Bioinspired Artificial Muscles Based on Sodium Alginate-Wrapped Multi-Walled Carbon Nanotubes and Molybdenum Disulfide Composite Electrode Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
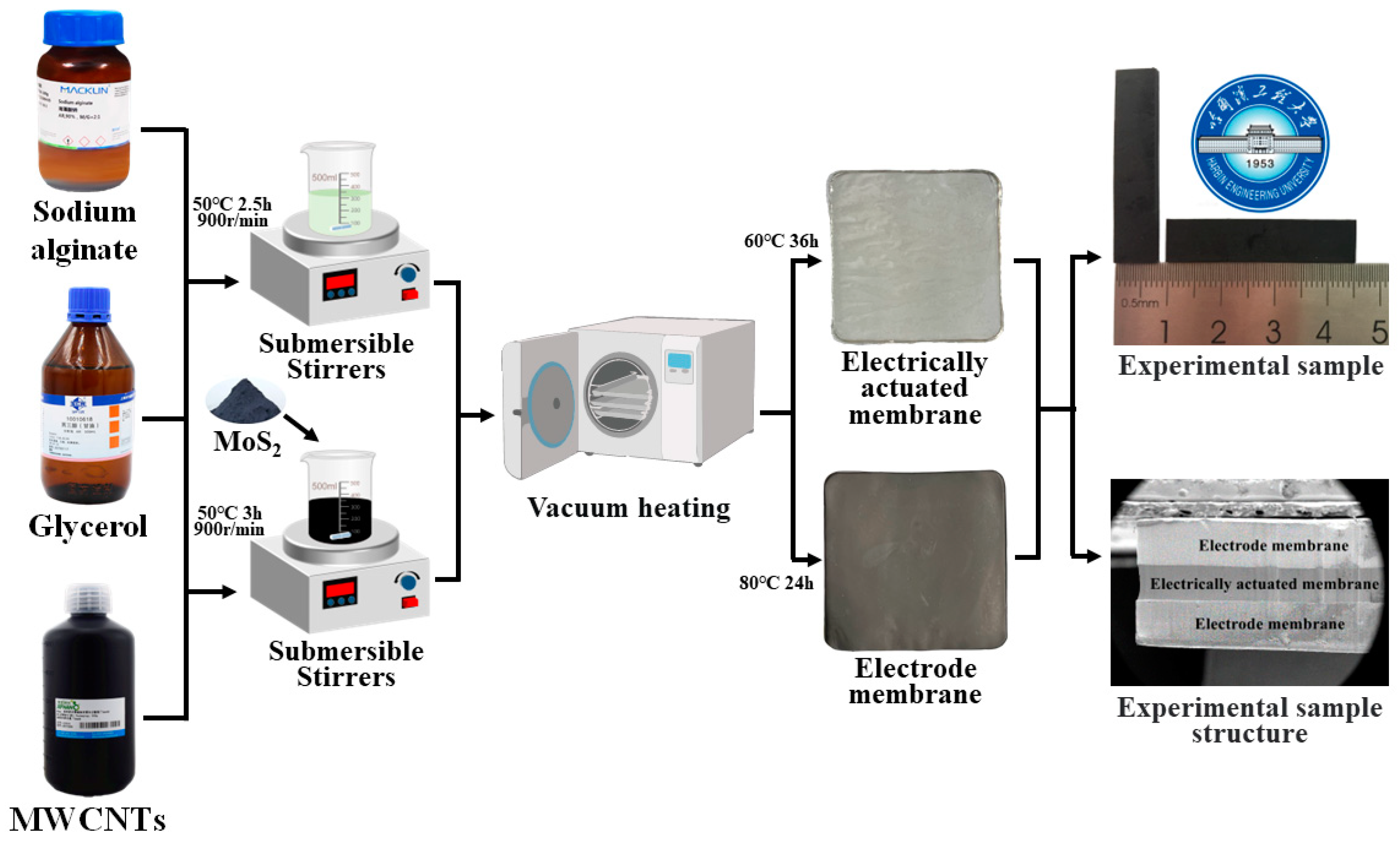
2.2. Preparation of BMAMs
2.2.1. Electroactive Film Fabrication
2.2.2. Electrode Membrane Fabrication
2.2.3. Assembly of the BMAM
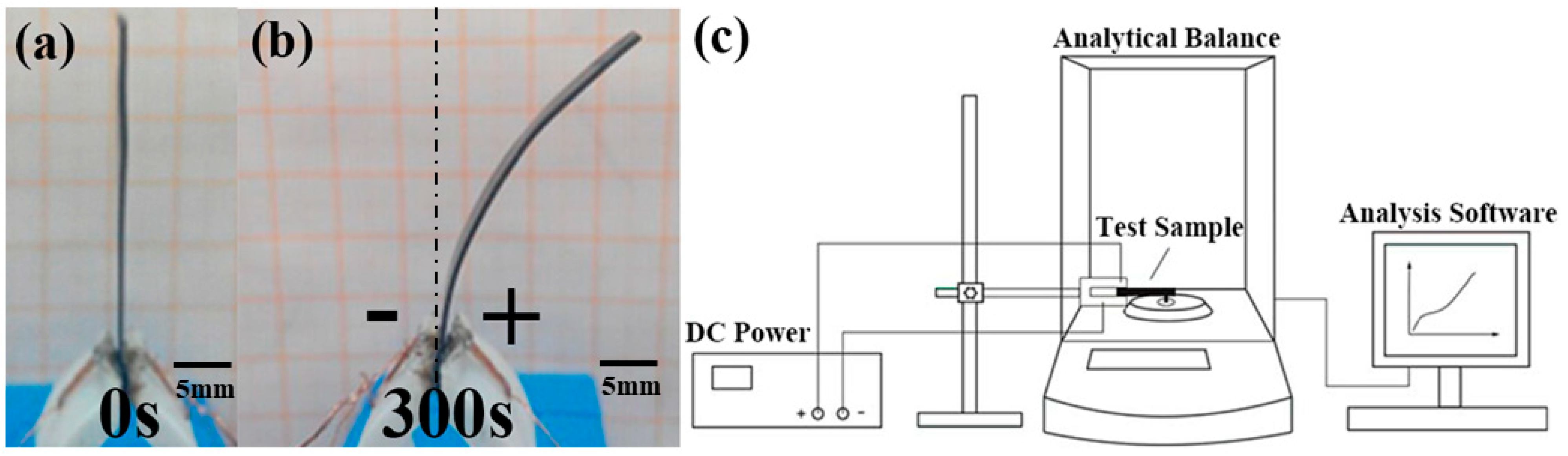
2.3. Testing Methods
3. Results and Discussion
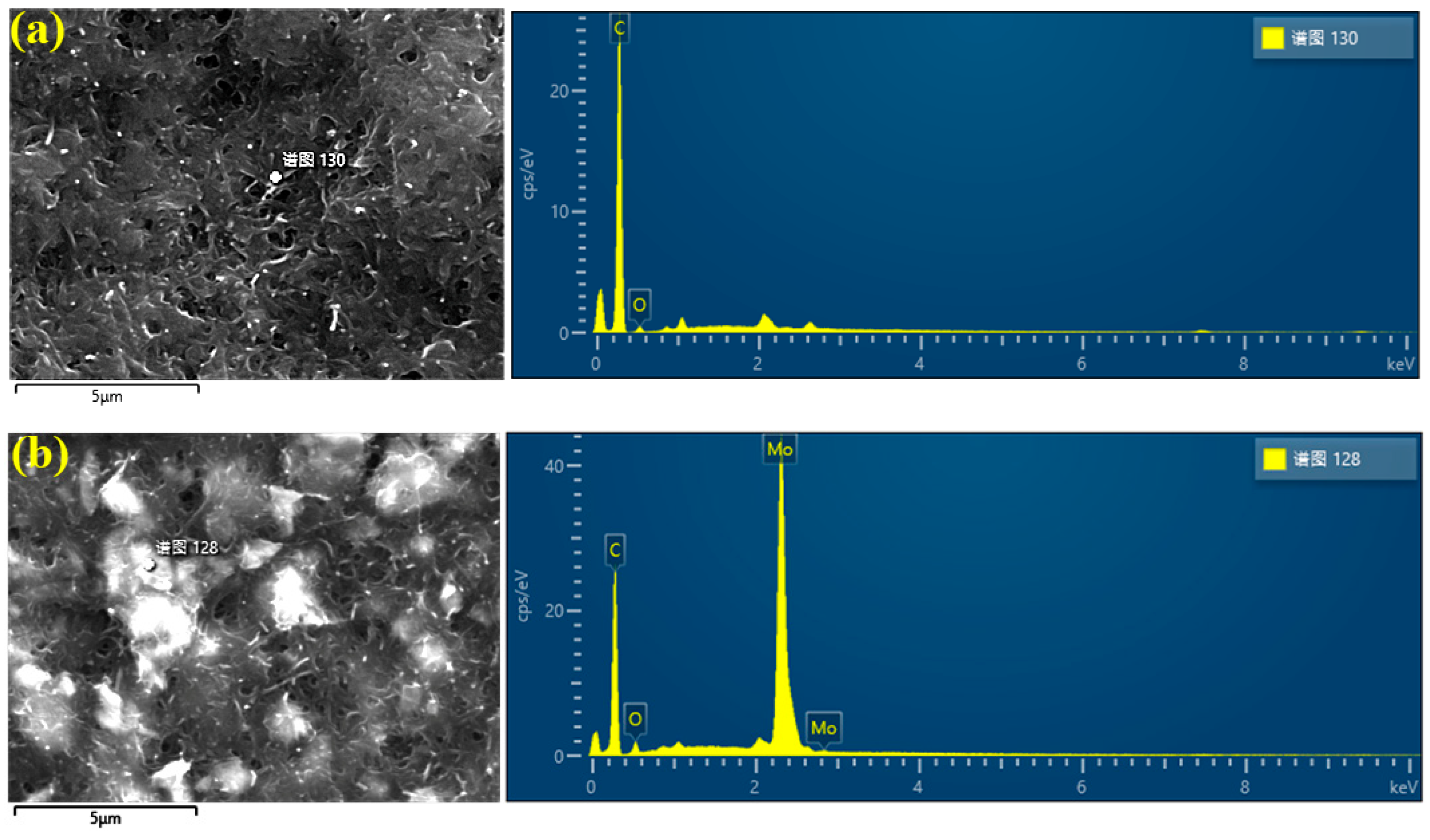
3.1. Analysis of Scanning Electron Microscope Results
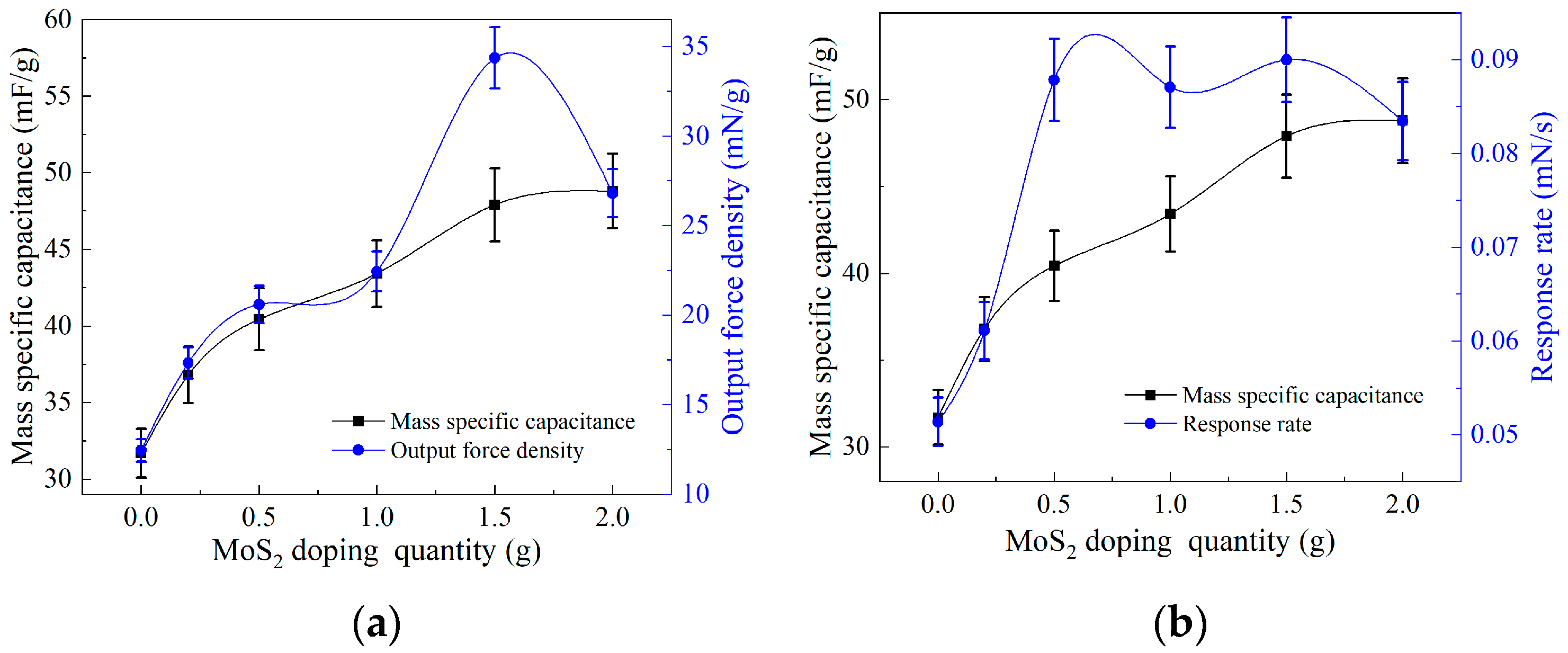
3.2. Analysis of Output Force Testing Results
3.3. Analysis of Electrochemical Testing Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, K.; Shen, H.; Xue, W. Wet-Driven Bionic Actuators from Wool Artificial Yarn Muscles. ACS Appl. Mater. Interfaces 2023, 15, 16232–16243. [Google Scholar] [CrossRef] [PubMed]
- Orozco, F.; Salvatore, A.; Sakulmankongsuk, A.; Gomes, D.R.; Pei, Y.; Araya-Hermosilla, E.; Pucci, A.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Electroactive performance and cost evaluation of carbon nanotubes and carbon black as conductive fillers in self-healing shape memory polymers and other composites. Polymer 2022, 260, 125365. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, J.; He, B.; Hu, Z. Solution processable poly(vinylidene fluoride)-based ferroelectric polymers for flexible electronics. APL Mater. 2021, 9, 010902. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.D.; Shi, Q.; Liang, Y. Electroactive dielectric polymer gels as new-generation soft actuators: A review. J. Mater. Sci. 2021, 56, 14943–14963. [Google Scholar] [CrossRef]
- Liman, M.L.R.; Islam, M.T.; Hossain, M.M. Mapping the Progress in Flexible Electrodes for Wearable Electronic Textiles: Materials, Durability, and Applications. Adv. Electron. Mater. 2021, 8, 2100578. [Google Scholar] [CrossRef]
- Yang, J.; Yao, J.; Ma, Y. A highly flexible, renewable and green alginate polymer for electroactive biological gel paper actuators reinforced with a double-side casting approach. Cellulose 2021, 28, 3647–3662. [Google Scholar] [CrossRef]
- Wang, T.; Farajollahi, M.; Choi, Y.S.; Lin, I.T.; Marshall, J.E.; Thompson, N.M.; Kar-Narayan, S.; Madden, J.D.; Smoukov, S.K. Electroactive polymers for sensing. Interface Focus 2016, 6, 20160026. [Google Scholar] [CrossRef]
- Kanaan, A.F.; Pinho, A.C.; Piedade, A.P. Electroactive Polymers Obtained by Conventional and Non-Conventional Technologies. Polymers 2021, 13, 2713. [Google Scholar] [CrossRef]
- Huang, X.; Wei, J.; Wei, H.; Zhang, L. Magnetocaloric actuation of soft polymer robots. J. Mater. Chem. C 2021, 9, 13635–13639. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Wang, H. Modeling and control of ionic polymer metal composite actuators: A review. Eur. Polym. J. 2023, 186, 111821. [Google Scholar] [CrossRef]
- Feng, C.; Hemantha Rajapaksha, C.P.; Jákli, A. Ionic Elastomers for Electric Actuators and Sensors. Engineering 2021, 7, 581–602. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Y.; Wang, M.; Liang, Y.; Ren, L.; Ren, L. Recent progress in 4D printing of stimuli-responsive polymeric materials. Sci. China Technol. Sci. 2019, 63, 532–544. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- James, R.; Nagarale, R.K.; Sachan, V.K.; Badalucco, C.; Bhattacharya, P.K.; Kumbar, S.G. Synthesis and characterization of electrically conducting polymers for regenerative engineering applications: Sulfonated ionic membranes. Polym. Adv. Technol. 2014, 25, 1439–1445. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. (Weinh) 2019, 6, 1900813. [Google Scholar] [CrossRef]
- Chang, K.-C.; Huang, K.-Y.; Hsu, C.-H.; Ji, W.-F.; Lai, M.-C.; Hung, W.-I.; Chuang, T.-L.; Yeh, J.-M. Synthesis of ultra-high-strength electroactive polyimide membranes containing oligoaniline in the main chain by thermal imidization reaction. Eur. Polym. J. 2014, 56, 26–32. [Google Scholar] [CrossRef]
- Jung, K.; Corrigan, N.; Wong, E.H.H.; Boyer, C. Bioactive Synthetic Polymers. Adv. Mater. 2022, 34, e2105063. [Google Scholar] [CrossRef]
- Szabo, L.; Gerber-Lemaire, S.; Wandrey, C. Strategies to Functionalize the Anionic Biopolymer Na-Alginate without Restricting Its Polyelectrolyte Properties. Polymers 2020, 12, 919. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Lin, Z.; Tang, Z.; Lin, B. Carboxymethyl chitosan/sodium alginate hydrogel films with good biocompatibility and reproducibility by in situ ultra-fast crosslinking for efficient preservation of strawberry. Carbohydr. Polym. 2023, 316, 121073. [Google Scholar] [CrossRef]
- Amare, M.; Admassie, S. Potentiodynamic fabrication and characterization of poly(4-amino-3-hydroxynaphthalene sulfonic acid) modified glassy carbon electrode. J. Mater. Res. Technol. 2020, 9, 11484–11496. [Google Scholar] [CrossRef]
- Bolduc, A.; Skene, W.G. Direct preparation of electroactive polymers on electrodes and their use in electrochromic devices. Polym. Chem. 2014, 5, 1119–1123. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. Characteristics of MOF, MWCNT and graphene containing materials for hydrogen storage: A review. J. Energy Chem. 2019, 30, 132–144. [Google Scholar] [CrossRef]
- Sun, B.; Huang, K.; Qi, X.; Wei, X.; Zhong, J. Rational Construction of a Functionalized V2O5Nanosphere/MWCNT Layer-by-Layer Nanoarchitecture as Cathode for Enhanced Performance of Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 5633–5639. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Nithyadharseni, P.; Lee, S.J.; Durai, G.; Kuppusami, P.; Madhavan, J.; Choi, M.Y. Recent progress and emerging challenges of transition metal sulfides based composite electrodes for electrochemical supercapacitive energy storage. Ceram. Int. 2020, 46, 14317–14345. [Google Scholar] [CrossRef]
- Jamal, F.; Rafique, A.; Moeen, S.; Haider, J.; Nabgan, W.; Haider, A.; Imran, M.; Nazir, G.; Alhassan, M.; Ikram, M.; et al. Review of Metal Sulfide Nanostructures and their Applications. ACS Appl. Nano Mater. 2023, 6, 7077–7106. [Google Scholar] [CrossRef]
- Gao, Y.; Mao, J.; Ye, H.; Meng, F.; Li, Y. A review of the geological characteristics and geodynamic setting of the late Early Cretaceous molybdenum deposits in the East Qinling–Dabie molybdenum belt, East China. J. Asian Earth Sci. 2015, 108, 81–96. [Google Scholar] [CrossRef]
- Yang, J.; Yao, J.; Guan, S. Enhanced electroresponsive and electrochemical properties of the biological gel artificial muscle prepared by sodium alginate and carboxylated chitosan. Sens. Actuators B Chem. 2020, 322, 128526. [Google Scholar] [CrossRef]
- Yang, J.; Yao, J.; Wang, S. Electromechanical response performance of a reinforced biomass gel artificial muscle based on natural polysaccharide of sodium alginate doped with an ionic liquid for micro-nano regulation. Carbohydr. Polym. 2022, 275, 118717. [Google Scholar] [CrossRef]





| Element | Position 1 | Position 2 | ||
|---|---|---|---|---|
| Mass (%) | Atomicity (%) | Mass (%) | Atomicity (%) | |
| C | 96.7 | 97.02 | 70.58 | 92.73 |
| O | 3.93 | 2.98 | 2.96 | 2.92 |
| Mo | 0 | 0 | 26.46 | 4.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Wang, K.; Zhao, G. Bioinspired Artificial Muscles Based on Sodium Alginate-Wrapped Multi-Walled Carbon Nanotubes and Molybdenum Disulfide Composite Electrode Membrane. Polymers 2023, 15, 3535. https://doi.org/10.3390/polym15173535
Ji Y, Wang K, Zhao G. Bioinspired Artificial Muscles Based on Sodium Alginate-Wrapped Multi-Walled Carbon Nanotubes and Molybdenum Disulfide Composite Electrode Membrane. Polymers. 2023; 15(17):3535. https://doi.org/10.3390/polym15173535
Chicago/Turabian StyleJi, Yingxin, Keyi Wang, and Gang Zhao. 2023. "Bioinspired Artificial Muscles Based on Sodium Alginate-Wrapped Multi-Walled Carbon Nanotubes and Molybdenum Disulfide Composite Electrode Membrane" Polymers 15, no. 17: 3535. https://doi.org/10.3390/polym15173535





