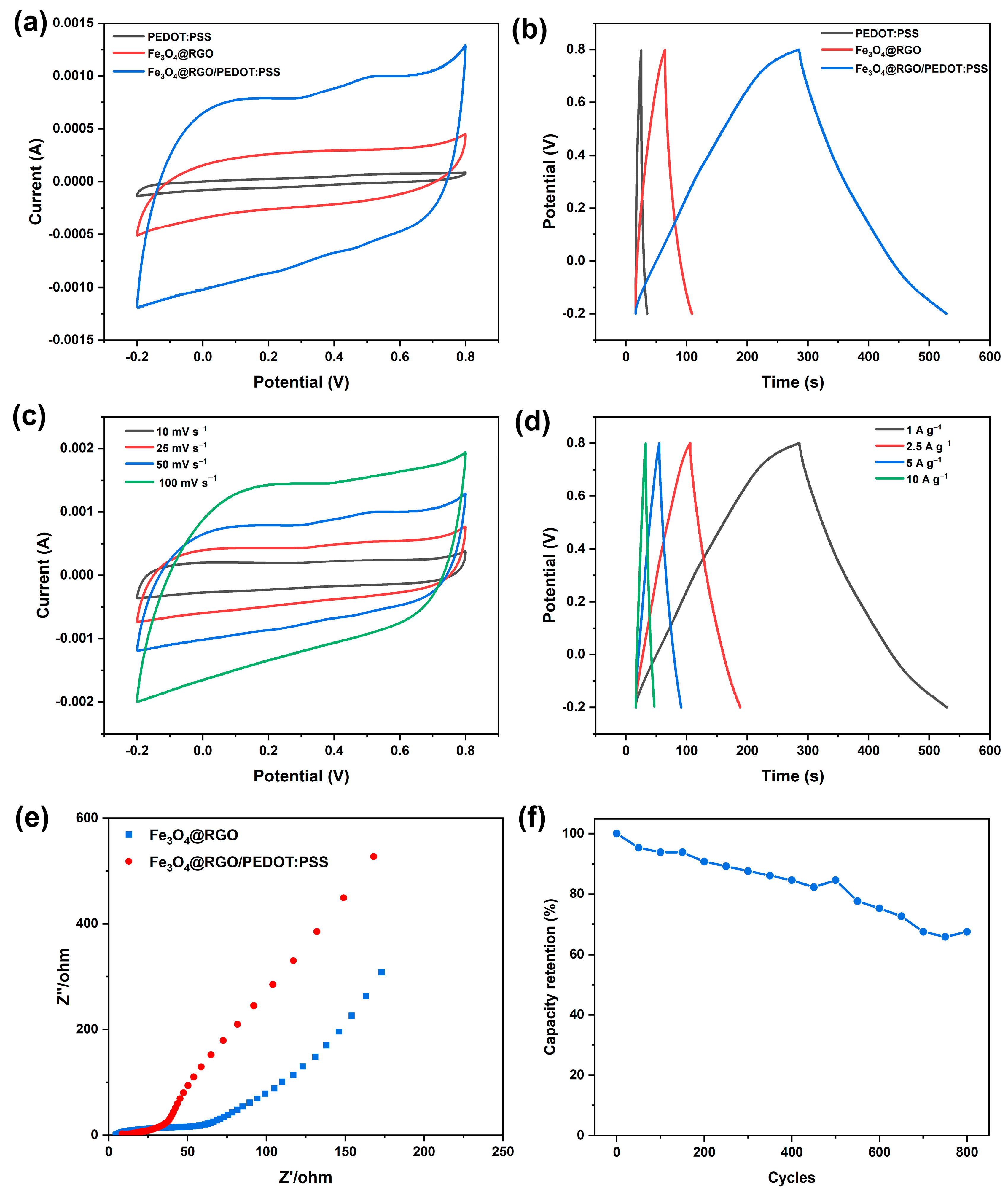
3.3. Electrochemical Properties of Fe3O4@RGO/PEDOT:PSS Free-Standing Films
3.4. Thermoelectric Performance of Fe3O4@RGO/PEDOT:PSS Free-Standing Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Xiao, F.; Lei, Y.; Lu, H.; Zhang, J.; Yan, M.; Xu, J. A novel approach for synthesis of expanded graphite and its enhanced lithium storage properties. J. Energy Chem. 2021, 59, 292–298. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, H.; Yang, J.; Bo, Z.; Huang, F.; Islam, M.S.; Lu, X.; Dai, L.; Amal, R.; Wang, C.H.; et al. Two-birds-one-stone: Multifunctional supercapacitors beyond traditional energy storage. Energy Environ. Sci. 2021, 14, 1854–1896. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lu, Y.; Yang, Y.; Zhang, M.; Yue, Z.; Zhang, N.; Peng, T.; Liu, X.; Luo, Y. A vanadium-based oxide-nitride heterostructure as a multifunctional sulfur host for advanced Li-S batteries. Nanoscale 2021, 13, 13085–13094. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Q.; Wang, W.; Xu, Z.; Li, N.; Li, M.; Jia, L.; Zhu, W.; Cao, Z.; Xu, J. Carbon framework microbelt supporting SnO(x) as a high performance electrode for lithium ion batteries. Nanotechnology 2019, 30, 325405. [Google Scholar] [CrossRef]
- Peng, T.; Guo, Y.; Zhang, Y.; Wang, Y.; Zhang, D.; Yang, Y.; Lu, Y.; Liu, X.; Chu, P.K.; Luo, Y. Uniform cobalt nanoparticles-decorated biscuit-like VN nanosheets by in situ segregation for Li-ion batteries and oxygen evolution reaction. Appl. Surf. Sci. 2021, 536, 147982. [Google Scholar] [CrossRef]
- Jiang, Y.; Ou, J.; Luo, Z.; Chen, Y.; Wu, Z.; Wu, H.; Fu, X.; Luo, S.; Huang, Y. High Capacitive Antimonene/CNT/PANI Free-Standing Electrodes for Flexible Supercapacitor Engaged with Self-Healing Function. Small 2022, 18, 2201377. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, D.; Cheng, M.; Zhou, J.; Owusu, K.A.; Mai, L.; Yu, Y. A New View of Supercapacitors: Integrated Supercapacitors. Adv. Energy Mater. 2019, 9, 1901081. [Google Scholar] [CrossRef]
- Bertana, V.; Scordo, G.; Camilli, E.; Ge, L.; Zaccagnini, P.; Lamberti, A.; Marasso, S.L.; Scaltrito, L. 3D Printed Supercapacitor Exploiting PEDOT-Based Resin and Polymer Gel Electrolyte. Polymers 2023, 15, 2657. [Google Scholar] [CrossRef]
- Chang, J.; Adhikari, S.; Lee, T.H.; Li, B.; Yao, F.; Pham, D.T.; Le, V.T.; Lee, Y.H. Leaf vein-inspired nanochanneled graphene film for highly efficient micro-supercapacitors. Adv. Energy Mater. 2015, 5, 1500003. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Meng, W.; Fu, Y.; Wang, Z.; Huang, Y.; Pei, Z.; Zhi, C. Robust reduced graphene oxide paper fabricated with a household non-stick frying pan: A large-area freestanding flexible substrate for supercapacitors. RSC Adv. 2015, 5, 33981–33989. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Guan, C.; Elshahawy, A.M.; Dong, Y.; Pennycook, S.J.; Wang, J. (Ni,Co)Se2/NiCo-LDH Core/Shell Structural Electrode with the Cactus-Like (Ni,Co)Se2Core for Asymmetric Supercapacitors. Small 2018, 15, 1803895. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiu, F.; Lu, C.; Zhu, J.; Ke, C.; Han, S.; Zhuang, X. A Terpyridine-Fe(2+)-Based Coordination Polymer Film for On-Chip Micro-Supercapacitor with AC Line-Filtering Performance. Polymers 2021, 13, 1002. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Wang, D.-W.; Ren, W.; Zhao, J.; Zhou, G.; Li, F.; Cheng, H.-M. Anchoring Hydrous RuO2 on Graphene Sheets for High-Performance Electrochemical Capacitors. Adv. Funct. Mater. 2010, 20, 3595–3602. [Google Scholar] [CrossRef]
- Yu, G.; Hu, L.; Liu, N.; Wang, H.; Vosgueritchian, M.; Yang, Y.; Cui, Y.; Bao, Z. Enhancing the supercapacitor performance of graphene/MnO2 nanostructured electrodes by conductive wrapping. Nano Lett. 2011, 11, 4438–4442. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Cai, S.; Tao, K.; Xu, Y. A Solid-State Wire-Shaped Supercapacitor Based on Nylon/Ag/Polypyrrole and Nylon/Ag/MnO2 Electrodes. Polymers 2023, 15, 1627. [Google Scholar] [CrossRef]
- Fang, L.; Lan, M.; Liu, B.; Cao, Y. Synthesis and Electrochemical Performance of Flower-like Zn0.76Co0.24S. J. Xinyang Norm. Univ. (Nat. Sci. Ed.) 2022, 35, 615–620. [Google Scholar]
- Liu, D.; Wang, X.; Wang, X.; Tian, W.; Liu, J.; Zhi, C.; He, D.; Bando, Y.; Golberg, D. Ultrathin nanoporous Fe3O4–carbon nanosheets with enhanced supercapacitor performance. J. Mater. Chem. A 2013, 1, 1952–1955. [Google Scholar] [CrossRef]
- Khoh, W.-H.; Hong, J.-D. Layer-by-layer self-assembly of ultrathin multilayer films composed of magnetite/reduced graphene oxide bilayers for supercapacitor application. Colloids Surf. A 2013, 436, 104–112. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Wu, Q.; Xu, H. Synthesis and electrochemical properties of Fe3O4/MnO2/RGOs sandwich-like nano-superstructures. J. Alloy. Compd. 2017, 693, 373–380. [Google Scholar] [CrossRef]
- Guan, D.; Gao, Z.; Yang, W.; Wang, J.; Yuan, Y.; Wang, B.; Zhang, M.; Liu, L. Hydrothermal synthesis of carbon nanotube/cubic Fe3O4 nanocomposite for enhanced performance supercapacitor electrode material. Mater. Sci. Eng. B 2013, 178, 736–743. [Google Scholar] [CrossRef]
- Ramanathan, S.; SasiKumar, M.; Radhika, N.; Obadiah, A.; Durairaj, A.; Helen Swetha, G.; Santhoshkumar, P.; Sharmila Lydia, I.; Vasanthkumar, S. Musa paradisiaca reduced graphene oxide (BRGO) /MWCNT-Fe3O4 nanocomposite for supercapacitor and photocatalytic applications. Mater. Today Proc. 2021, 47, 843–852. [Google Scholar] [CrossRef]
- Fang, L.; Qiu, Y.; Zhai, T.; Wang, F.; Zhou, H. Synthesis of Ni(OH)2-VS2 Nanocomposite and Their Application in Supercapacitors. J. Xinyang Norm. Univ. (Nat. Sci. Ed.) 2017, 30, 109–113. [Google Scholar]
- Mondal, S.; Thakur, S.; Maiti, S.; Bhattacharjee, S.; Chattopadhyay, K.K. Self-Charging Piezo-Supercapacitor: One-Step Mechanical Energy Conversion and Storage. ACS Appl. Mater. Interfaces 2023, 15, 8446–8461. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Chu, C.W.; Chen, F.C.; Xu, Q.; Yang, Y. High-Conductivity Poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) Film and Its Application in Polymer Optoelectronic Devices. Adv. Funct. Mater. 2005, 15, 203–208. [Google Scholar] [CrossRef]
- Ouyang, J.; Xu, Q.; Chu, C.-W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Badre, C.; Marquant, L.; Alsayed, A.M.; Hough, L.A. Highly Conductive Poly(3,4-ethylenedioxythiophene):Poly (styrenesulfonate) Films Using 1-Ethyl-3-methylimidazolium Tetracyanoborate Ionic Liquid. Adv. Funct. Mater. 2012, 22, 2723–2727. [Google Scholar] [CrossRef]
- Fan, Z.; Du, D.; Yu, Z.; Li, P.; Xia, Y.; Ouyang, J. Significant Enhancement in the Thermoelectric Properties of PEDOT:PSS Films through a Treatment with Organic Solutions of Inorganic Salts. ACS Appl. Mater. Int. 2016, 8, 23204–23211. [Google Scholar] [CrossRef]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, H.; Kim, S.; Son, W.; Cheong, I.W.; Kim, J.H. Transparent and flexible organic semiconductor nanofilms with enhanced thermoelectric efficiency. J. Mater. Chem. A 2014, 2, 7288–7294. [Google Scholar] [CrossRef]
- Takano, T.; Masunaga, H.; Fujiwara, A.; Okuzaki, H.; Sasaki, T. PEDOT Nanocrystal in Highly Conductive PEDOT:PSS Polymer Films. Macromolecules 2012, 45, 3859–3865. [Google Scholar] [CrossRef]
- Crispin, X.; Jakobsson, F.L.E.; Crispin, A.; Grim, P.C.M.; Andersson, P.; Volodin, A.; van Haesendonck, C.; Van der Auweraer, M.; Salaneck, W.R.; Berggren, M. The Origin of the High Conductivity of Poly(3,4-ethylenedioxythiophene)-Poly(styrenesulfonate) (PEDOT-PSS) Plastic Electrodes. Chem. Mater. 2006, 18, 4354–4360. [Google Scholar] [CrossRef]
- Liu, F.; Xie, L.; Wang, L.; Chen, W.; Wei, W.; Chen, X.; Luo, S.; Dong, L.; Dai, Q.; Huang, Y.; et al. Hierarchical Porous RGO/PEDOT/PANI Hybrid for Planar/Linear Supercapacitor with Outstanding Flexibility and Stability. Nano Micro Lett. 2020, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Bießmann, L.; Saxena, N.; Hohn, N.; Hossain, M.A.; Veinot, J.G.C.; Müller-Buschbaum, P. Highly Conducting, Transparent PEDOT:PSS Polymer Electrodes from Post-Treatment with Weak and Strong Acids. Adv. Electron. Mater. 2019, 5, 1800654. [Google Scholar] [CrossRef]
- Song, H.; Liu, C.; Xu, J.; Jiang, Q.; Shi, H. Fabrication of a layered nanostructure PEDOT:PSS/SWCNTs composite and its thermoelectric performance. RSC Adv. 2013, 3, 22065–22071. [Google Scholar] [CrossRef]
- Ely, F.; Matsumoto, A.; Zoetebier, B.; Peressinotto, V.S.; Hirata, M.K.; de Sousa, D.A.; Maciel, R. Handheld and automated ultrasonic spray deposition of conductive PEDOT:PSS films and their application in AC EL devices. Org. Electron. 2014, 15, 1062–1070. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Pal, A.; Ning, Y.; Wang, Z.; Zhao, B.; Bradley, R.; Wu, W. Ultra-small Fe3O4 nanoparticles encapsulated in hollow porous carbon nanocapsules for high performance supercapacitors. Carbon 2021, 179, 327–336. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Wu, H.; Qian, X.; Chen, L.; Dan, Y.; Yu, Q. Porous Fe3O4/C nanoaggregates by the carbon polyhedrons as templates derived from metal organic framework as battery-type materials for supercapacitors. Electrochim. Acta 2020, 337, 135818. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Chen, M.; Jiao, Y.; Wang, J. Electrochemical Performances of Nanoparticle Fe3O4/Activated Carbon Supercapacitor Using KOH Electrolyte Solution. J. Phys. Chem. C 2009, 113, 2643–2646. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, D.; Jing, C.; Liu, X.; Li, K.; Yu, M.; Qi, S.; Zhang, Y. Biotemplate Synthesis of Fe3O4/Polyaniline for Supercapacitor. J. Energy Storage 2020, 30, 101554. [Google Scholar] [CrossRef]
- Li, J.; Lu, W.; Yan, Y.; Chou, T.-W. High performance solid-state flexible supercapacitor based on Fe3O4/carbon nanotube/polyaniline ternary films. J. Mater. Chem. A 2017, 5, 11271–11277. [Google Scholar] [CrossRef]
- Qiu, C.; Jiang, L.; Gao, Y.; Sheng, L. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Des. 2023, 230, 111952. [Google Scholar] [CrossRef]
- Du, Y.; Shi, Y.; Meng, Q.; Shen, S.Z. Preparation and thermoelectric properties of flexible SWCNT/PEDOT:PSS composite film. Synth. Met. 2020, 261, 116318. [Google Scholar] [CrossRef]
- Wei, S.; Huang, X.; Deng, L.; Yan, Z.-C.; Chen, G. Facile preparations of layer-like and honeycomb-like films of poly(3,4-ethylenedioxythiophene)/carbon nanotube composites for thermoelectric application. Compos. Sci. Technol. 2021, 208, 108759. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Liu, F.; Wei, Q.; Cai, Z.; Duan, J.; Li, F.; Li, H.; Lv, R.; Wang, M.; Li, J.; et al. Fabrication of Highly Conductive Porous Fe3O4@RGO/PEDOT:PSS Composite Films via Acid Post-Treatment and Their Applications as Electrochemical Supercapacitor and Thermoelectric Material. Polymers 2023, 15, 3453. https://doi.org/10.3390/polym15163453
Gao L, Liu F, Wei Q, Cai Z, Duan J, Li F, Li H, Lv R, Wang M, Li J, et al. Fabrication of Highly Conductive Porous Fe3O4@RGO/PEDOT:PSS Composite Films via Acid Post-Treatment and Their Applications as Electrochemical Supercapacitor and Thermoelectric Material. Polymers. 2023; 15(16):3453. https://doi.org/10.3390/polym15163453
Chicago/Turabian StyleGao, Luyao, Fuwei Liu, Qinru Wei, Zhiwei Cai, Jiajia Duan, Fuqun Li, Huiying Li, Ruotong Lv, Mengke Wang, Jingxian Li, and et al. 2023. "Fabrication of Highly Conductive Porous Fe3O4@RGO/PEDOT:PSS Composite Films via Acid Post-Treatment and Their Applications as Electrochemical Supercapacitor and Thermoelectric Material" Polymers 15, no. 16: 3453. https://doi.org/10.3390/polym15163453
APA StyleGao, L., Liu, F., Wei, Q., Cai, Z., Duan, J., Li, F., Li, H., Lv, R., Wang, M., Li, J., & Wang, L. (2023). Fabrication of Highly Conductive Porous Fe3O4@RGO/PEDOT:PSS Composite Films via Acid Post-Treatment and Their Applications as Electrochemical Supercapacitor and Thermoelectric Material. Polymers, 15(16), 3453. https://doi.org/10.3390/polym15163453






