Polymer-Derived Carbon Nanofiber and Its Photocurrent-Switching Responses of Carbon Nanofiber/Cu Nanocomposite in Wide Ranges of Excited Light Wavelength
Abstract
:1. Introduction
2. Experimental Details
2.1. Raw Materials
2.2. Synthesis of Carbon Nanofiber with Carboxymethyl Cellulose Hydrothermal Carbonization
2.3. Defects Passivation of Carbon Nanofiber Derived with Carboxymethyl Cellulose with Cu Nanoparticles
2.4. Morphology Observation with SEM
2.5. Energy Dispersive Spectroscopy (EDS) Measurements
2.6. Morphology Observation with TEM
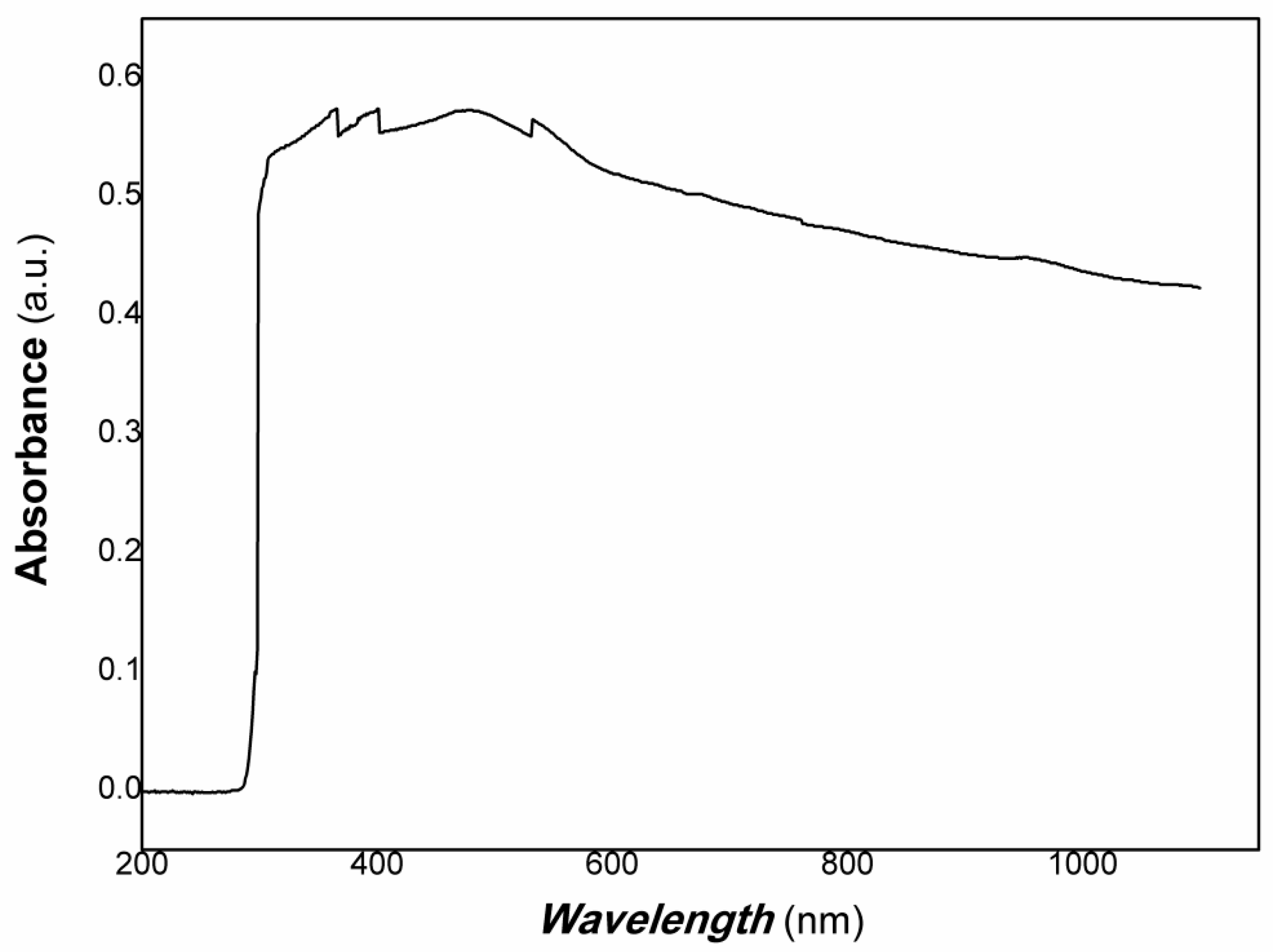
2.7. Measurement of UV-Vis-NIR Spectrum
2.8. XRD Characterization
2.9. Photocurrent Response of Nanocomposite to Visible Light and NIR
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Roy, S.; Sarkar, S.; Xu, J.; Zhao, J.; Zhang, Y. A review of carbon dots and their composite materials for electrochemical energy technologies. Carbon Energy 2021, 3, 795–826. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, J.; Song, T.; Ni, J.; Wang, F.; Xiong, H. Carbon dots crosslinked gel polymer electrolytes for dendrite-free and long-cycle lithium metal batteries. SmartMat 2022, 3, 323–336. [Google Scholar] [CrossRef]
- Kumar, P.; Dua, S.; Kaur, R.; Kumar, M.; Bhatt, G. A review on advancements in carbon quantum dots and their application in photovoltaics. RSC Adv. 2022, 12, 4714. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, A.; Dharmadhikari, B.; Debnath, D.; Patra, P.; Kumar, C.V. Advances in Structural Modifications and Properties of Graphene Quantum Dots for Biomedical Applications. ACS Omega 2023, 8, 21358–21376. [Google Scholar] [CrossRef]
- Lu, H.; Xie, Y.; Zhang, Z.; Zhang, Z.; Sun, J.; Zhang, H.; Tang, B. Emerging Clusteroluminescence from Complexes between Carbonyl-Based Polymers and Organic Base. Chin. J. Chem. 2023, in press. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Yang, G. A facile strategy for the preparation of carboxymethylcellulose-derived polymer dots and their application to detect tetracyclines. Macromol. Chem. Phys. 2021, 222, 2100267. [Google Scholar] [CrossRef]
- Bazazi, S.; Hosseini, S.P.; Hashemi, E.; Rashidzadeh, B.; Liu, Y.; Reza Saeb, M.; Xiao, H.; Seidi, F. Polysaccharide-based C-dots and polysaccharide/C-dot nanocomposites: Fabrication strategies and applications. Nanoscale 2023, 15, 3630. [Google Scholar] [CrossRef]
- He, X.; Chen, P.; Zhang, J.; Luo, T.; Wang, H.; Liu, Y.; Yu, X. Cationic Polymer-Derived Carbon Dots for Enhanced Gene Delivery and Cell Imaging. Biomater Sci. 2019, 7, 1940–1948. [Google Scholar] [CrossRef]
- Bhandari, S.; Mondal, D.; Nataraj, S.K.; Balakrishna, R.G. Biomolecule-derived quantum dots for sustainable optoelectronics. Nanoscale Adv. 2019, 1, 913–936. [Google Scholar] [CrossRef]
- Sabet, M.; Mahdavi, K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Monday, Y.N.; Abdullah, J.; Yusof, N.A.; Abdul Rashid, S.; Shueb, R.H. Facile Hydrothermal and Solvothermal Synthesis and Characterization of Nitrogen-Doped Carbon Dots from Palm Kernel Shell Precursor. Appl. Sci. 2021, 11, 1630. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Chuangchote, S.; Wongyao, N.; Surareungchai, W. Biomass-derived Carbon Quantum Dots—A Review Part 1: Preparation and Characterization. ChemBioEng Rev. 2021, 8, 265–301. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Lu, H.; Liang, X.; Wang, H.; Zhang, M.; Xia, K.; Yin, Z.; Zhang, Y.; Zhang, X.; et al. Sustainable Silk-Derived Multimode Carbon Dots. Small 2021, 17, 2103623. [Google Scholar] [CrossRef]
- Lou, Y.; Hao, X.; Liao, L.; Zhang, K.; Chen, S.; Li, Z.; Ou, J.; Qin, A.; Li, Z. Recent advances of biomass carbon dots on syntheses, characterization, luminescence mechanism, and sensing applications. Nano Sel. 2021, 2, 1117–1145. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Wang, R.; Han, T.; Liu, X.; Wang, B.; Huang, C.; Zhu, S.; Chen, J. Indole Carbonized Polymer Dots Boost Full-Color Emission by Regulating Surface State. iScience 2020, 23, 101546. [Google Scholar] [CrossRef]
- Raveendran, V.; Babu, A.R.S.; Renuka, N.K. Mint leaf derived carbon dots for dual analyte detection of Fe(III) and ascorbic acid. RSC Adv. 2019, 9, 12070. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Tian, L.; Xiang, W.; Wang, T.; Hu, W.; Hu, Y.; Chen, S.; Chen, J.; Dai, Z. Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chem. 2018, 20, 4438–4442. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Xue, Y.; Lu, S.; Yang, H.; Yang, L.; Ding, C.; Yu, S. Cross-Linked Polyamide Chains Enhanced the Fluorescence of Polymer Carbon Dots. ACS Omega 2020, 5, 8219–8229. [Google Scholar] [CrossRef]
- Rocco, D.; Moldoveanu, V.G.; Feroci, M.; Bortolami, M.; Vetica, F. Electrochemical Synthesis of Carbon Quantum Dots. Chem. Electro. Chem. 2023, 10, e202201104. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, M.; Na, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Review of natural product derived carbon dots: From natural products to functional materials. Chem. Sus. Chem. 2017, 11, 11–24. [Google Scholar] [CrossRef]
- Dezfuli, A.S.; Kohan, E.; Fateh, S.T.; Alimirzaei, N.; Arzaghi, H.; Hamblin, M.R. Organic dots (O-dots) for theranostic applications: Preparation and surface engineering. RSC Adv. 2021, 11, 2253. [Google Scholar] [CrossRef]
- Döring, A.; Ushakova, E.; Rogach, A.L. Chiral carbon dots: Synthesis, optical properties, and emerging applications. Light Sci. Appl. 2022, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Zhou, C.; Kang, C.; Zhu, S.; Feng, T.; Zhang, S.; Ding, Z.; Zheng, C.; Xia, C.; Yang, B. Confined-domain crosslink-enhanced emission effect in carbonized polymer dots. Light Sci. Appl. 2022, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xue, S.; Sun, L.; Zong, X.; An, L.; Qu, D.; Wang, X.; Sun, Z. Formation and fluorescent mechanism of red emissive carbon dots from o-phenylenediamine and catechol system. Light Sci. Appl. 2022, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kundelev, E.V.; Vedernikova, A.A.; Cherevkov, S.A.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; Tsypkin, A.N.; Litvin, A.P.; Baranov, A.V.; et al. Revealing the nature of optical activity in carbon dots produced from different chiral precursor molecules. Light: Sci. Appl. 2022, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, J.; Wang, Z.; Liu, X.; Xu, Y.; Zhao, H.; Astruc, D. Turning waste into wealth: Facile and green synthesis of carbon nanodots from pollutants and applications to bioimaging. Chem. Sci. 2021, 12, 11722–11729. [Google Scholar] [CrossRef]
- Guo, L.; Ge, J.; Liu, W.; Niu, G.; Jia, Q.; Wang, H.; Wang, P. Tunable Multicolor Carbon Dots Prepared from Well-defined Polythiophene Derivatives and their Emission Mechanism. Nanoscale 2016, 8, 729–734. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Q.; Li, Y.; Yu, Y.; Li, Z.; Lin, H. Tumor-specific and photothermal-augmented chermodynamic therapy by ferrocene-carbon dot-crosslinked nanoparticles. Smart Mat. 2022, 3, 311–322. [Google Scholar]
- Gentile, G.; Mamone, M.; Rosso, C.; Amato, F.; Lanfrit, C.; Filippini, G.; Prato, M. Tailoring the Chemical Structure of Nitrogen-Doped Carbon Dots for Nano-Aminocatalysis in Aqueous Media. ChemSusChem 2023, 16, e202202399. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Nan, F.; Wang, J.; Zhang, Y.; Liang, K.; Xue, X.; Chen, T.; Kong, L.; Ge, J.; et al. Polythiophene Derivatives Carbonized Polymer Dots: Aggregation Induced Solid-State Fluorescence Emission. Chin. J. Chem. 2023, 41, 1950–1956. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, J.H.; Wang, G.; Zhang, Z.; Wu, J.; Zhang, B.; Zhang, H.; Liu, E.; Wang, L.; Liu, T.; et al. Toward Strong Near-Infrared Absorption/Emission from Carbon Dots in Aqueous Media through Solvothermal Fusion of Large Conjugated Perylene Derivatives with Post-Surface Engineering. Adv. Sci. 2022, 9, 2202283. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wei, Z.; Sui, L.; Yu, J.; Zhang, B.; Wang, X.; Feng, S.; Song, H.; Yong, X.; Tian, Y.; et al. Electron–phonon coupling-assisted universal red luminescence of o-phenylenediamine-based carbon dots. Light Sci. Appl. 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Wang, Y.; Lin, C.; Zheng, L.; Du, J.; Zhuang, Y.; Xie, R.; Li, Z.; Lin, H. Enabling robust and hour-level organic long persistent luminescence from carbon dots by covalent fixation. Light Sci. Appl. 2022, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Rafieea, Z.; Omidi, S. Modification of carbon-based nanomaterials by polyglycerol: Recent advances and applications. RSC Adv. 2022, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Arcudi, F.; Đorđević, L. Supramolecular Chemistry of Carbon-Based Dots Offers Widespread Opportunities. Small 2023, 19, 2300906. [Google Scholar] [CrossRef]
- Stergiou, A.; Tagmatarchis, N. Interfacing Carbon Dots for Charge-Transfer Processes. Small 2021, 17, 2006005. [Google Scholar] [CrossRef]
- Tsai, W.; Chan, Y. Semiconducting polymer dots as near-infrared fluorescent probes for bioimaging and sensing. J. Chin. Chem. Soc. 2018, 66, 9–20. [Google Scholar] [CrossRef]
- Wang, H.; Ai, L.; Song, H.; Song, Z.; Yong, X.; Qu, S.; Lu, S. Innovations in the Solid-State Fluorescence of Carbon Dots: Strategies, Optical Manipulations, and Applications. Adv. Funct. Mater. 2023, 33, 2303756. [Google Scholar] [CrossRef]
- Xu, A.; Wang, G.; Li, Y.; Dong, H.; Yang, S.; He, P.; Ding, G. Carbon-Based Quantum Dots with Solid-State Photoluminescent: Mechanism, Implementation, and Application. Small 2020, 16, 2004621. [Google Scholar] [CrossRef]
- Kang, C.; Tao, S.; Yang, F.; Yang, B. Aggregation and luminescence in carbonized polymer dots. Aggregate 2022, 3, e169. [Google Scholar] [CrossRef]
- Ru, Y.; Waterhouse, G.I.N.; Lu, S. Aggregation in carbon dots. Aggregate 2022, 3, e296. [Google Scholar] [CrossRef]
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon dot/polymer nanocomposites: From green synthesis to energy, environmental and biomedical applications. Sustain. Mater. Technol. 2021, 29, e00304. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, H.; Yuan, T.; Meng, T.; Wu, H.; Chang, J.; Wang, H.; Song, X.; Li, Y.; Li, X.; et al. Carbon dots: An innovative luminescent nanomaterial. Aggregate 2022, 3, e108. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, Q.; Tao, S.; Xia, C.; Liu, C.; Liu, P.; Yang, B. Carbon Dots Based Photoinduced Reactions: Advances and Perspective. Adv. Sci. 2023, 10, 2207621. [Google Scholar] [CrossRef]
- Pandit, S.; Mondal, S.; De, M. Surface engineered amphiphilic carbon dots: Solvatochromic behavior and applicability as a molecular probe. J. Mater. Chem. B 2021, 9, 14321440. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Hu, G.; Mo, L.; Zheng, M.; Lei, B.; Zhang, X.; Hu, C.; Zhuang, J.; Liu, Y. Surface functional carbon dots: Chemical engineering applications beyond their optical property. J. Mater. Chem. C 2020, 8, 16282–16294. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Wu, M.; Zhu, J.; Zhang, X. Multifunctional Carbon-Based Nanomaterials: Applications in Biomolecular Imaging and Therapy. ACS Omega 2018, 3, 9126–9145. [Google Scholar] [CrossRef]
- Srinivasan, V.; Jhonsi, M.A.; Kathiresan, M.; Kathiravan, A. Nanostructured Graphene Oxide Dots: Synthesis, Characterization, Photoinduced Electron Transfer Studies, and Detection of Explosives/Biomolecules. ACS Omega 2018, 3, 9096–9104. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, H.; Zheng, M.; Xu, K.; Zou, C.; Qu, S.; Tan, Z. Narrow-bandwidth emissive carbon dots: A rising star in the fluorescent material family. Carbon Energy 2022, 4, 88–114. [Google Scholar] [CrossRef]
- Zheng, C.; Tao, S.; Yang, B. Polymer-Structure-Induced Room-Temperature Phosphorescence of Carbon Dot Materials. Small Struct. 2023, 4, 2200327. [Google Scholar] [CrossRef]
- Hao, H.; Yan, L.; Wang, M.; Cao, Y.; He, J.; Yang, Y. Research Process of Carbon Dots in Memristors. Adv. Electron. Mater. 2023, 9, 2201195. [Google Scholar] [CrossRef]
- Xue, S.; Li, P.; Sun, L.; An, L.; Qu, D.; Wang, X.; Sun, Z. The Formation Process and Mechanism of Carbon Dots Prepared from Aromatic Compounds as Precursors: A Review. Small 2023, 19, 2206180. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, C.; Zhang, X.; Gao, M.; Li, G. Broadband Spectrum Light-Driven PANI/Au/Beta-Cyclodextrin Nanocomposite and Its Light-Triggered Interfacial Carrier Transfer. Coatings 2022, 12, 1401. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.; Gao, M.; Zhang, X.; Wang, Y.; Li, G. Interface Optimization of Metal Quantum Dots/Polymer Nanocomposites and Their Properties: Studies of Multi-Functional Organic/Inorganic Hybrid. Materials 2023, 16, 150. [Google Scholar] [CrossRef]
- Ma, X.; Wang, M.; Li, G.; Chen, H.; Bai, R. Preparation of Polyaniline-TiO2 Composite Film with in-situ Polymerization Approach and Its Gas-sensitivity at Room Temperature. Mater. Chem. Phys. 2006, 98, 241–247. [Google Scholar] [CrossRef]
- Dou, L.; Cui, F.; Yu, Y.; Khanarian, G.; Eaton, S.W.; Yang, Q.; Resasco, J.; Schildknecht, C.; Schierle-Arndt, K.; Yang, P. Solution-Processed Copper/Reduced-Graphene-Oxide Core/Shell Nanowire Transparent Conductors. ACS Nano 2016, 10, 2600–2606. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Domingues, S.H.; Chou, H.; Wang, X.; Tan, C.; Kim, J.; Li, H.; Piner, R.; Zarbin, A.J.G.; Ruoff, R.S. Reduced Graphene Oxide/Copper Nanowire Hybrid Films as High-Performance Transparent Electrodes. ACS Nano 2013, 7, 1811–1816. [Google Scholar] [CrossRef]
- Li, W.; Hu, D.; Li, L.; Li, C.; Jiu, J.; Chen, C.; Ishina, T.; Sugahara, T.; Suganuma, K. Printable and Flexible Copper–Silver Alloy Electrodes with High Conductivity and Ultrahigh Oxidation Resistance. ACS Appl. Mater. Interfaces 2017, 9, 24711–24721. [Google Scholar] [CrossRef]
- Maria, F.D.; Lodola, F.; Zucchetti, E.; Benfenati, F.; Lanzani, G. The evolution of artificial light actuators in living systems: From planar to nanostructured interfaces. Chem. Soc. Rev. 2018, 47, 4757–4780. [Google Scholar] [CrossRef] [PubMed]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Gao, M.; Zhang, X.; Wang, Y.; Li, G. Polymer-Derived Carbon Nanofiber and Its Photocurrent-Switching Responses of Carbon Nanofiber/Cu Nanocomposite in Wide Ranges of Excited Light Wavelength. Polymers 2023, 15, 3528. https://doi.org/10.3390/polym15173528
Ma X, Gao M, Zhang X, Wang Y, Li G. Polymer-Derived Carbon Nanofiber and Its Photocurrent-Switching Responses of Carbon Nanofiber/Cu Nanocomposite in Wide Ranges of Excited Light Wavelength. Polymers. 2023; 15(17):3528. https://doi.org/10.3390/polym15173528
Chicago/Turabian StyleMa, Xingfa, Mingjun Gao, Xintao Zhang, You Wang, and Guang Li. 2023. "Polymer-Derived Carbon Nanofiber and Its Photocurrent-Switching Responses of Carbon Nanofiber/Cu Nanocomposite in Wide Ranges of Excited Light Wavelength" Polymers 15, no. 17: 3528. https://doi.org/10.3390/polym15173528
APA StyleMa, X., Gao, M., Zhang, X., Wang, Y., & Li, G. (2023). Polymer-Derived Carbon Nanofiber and Its Photocurrent-Switching Responses of Carbon Nanofiber/Cu Nanocomposite in Wide Ranges of Excited Light Wavelength. Polymers, 15(17), 3528. https://doi.org/10.3390/polym15173528




_Ma.jpg)


