Synthesis and Comparative Study of Polyether-b-polybutadiene-b-polyether Triblock Copolymers for Use as Polyurethanes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of HTPBs
2.2.1. Synthesis of AHTPB via Living Anionic Polymerization
2.2.2. Synthesis of RHTPB via ROMP
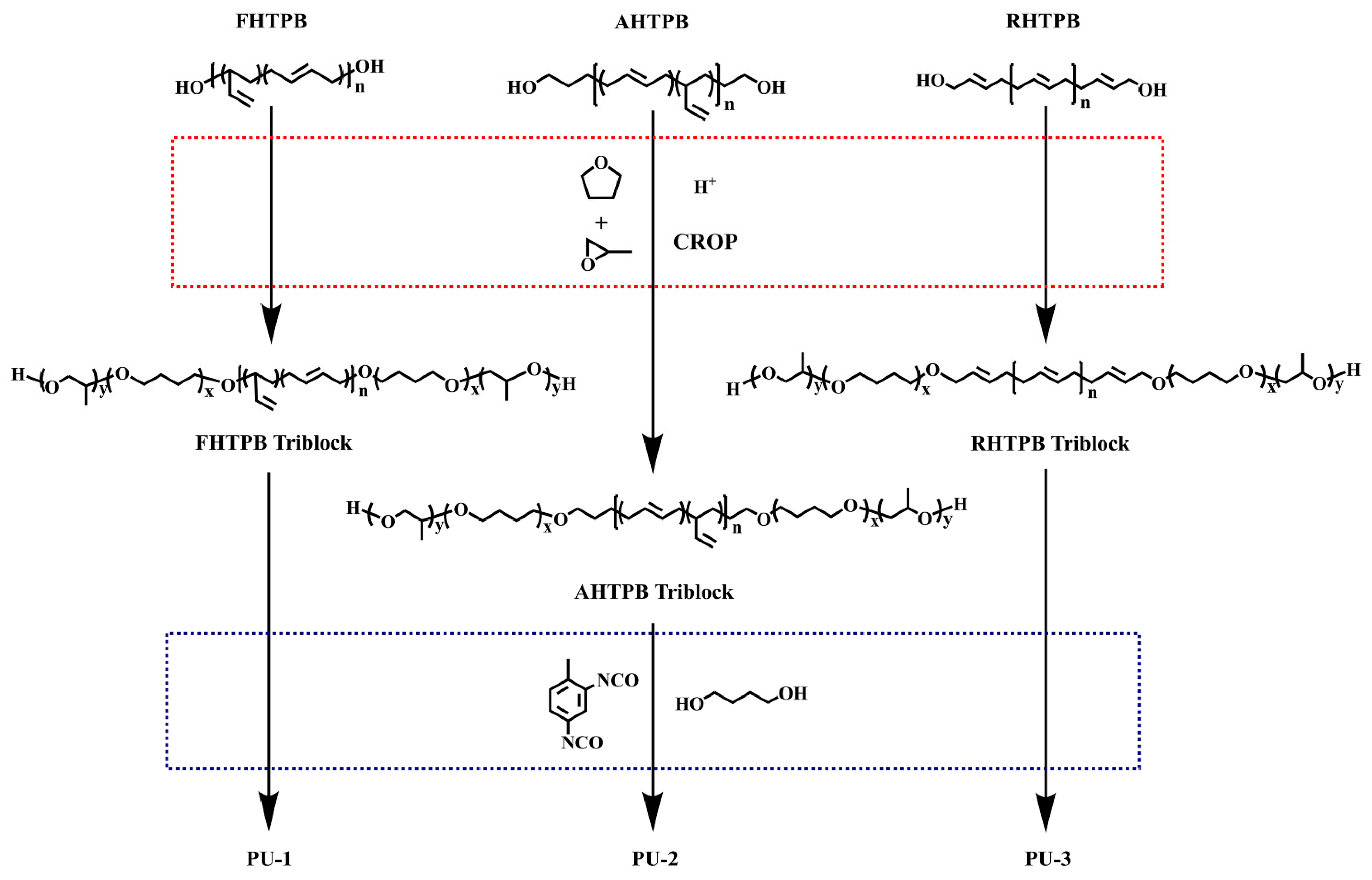
2.3. Synthesis of HTPB Triblock Copolymers
2.3.1. Synthesis of FHTPB Triblock Copolymers (TBCFHTPB)
2.3.2. Synthesis of AHTPB Triblock Copolymers (TBCAHTPB)
2.3.3. Synthesis of RHTPB Triblock Copolymers (TBCRHTPB)
2.4. Synthesis of Polyurethane Elastomer using HTPB Triblock Copolymers
2.5. Structural Characterization of HTPBs and HTPB Triblock Copolymers
2.5.1. FT-IR Test
2.5.2. NMR Test
2.5.3. SEC-MALLS Test
2.6. Comparative Study of the Properties of the Triblock Copolymers and Polyurethane Elastomers
2.6.1. DSC Test
2.6.2. TGA Test
2.6.3. Viscosity Test
2.6.4. Tensile Test
2.6.5. XRD Test
2.6.6. XPS Test
2.6.7. Contact Angle Test
3. Results and Discussion
3.1. Synthesis and Characterization of TBCFHTPB
3.2. Synthesis and Characterization of AHTPB and TBCAHTPB
3.3. Synthesis and Characterization of RHTPB and TBCRHTPB
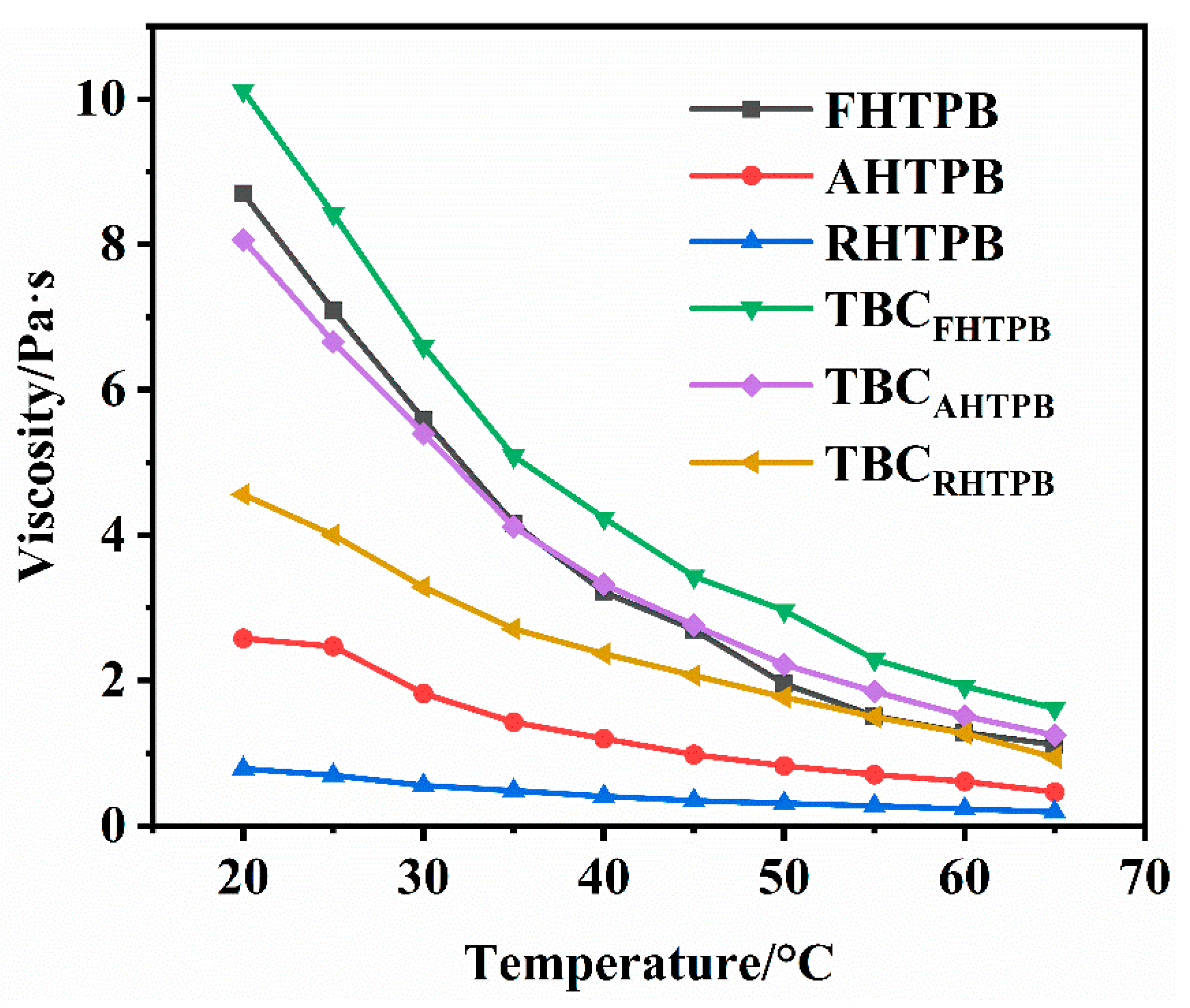
3.4. Viscosity of the HTPBs and Their Triblock Copolymers
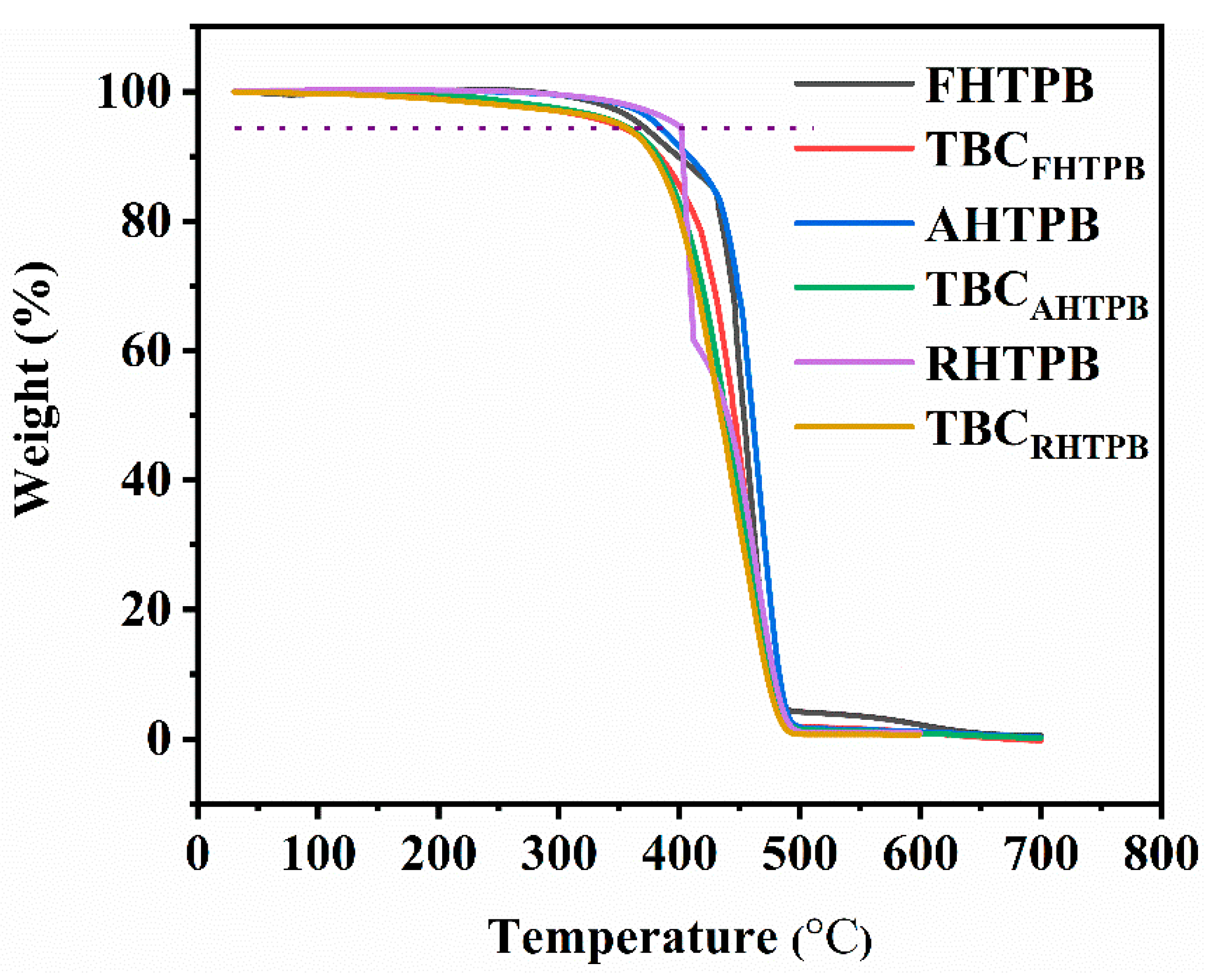
3.5. Thermal Properties of the HTPBs and Their Triblock Copolymers
3.6. Property Comparison of Polyurethane Elastomers
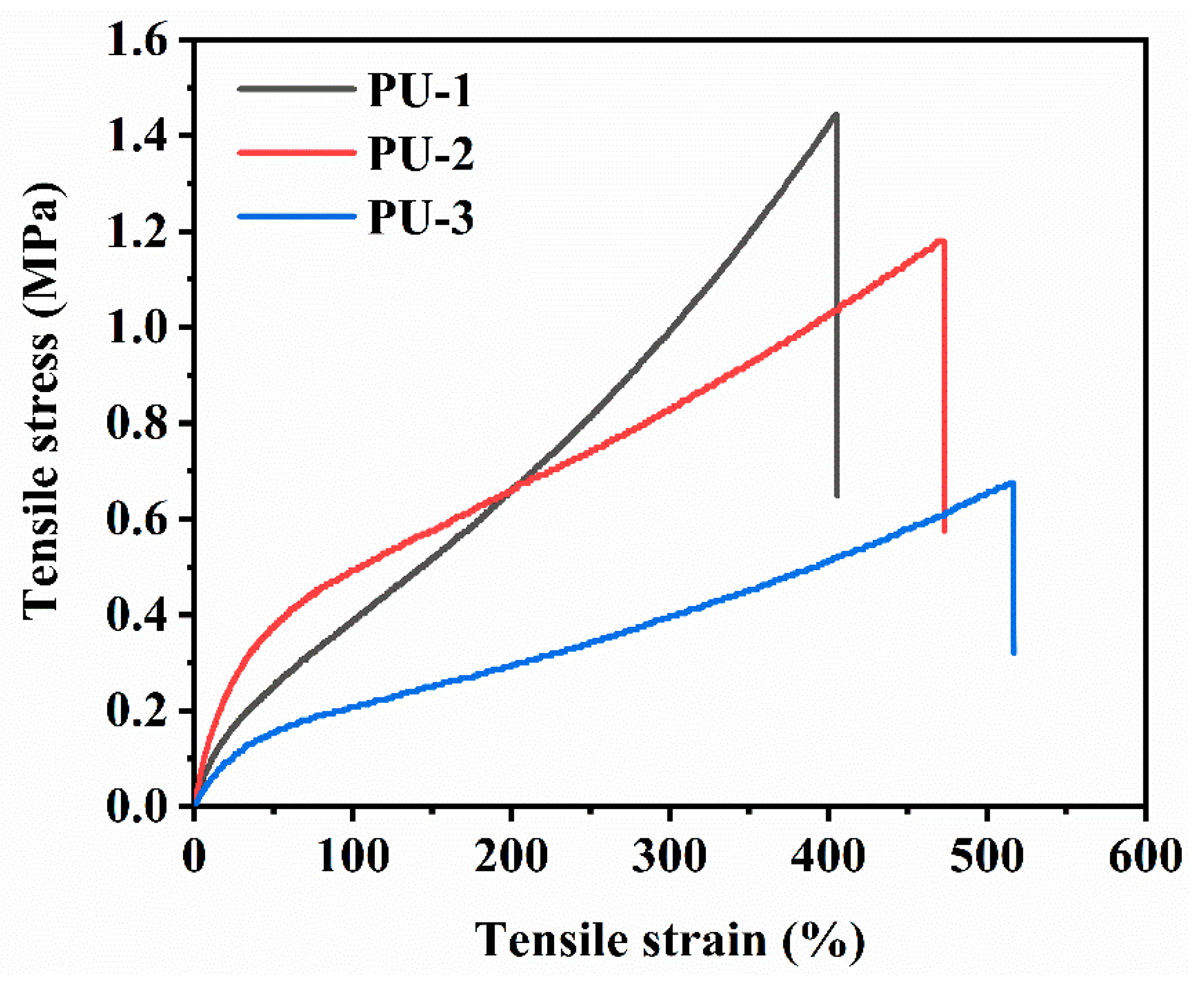
3.6.1. Tensile Properties
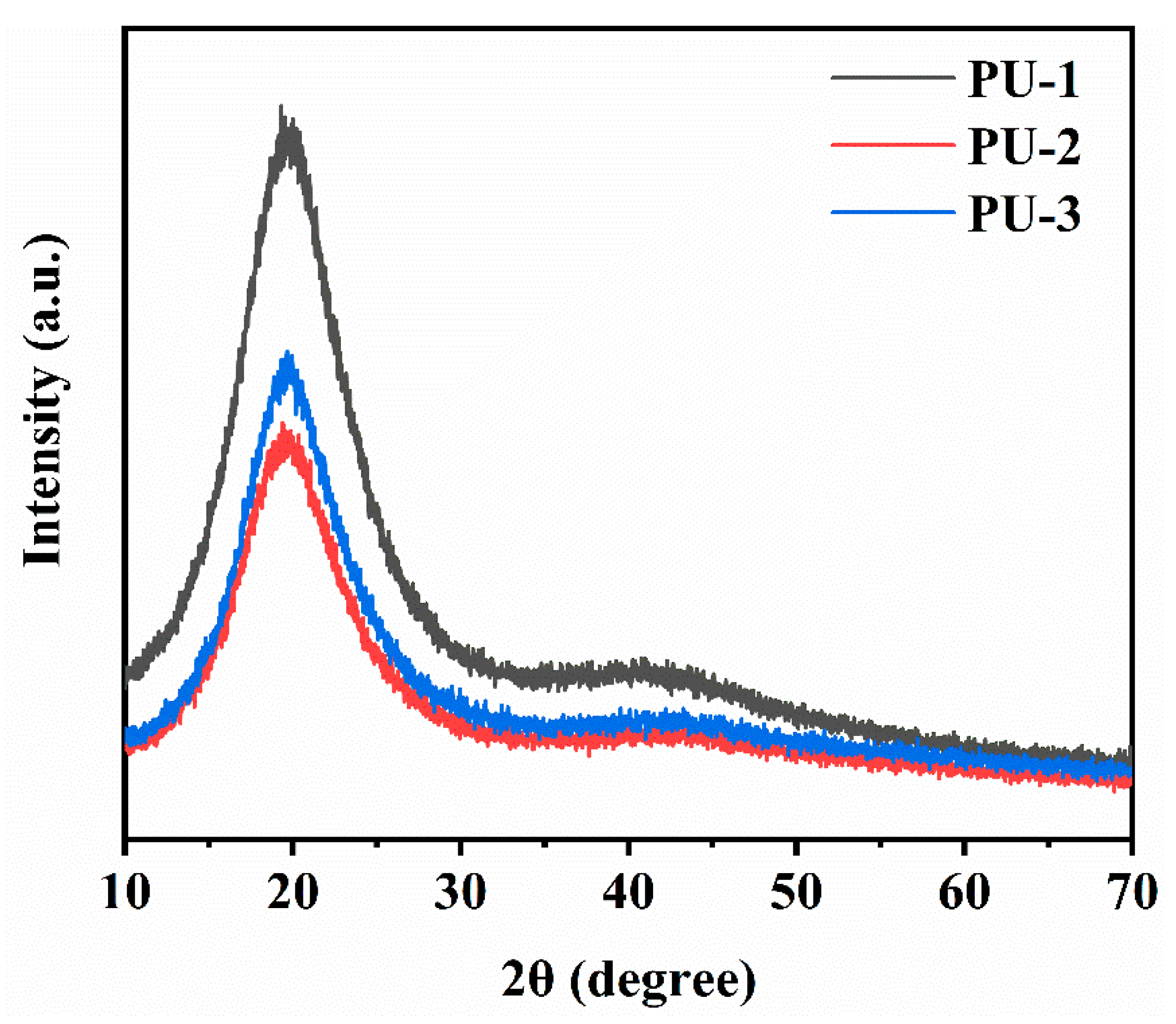
3.6.2. XRD Analysis
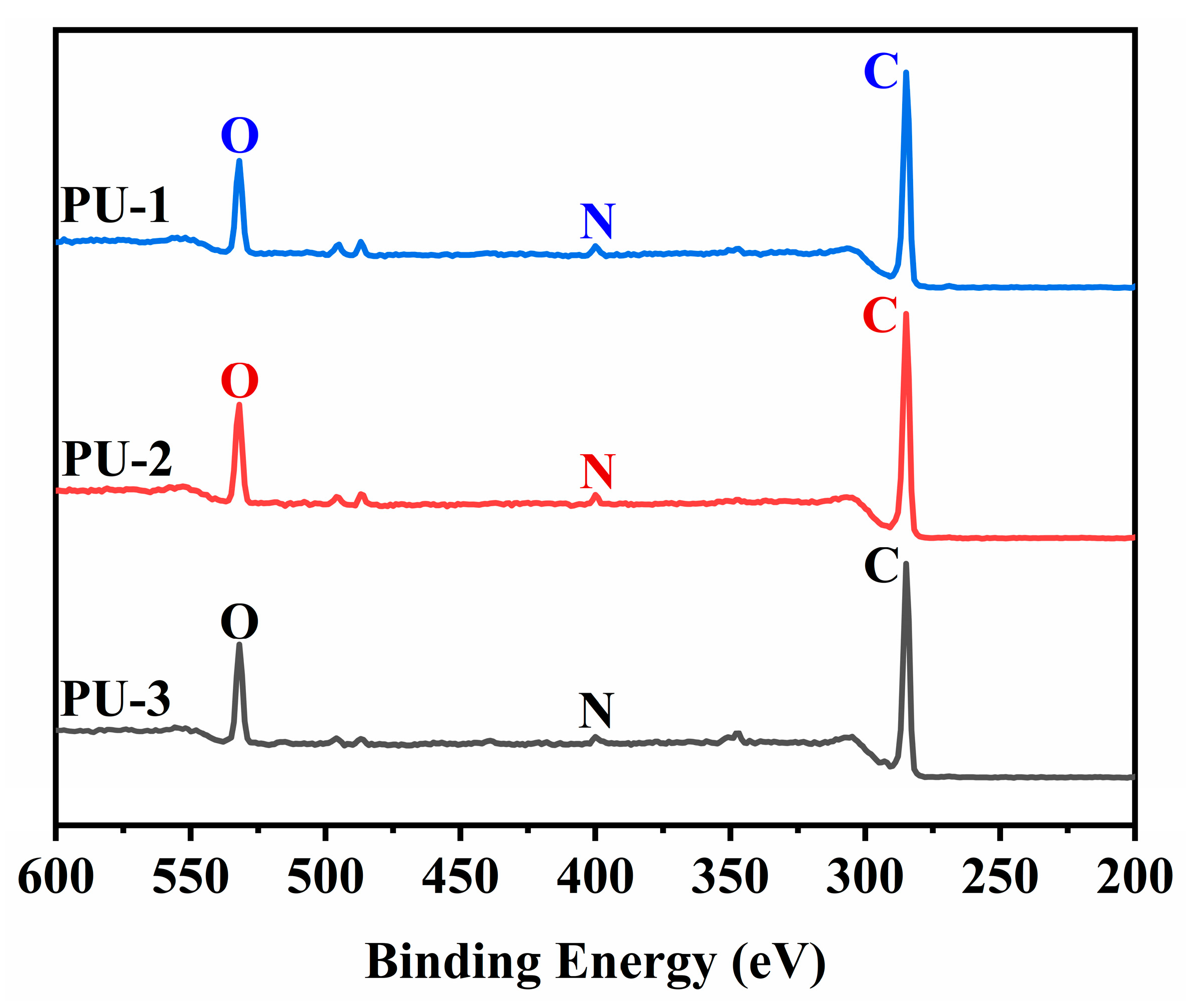
3.6.3. Surface Characterization of Polyurethane Elastomers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Selvakumar, S.; Rao, G.S.; Reddy, K.A. Diffusion of Labile Chemical Species in HTPB and HTPB-XT Solid Propellants and Its Effect over Solid Rocket Motor Properties on Aging—A Study. Propell. Explos. Pyrot. 2021, 46, 782–790. [Google Scholar]
- Chaturvedi, S.; Dave, P.N. Solid propellants: AP/HTPB composite propellants. Arab. J. Chem. 2019, 12, 2061–2068. [Google Scholar]
- Li, J.; Ning, Z.; Yang, W.; Yang, B.; Zeng, Y. Hydroxyl-Terminated Polybutadiene-Based Polyurethane with Self-Healing and Reprocessing Capabilities. ACS Omega 2022, 7, 10156–10166. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, X.; Zhang, W.; Zhang, H.; Wang, J.; Qu, J. Study on the preparation and performance comparison of side-chain hydroxyl-terminated polybutadiene derivatives with narrowly molecular weight distribution used for polyurethane. Polym. Test. 2021, 104, 107389. [Google Scholar]
- Sikder, B.K.; Jana, T. Effect of Solvent and Functionality on the Physical Properties of Hydroxyl-Terminated Polybutadiene (HTPB)-Based Polyurethane. ACS Omega 2018, 3, 3004–3013. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.R.; Nikje, M.M.A. Synthesis and characterization of novel epoxy-urethane coating and its graphene nanocomposites. Polym. Compos. 2023, 44, 2794–2803. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Liu, H.; Zheng, Y.; Zhong, Y.; Wang, G.; Zhu, Q.; Liu, X.; Zhang, L.; Li, H. Synthesis and characterization of a novel hydroxy telechelic polyfluoroether to enhance the properties of HTPB solid propellant binders. Colloids Surf. A 2022, 648, 129199. [Google Scholar]
- Zhang, P.; Tan, W.; Zhang, X.; Chen, J.; Yuan, J.; Deng, J. Chemical Modification of Hydroxyl-Terminated Polybutadiene and Its Application in Composite Propellants. Ind. Eng. Chem. Res. 2021, 60, 3819–3829. [Google Scholar]
- Yuan, B.; Wang, G.; Tian, W.; Zhou, L.; Li, C. Fabrication of Hydroxy-Terminated Polybutadiene with Piezoelectric Property by Functionalized Branch Chain Modification. Molecules 2023, 28, 1810. [Google Scholar]
- Zhang, Y.; Zheng, J.; Zhang, X.; Du, Y.; Li, K.; Yu, G.; Jia, Y.; Liu, Y. Effect of chemical copolymerization and mixed chain extenders on mechanical properties of HTPB polyurethane. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1167, 012012. [Google Scholar]
- Liang, J.; Nie, J.; Zhang, H.; Guo, X.; Yan, S.; Han, M. Interaction Mechanism of Composite Propellant Components under Heating Conditions. Polymers 2023, 15, 2485. [Google Scholar] [CrossRef]
- Liu, J.; Yang, D.; Li, S.; Bai, C.; Tu, C.; Zhu, F.; Xin, W.; Li, G.; Luo, Y. Synthesis and characterization of hydroxyl-terminated polybutadiene modified low temperature adaptive self-matting waterborne polyurethane. RSC Adv. 2023, 13, 7020–7029. [Google Scholar] [PubMed]
- Lemos, M.F.; Mendes, L.C.; Bohn, M.A. On the functionalization and characterization of hydroxyl-terminated polybutadiene with octyl-1-azide and the evaluation of polyurethane elastomers based on such modified HTPB. J. Appl. Polym. Sci. 2021, 138, e49981. [Google Scholar] [CrossRef]
- Kumar, D.; Mohammad, S.A.; Kumar, A.; Mane, S.R.; Banerjee, S. An amino acid-derived ABCBA-type antifouling biohybrid with multi-stimuli responsivity and contaminant removal capability. Polym. Chem. 2022, 13, 1960–1969. [Google Scholar]
- Li, L.; Peng, W.; Liu, L.; Zheng, S. Toughening of epoxy by nanostructures with ABA triblock copolymers: An influence of organosilicon modification of block copolymer. Polym. Eng. Sci. 2022, 62, 392–404. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Wei, Y. Syntheses and properties of tri- and multi-block copolymers consisting of polybutadiene and polylactide segments. RSC Adv. 2022, 12, 29777–29784. [Google Scholar]
- Min, X.; Fan, X. A New Strategy for the Synthesis of Hydroxyl Terminated Polystyrene-b-Polybutadiene-b-Polystyrene Triblock Copolymer with High Cis-1, 4 Content. Polymers 2019, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Earnshaw, J.; Ellison, L.; Santos, G.R.D.; Cavaye, H.; Cleaver, D.G. Understanding and controlling the glass transition of HTPB oligomers. Polym. Chem. 2021, 12, 2606–2617. [Google Scholar] [CrossRef]
- Garraza, A.L.R.; Mansilla, M.A.; Depaoli, E.L.; Macchi, C.; Cerveny, S.; Marzocca, A.J.; Somoza, A. Comparative study of thermal, mechanical and structural properties of polybutadiene rubber isomers vulcanized using peroxide. Polym. Test. 2016, 52, 117–123. [Google Scholar] [CrossRef]
- Wrana, C.; Schawe, J.E.K. Isothermal crystallization of cis-1.4-polybutadiene at low temperatures. Thermochim. Acta 2020, 690, 178669. [Google Scholar]
- Eslami, H.; Gharibi, A.; Plathe, F.M. Mechanisms of Nucleation and Solid−Solid-Phase Transitions in Triblock Janus Assemblies. J. Chem. Theory Comput. 2021, 17, 1742–1754. [Google Scholar] [PubMed]
- Sadeghi, G.M.M.; Morshedian, J.; Barikani, M. The effect of solvent on the microstructure, nature of hydroxyl end groups and kinetics of polymerization reaction in synthesize of hydroxyl terminated polybutadiene. React. Funct. Polym. 2006, 66, 255–266. [Google Scholar] [CrossRef]
- Grishchenko, V.K.; Boiko, V.P.; Svistova, E.I.; Yatsimirskaya, T.S.; Valuev, V.I.; Dmitrieva, T.S. Hydrogen-Peroxide-Initiated Polymerization of Isoprene in Alcohol Solutions. J. Appl. Polym. Sci. 1992, 46, 2081–2087. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Z.; Pan, G.; Qi, Y.; Yi, J.; Bai, H. Synthesis of hydroxyl-terminated polybutadiene possessing high content of 1,4-units via anionic polymerization. Chin. J. Polym. Sci. 2010, 28, 715–720. [Google Scholar] [CrossRef]
- Min, X.; Fan, X.; Liu, J. Utilization of steric hindrance of alkyl lithium-based initiator to synthesize high 1,4 unit- containing hydroxyl- terminated polybutadiene. R. Soc. Open Sci. 2018, 5, 180156. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fan, X.; Zhao, N.; Min, X.; Liu, J.; Wang, Z. Influence of mono-lithium based initiators with different steric volumes on 1,4 unit content of hydroxyl terminated polybutadiene using anionic polymerization. RSC Adv. 2017, 7, 52712–52718. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Zheng, S. Synthesis of POSS-terminated polycyclooctadiene telechelics via ring-opening metathesis polymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 223–233. [Google Scholar] [CrossRef]
- Torres-Rocha, O.L.; Wu, X.; Zhu, C.; Crudden, C.M.; Cunningham, M.F. Polymerization-Induced Self-Assembly (PISA) of 1,5-Cyclooctadiene Using Ring Opening Metathesis Polymerization. Macromol. Rapid Commun. 2019, 40, 1800326. [Google Scholar]
- Zhu, X.; Fan, X.; Zhao, N.; Liu, J.; Min, X.; Wang, Z. Comparative study of structures and properties of HTPBs synthesized via three different polymerization methods. Polym. Test. 2018, 68, 201–207. [Google Scholar] [CrossRef]
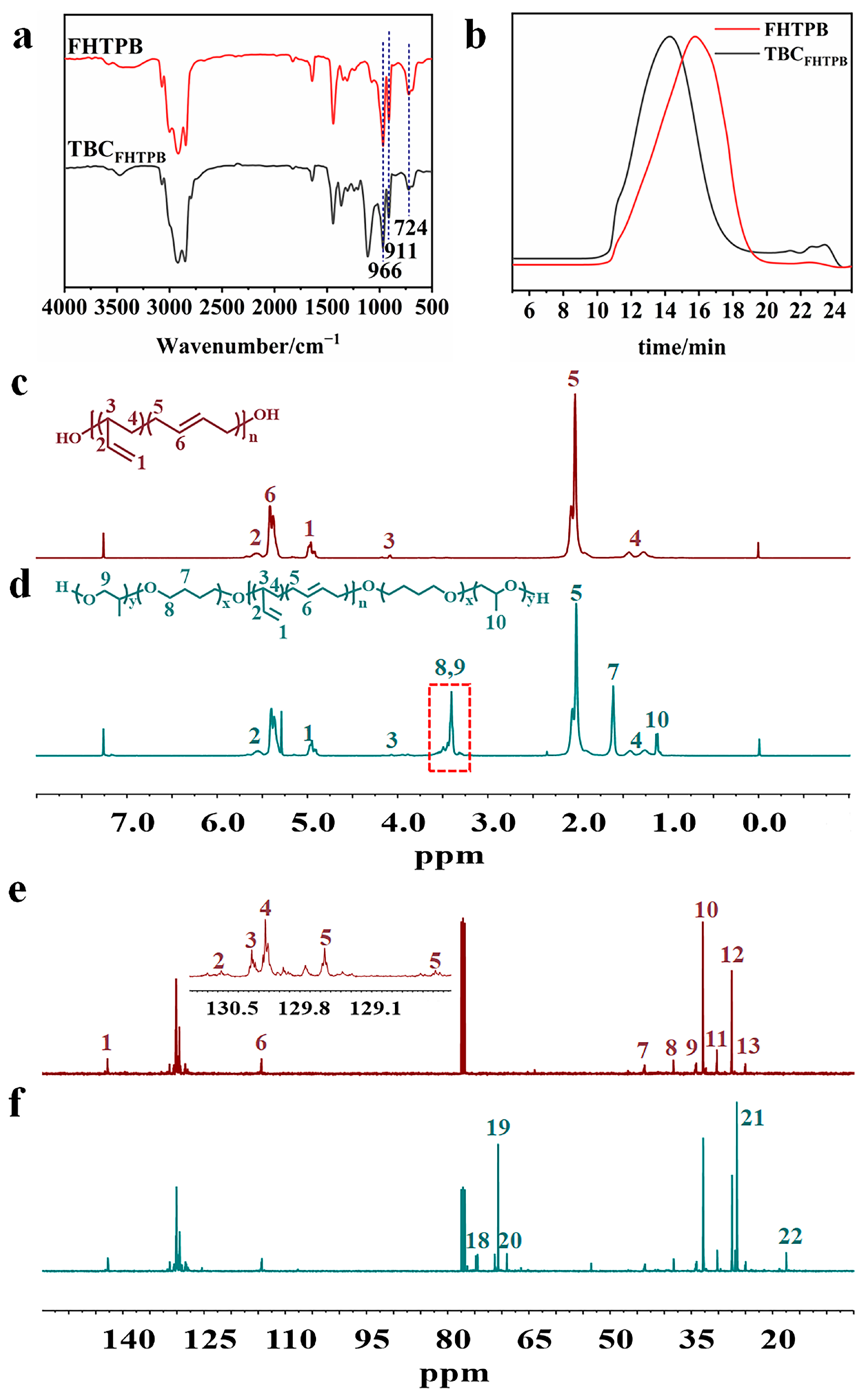
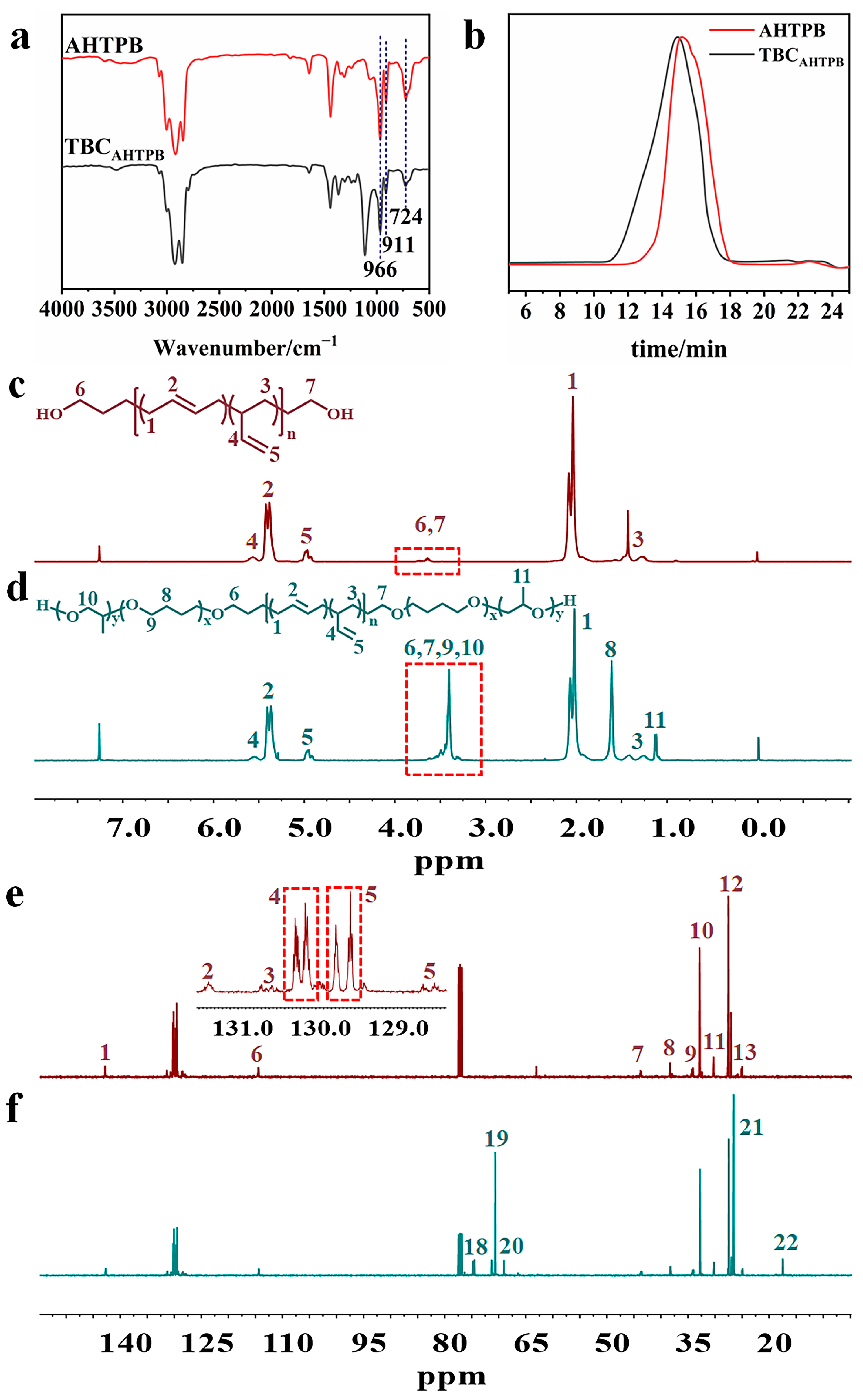



| Peak | Chemical Shift (ppm) | Carbon |
|---|---|---|
| 1 | 142.78 | -H=CH2 |
| 2 | 131.37 | -CH2-CH=H-CH2- |
| 3, 4 | 130.49–130.74, 128.78–130.32 | -CH2-H=H-CH2- |
| 5 | 128.39–129.78 | -CH2-H=CH-CH2- |
| 6 | 114.44 | -CH=H2 |
| 7 | 43.56 | -CH2-H- |
| 8 | 38.29 | -H2-CH=CH-CH2- |
| 9 | 34.15 | -H2-CH(C2H4)-H2-CH= |
| 10, 12 | 32.71, 27.44 | -H2-CH=CH-H2- |
| 11, 13 | 30.15, 24.95 | -CH2-CH=CH-H2- |
| 14, 15 | 132.59, 130.22 | -CH2-CH=H-CH2-OH |
| 16, 17 | 63.87, 58.61 | -CH2-CH=CH-H2-OH |
| 18 | 74.39–74.85 | -CH2-O-H-CH3 |
| 19 | 70.71–71.28 | -O-H2-CH2-CH2-H2-O- |
| 20 | 69.07 | -H2-O-CH-CH3 |
| 21 | 26.52–26.61 | -O-CH2-H2-H2-CH2-O- |
| 22 | 17.48 | -CH2-O-CH-H3 |
| Polymer | Mn/g mol−1 | Mw/Mn | % of Microstructure Determined via FT-IR | ||
|---|---|---|---|---|---|
| Cis-1,4 | 1,2 | Trans-1,4 | |||
| FHTPB | 3400 | 1.67 | 26.2 | 30.1 | 43.7 |
| AHTPB | 3500 | 1.03 | 58.9 | 15.7 | 25.4 |
| RHTPB | 3500 | 1.47 | 78.0 | 0 | 22.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, P.; Zhu, X.; Han, J.; Tian, L.; Zhang, W. Synthesis and Comparative Study of Polyether-b-polybutadiene-b-polyether Triblock Copolymers for Use as Polyurethanes. Polymers 2023, 15, 3486. https://doi.org/10.3390/polym15163486
Bi P, Zhu X, Han J, Tian L, Zhang W. Synthesis and Comparative Study of Polyether-b-polybutadiene-b-polyether Triblock Copolymers for Use as Polyurethanes. Polymers. 2023; 15(16):3486. https://doi.org/10.3390/polym15163486
Chicago/Turabian StyleBi, Pengzhi, Xiuzhong Zhu, Jinbang Han, Li Tian, and Wanbin Zhang. 2023. "Synthesis and Comparative Study of Polyether-b-polybutadiene-b-polyether Triblock Copolymers for Use as Polyurethanes" Polymers 15, no. 16: 3486. https://doi.org/10.3390/polym15163486
APA StyleBi, P., Zhu, X., Han, J., Tian, L., & Zhang, W. (2023). Synthesis and Comparative Study of Polyether-b-polybutadiene-b-polyether Triblock Copolymers for Use as Polyurethanes. Polymers, 15(16), 3486. https://doi.org/10.3390/polym15163486






