Highly Efficient One-Pot Synthesis of Hexakis(m-phenyleneimine) Macrocyle Cm6 and the Thermostimulated Self-Healing Property through Dynamic Covalent Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of Monomer: m-Aminobenzaldehyde Diethylacetal
2.2.1. Synthesis of m-Nitrobenzaldehyde Diethylacetal (1)

2.2.2. Synthesis of m-Aminobenzaldehyde Diethylacetal (2) via Catalytic Hydrogenation of m-Nitrobenzaldehyde Diethylacetal

2.3. Polymerization: Synthesis of Hexakis(m-phenyleneimine) Macrocycle (Cm6)
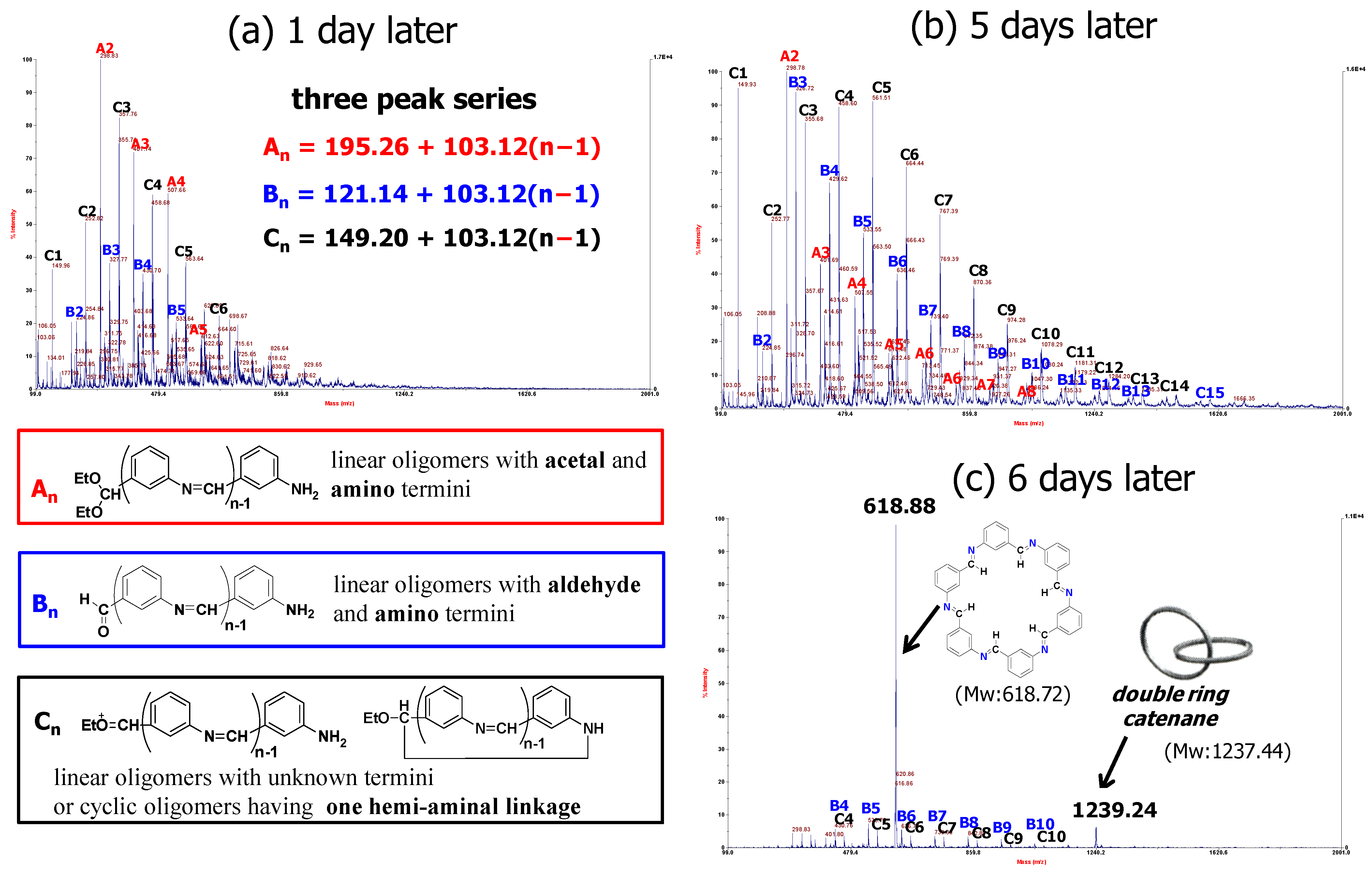
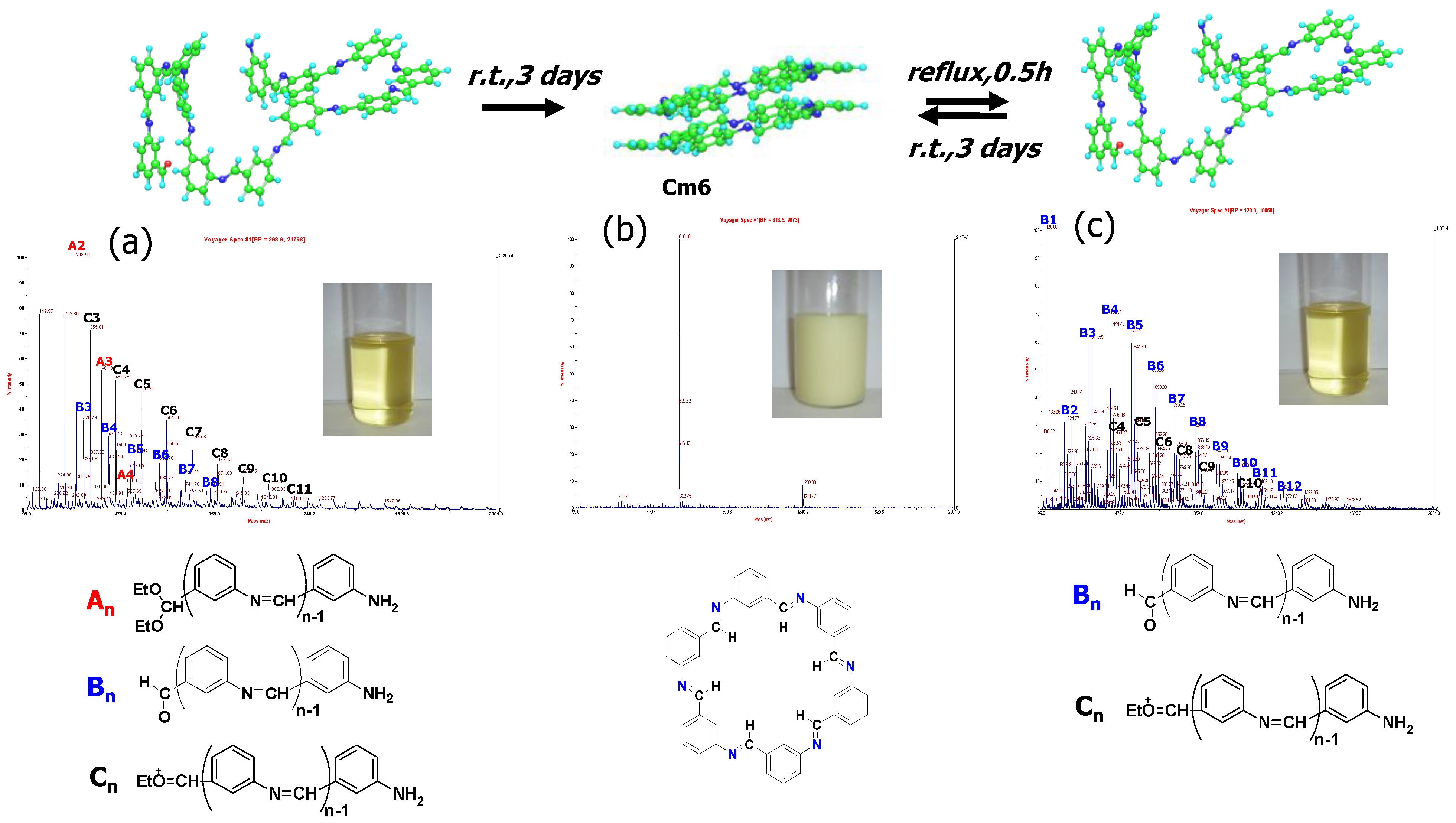
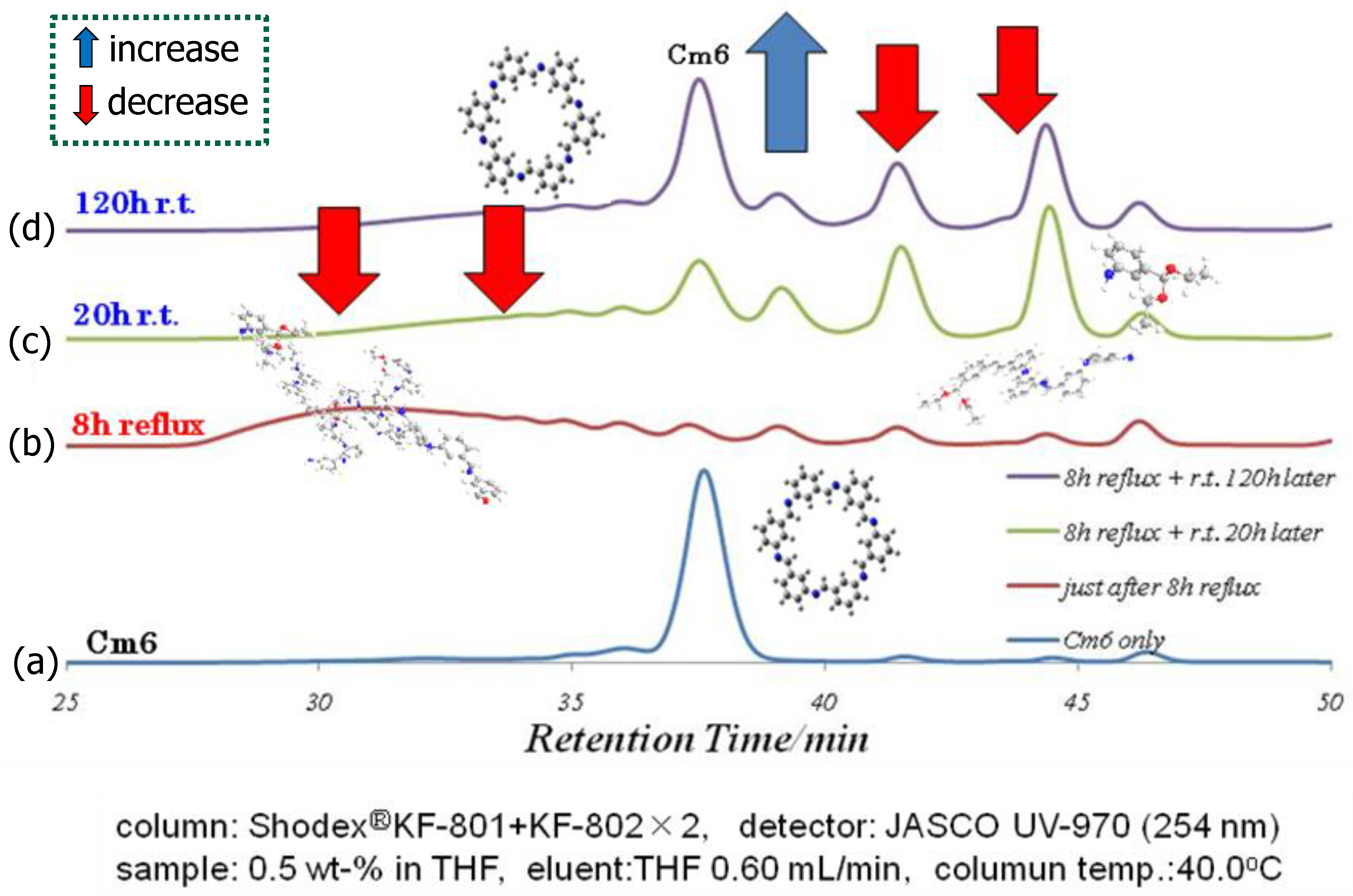
2.4. Self-Healing Analysis of Cm6 Using MALDI-TOF MS and GPC
3. Results and Discussion
3.1. Monomer Synthesis
3.2. Polymerization: Synthesis of Macrocycle Cm6
3.3. Structure of Cm6
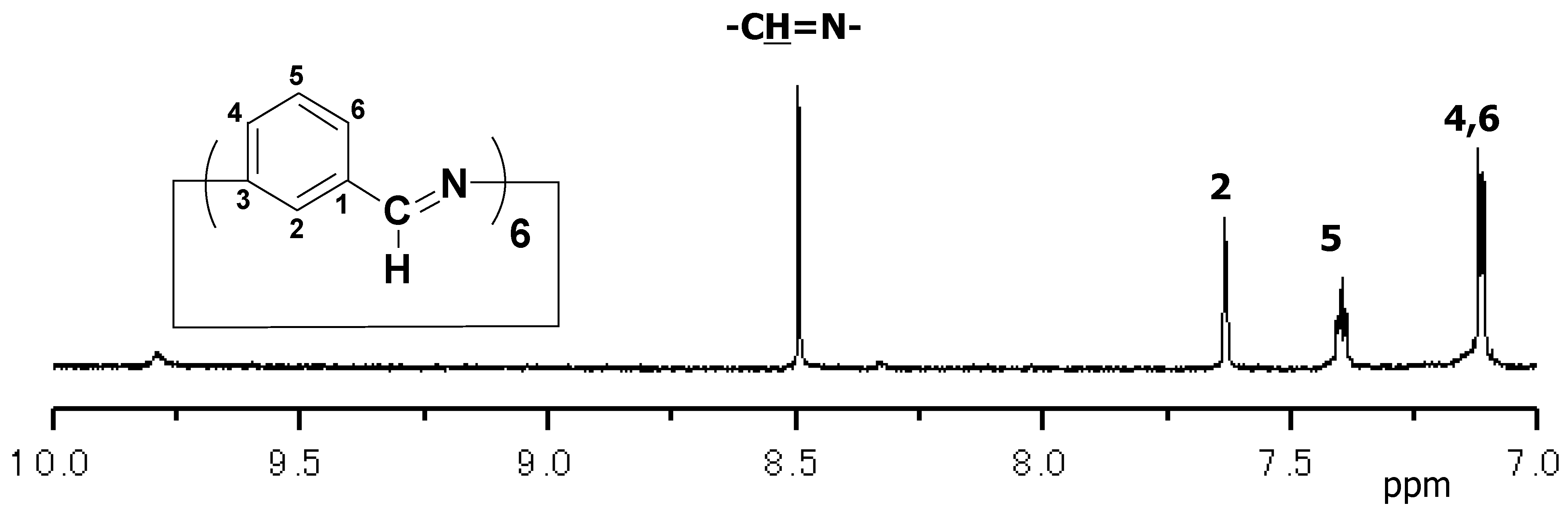
3.3.1. NMR Analysis
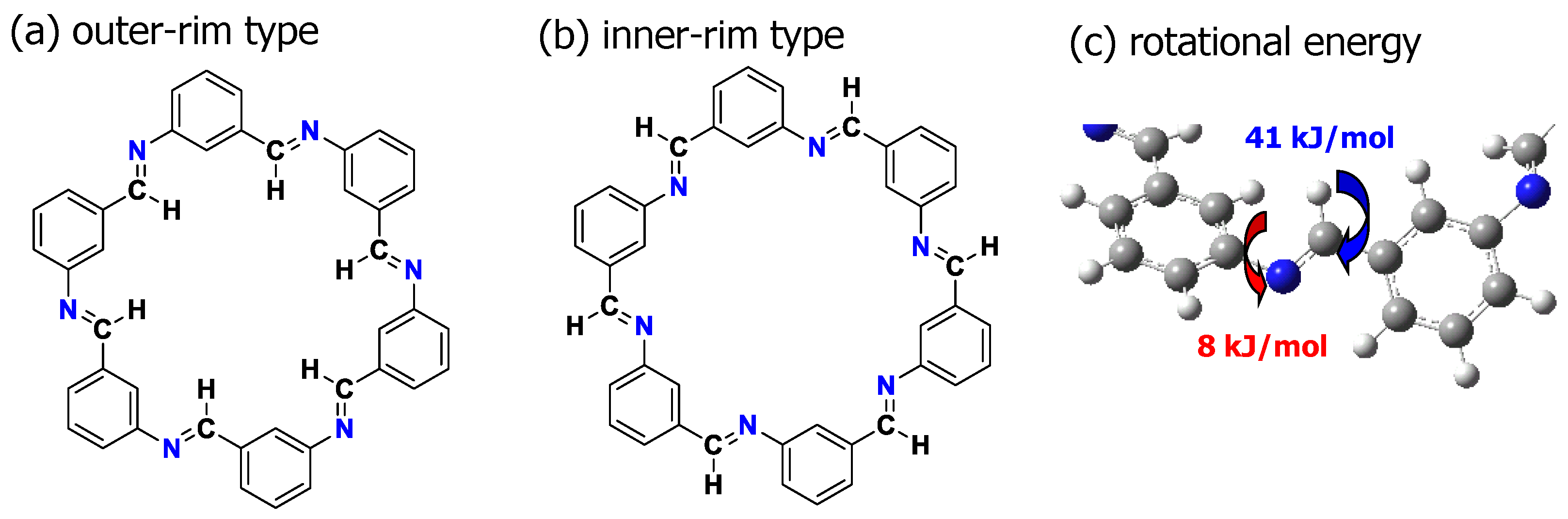
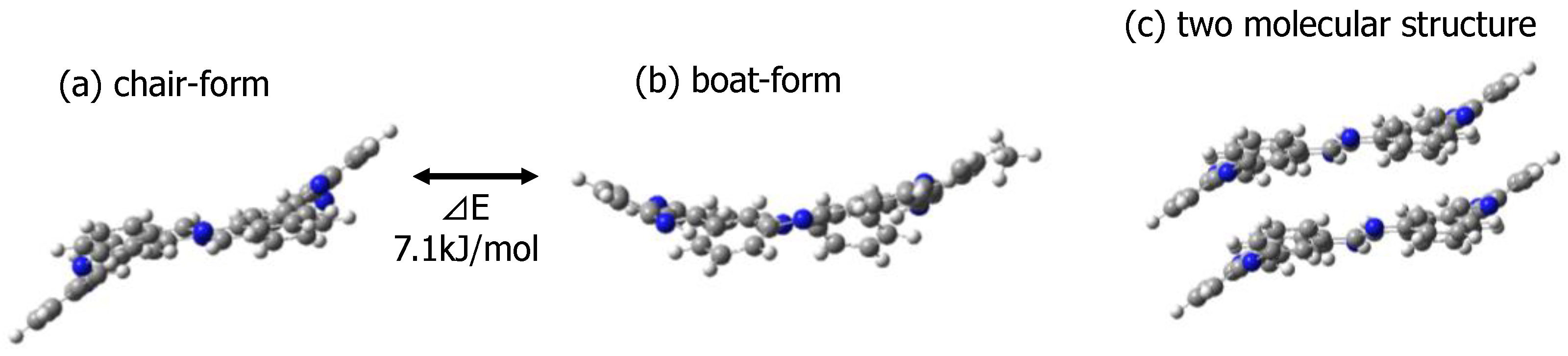
3.3.2. Prediction of Optimal Structure Using MO Calculation
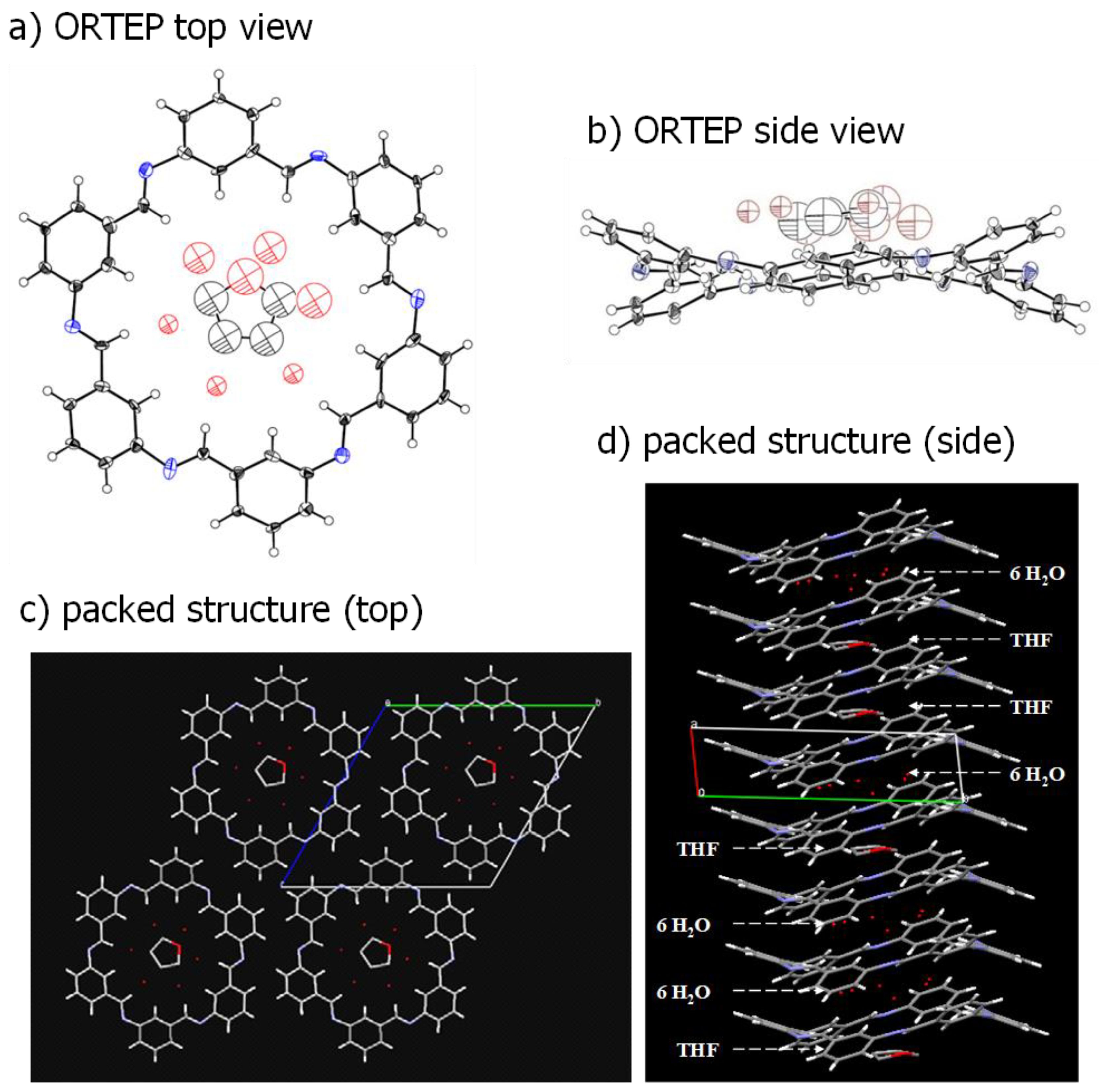
3.3.3. X-ray Analysis of Cm6 Crystal
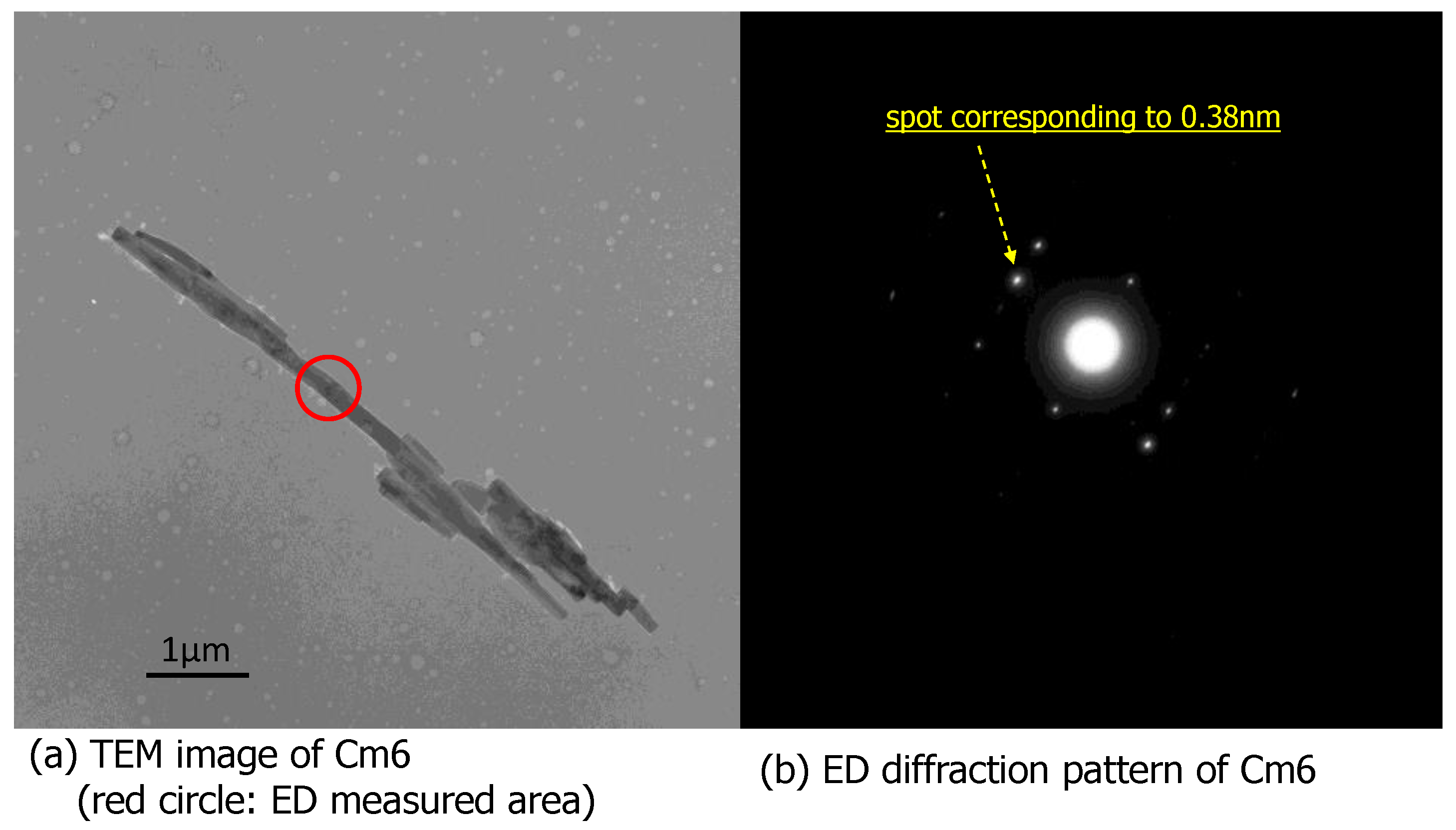
3.3.4. TEM-ED Analysis of Cm6
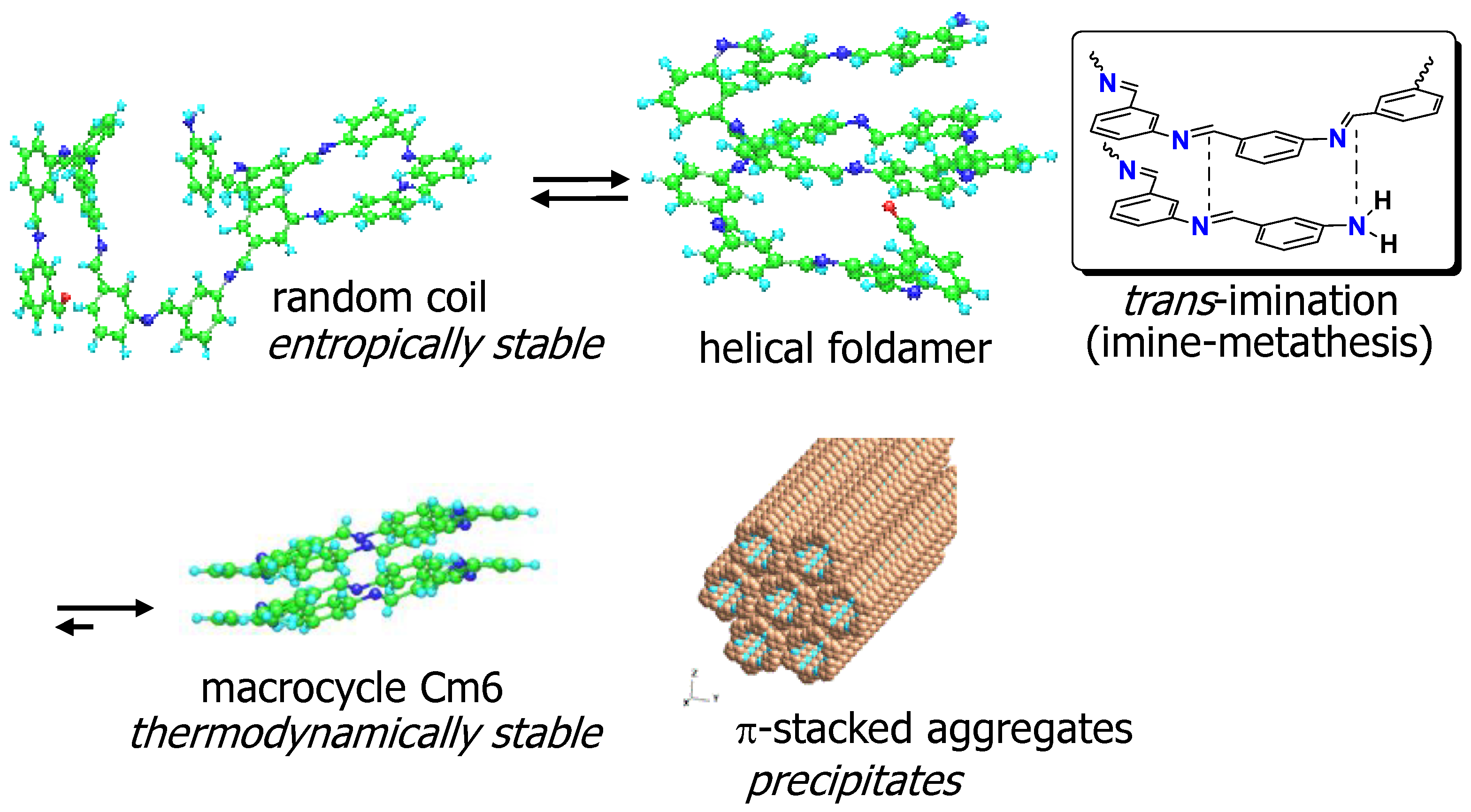
3.4. Possible Mechanism for Quantitative Formation of Macrocycles
3.5. Self-Healing Property
3.5.1. MALDI-TOF MS Analysis
3.5.2. GPC Analysis
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Höger, S. Shape-persistent rings and wheels. Pure Appl. Chem. 2010, 82, 821–830. [Google Scholar] [CrossRef]
- Zhang, W.; Moore, J.S. Shape-persistent macrocycles: Structures and synthetic approaches from arylene and ethynylene building blocks. Angew. Chem. Int. Ed. 2006, 45, 4416–4439. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yamato, K.; Yang, L.; Ferguson, J.S.; Zhong, L.; Zou, S.; Yuan, L.; Zeng, X.C.; Gong, B. Efficient kinetic macrocyclization. J. Am. Chem. Soc. 2009, 131, 2629–2637. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, W.; Hu, J.; Zou, S.; Gao, R.; Yamato, K.; Kline, M.; Cai, Z.; Gao, Y.; Wang, Y.; et al. Strong aggregation and directional assembly of aromatic oligoamide macrocycles. J. Am. Chem. Soc. 2011, 133, 18590–18593. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.E.; Moore, J.S. Arylene-ethynylene macrocycles via depolymerization-macrocyclization. Macromolecules 2011, 44, 3685–3687. [Google Scholar] [CrossRef]
- Zhang, J.; Moore, J.S. Nanoarchitectures. 6. Liquid crystals based on shape-persistent macrocyclic mesogens. J. Am. Chem. Soc. 1994, 116, 2655–2656. [Google Scholar] [CrossRef]
- Islam, M.F.; Adame-Ramirez, E.; Williams, E.R.; Kittikhunnatham, P.; Wijesekera, A.; Zhang, S.; Ge, T.; Stefik, M.; Smith, M.D.; Pellechia, P.J.; et al. Inclusion polymerization of pyrrole and ehylenedioxythiophene in assembled triphenylamine bis-urea macrocycles. Macromolecules 2022, 55, 11013–11022. [Google Scholar] [CrossRef]
- MacLachlan, M.J. Conjugated shape-persistent macrocycles via Schiff-base condensation: New motifs forsupramolecular chemistry. Pure Appl. Chem. 2006, 78, 873–888. [Google Scholar] [CrossRef]
- Lisowski, J. Imine- and amine-type macrocycles derived from chiral diamines and aromatic dianhydrides. Molecules 2022, 27, 4097. [Google Scholar] [CrossRef]
- Zhang, W.; Moore, J.S. Arylene ethynylene macrocycles prepared by precipitation-driven alkyne metathesis. J. Am. Chem. Soc. 2004, 126, 12796. [Google Scholar] [CrossRef]
- Zhang, W.; Moore, J.S. Reaction pathways leading to arylene ethynylene macrocycles via alkyne metathesis. J. Am. Chem. Soc. 2005, 127, 11863–11870. [Google Scholar] [CrossRef]
- Okochi, K.D.; Jin, Y.; Zhang, W. Highly efficient one-pot synthesis of hetero-sequenced shape-persistent macrocycles through orthogonal dynamic covalent chemistry (ODCC). Chem. Commun. 2013, 49, 4418–4420. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Mastalerz, M. Porous shape-persistent organic cage compounds of different size, geometry, and function. Acc. Chem. Res. 2018, 51, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.S.; Moore, J.S. Programmed dynamic covalent assembly of unsymmetrical macrocycles. J. Am. Chem. Soc. 2007, 129, 11682–11683. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, Q.; Taynton, P.; Zhang, W. Dynamic covalent chemistry approaches toward macrocycles, molecular cages, and polymers. Acc. Chem. Res. 2014, 47, 1575–1586. [Google Scholar] [CrossRef]
- Corbett, P.T.; Leclaire, J.; Vial, L.; West, K.R.; Wietor, J.L.; Sanders, J.K.M.; Otto, S. Dynamic combinatorial chemistry. Chem. Rev. 2006, 106, 3652–3711. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, C.; Denman, R.J.; Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6634–6654. [Google Scholar] [CrossRef]
- Janczak, J.; Prochowicz, D.; Lewiński, J.; Fairen-Jimenez, D.; Bereta, T.; Lisowski, J. Trinuclear cage-like ZnII macrocyclic complexes: Enantiomeric recognition and gas adsorption properties. Chem. Eur. J. 2016, 22, 598–609. [Google Scholar] [CrossRef]
- Sarnicka, A.; Starynowicz, P.; Lisowski, J. Controlling the macrocycle size by the stoichiometry of the applied template ion. Chem. Commun. 2012, 48, 2237–2239. [Google Scholar] [CrossRef]
- Byun, J.C.; Lee, N.H.; Mun, D.H.; Park, K.M. Synthesis and characterization of dinuclear copper(II) complexes, [Cu2([20]-DCHDC)(La)2 (La = N3−, NCS− or S2O32−) with tetraazadiphenol macrocyclic ligand having cyclohexane rings. Inorg. Chem. Commun. 2010, 13, 1156–1159. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.-G.; Zhou, Y.; Boxer, L.M.; Woolley, F.R.; Zingaro, R.A. Artificial Zinc(II) complexes regulate cell cycle and apoptosis-related genes in tumor cell lines. ChemBioChem 2007, 8, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Woolley, F.R.; Zingaro, R.A. Catalytic asymmetric cyclopropanation at a chiral platform. Org. Biomol. Chem. 2005, 3, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Reibenspies, J.H.; Martell, A.E. Structurally defined catalysts for enantioselective oxidative coupling reactions. Angew. Chem. 2003, 115, 6190–6194. [Google Scholar] [CrossRef]
- Chinnaraja, E.; Arunachalam, R.; Samanta, J.; Natarajan, R.; Subramanian, P.S. Desymmetrization of meso diols using enantiopure zinc (II) dimers: Synthesis and chiroptical properties. Appl. Organomet. Chem. 2019, 33, e4827. [Google Scholar] [CrossRef]
- Chinnaraja, E.; Arunachalam, R.; Pillai, R.S.; Peuronen, A.; Rissanen, K.; Subramanian, P.S. One-pot synthesis of [2 + 2]-helicate-like macrocycle and 2 + 4-μ4-oxo tetranuclear open frame complexes: Chiroptical properties and asymmetric oxidative coupling of 2-naphthols. Appl. Organomet. Chem. 2020, 34, e5666. [Google Scholar] [CrossRef]
- Yang, Z.; Esteve, F.; Antheaume, C.; Lehn, J.-M. Dynamic covalent self-assembly and self-sorting processes in the formation of imine-based macrocycles and macrobicyclic cages. Chem. Sci. 2023, 14, 6631–6642. [Google Scholar] [CrossRef]
- Chao, A.; Negulescu, I.; Zhang, D. Dynamic covalent polymer networks based on degenerative imine bond exchange: Tuning the malleability and self-healing properties by solvent. Macromolecules 2016, 17, 6277–6284. [Google Scholar] [CrossRef]
- Hughes, T.S.; KoriZhaoch, A.L. Efficient synthesis of an ortho-phenylene-para-phenylene-imine macrocycle; Abstracts of Papers. In Proceedings of the 230th ACS National Meeting, Washington, DC, USA, 28 August–1 September 2005. [Google Scholar]
- Moriya, Y.; Yamanaka, M.; Mori, K. Synthesis of C3-symmetric macrocyclic triimines from monomers having Boc-protected amine and formyl group. Chem. Lett. 2022, 51, 217–220. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. NMR and EPR, 1st ed.; Elsevier Science Publishing Co., Inc.: San Diego, CA, USA, 1999; Volume 5, p. 360. [Google Scholar]
- Ilche Gjuroski, I.; Furrer, J.; Martina Vermathen, M. Probing the interactions of porphyrins with macromolecules using NMR spectroscopy techniques. Molecules 2021, 26, 1942. [Google Scholar] [CrossRef]
- Noggle, J.H.; Schirmer, R.E. The Nuclear Overhouser Effect: Chemistry Applications; Academic Press: New York, NY, USA; London, UK, 1971. [Google Scholar]
- Frisch, M.J. Gaussian03, Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Zhao, D.; Moore, J.S. Folding-driven reversible polymerization of oligo(m-phenylene ethynylene) imines: Solvent and starter sequence studies. Macromolecules 2003, 36, 2712–2720. [Google Scholar] [CrossRef]
- Zhao, D.; Moore, J.S. Synthesis and self-association of an imine-containing m-phenylene ethynylene macrocycle. J. Org. Chem. 2002, 67, 3548–3554. [Google Scholar] [CrossRef] [PubMed]
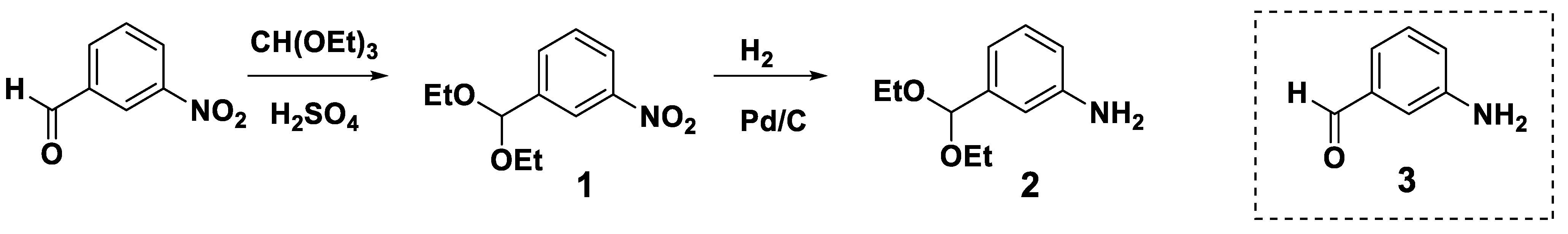




| Entry | Conc. (M) | Solvent | Initiator | Time (d) b | Yield (%) c |
|---|---|---|---|---|---|
| 1 | 1.28 | THF | H2O | 6 | 82 |
| 2 | 0.67 | THF | H2O | 30 | 71 |
| 3 | 0.46 | THF | H2O | 36 | 61 |
| 4 | 0.36 | THF | H2O | 38 | 52 |
| 5 | 0.26 | THF | H2O | 60 | 8 |
| 6 | 0.20 | THF | H2O | 120 | ND |
| 7 | 0.15 | THF | H2O | d | - |
| 8 | 0.10 | THF | H2O | d | - |
| 9 | 0.05 | THF | H2O | d | - |
| 10 | 0.51 | THF | H2O | 3 e | 70 |
| 11 | 1.29 | Dioxane | H2O | 6 | 73 |
| 12 | 1.29 | EtOH | H2O | 11 | ND |
| 13 | 1.28 | THF | 1.0M AcOH | 8 | 70 |
| Empirical formula | C42H30N6·0.5THF(C4H8O)·3H2O |
| Formula weight | 708.84 |
| Temperature | 100(2) K |
| Wavelength(Mo Ka) | 0.71073 Å |
| Crystal system, space group | Triclinic, P1 |
| Unit cell dimensions | a = 3.7348(9) Å a = 119.426(3) deg. |
| b = 16.541(4) Å b = 94.368(3) deg. | |
| c = 16.546(4) Å g = 94.269(3) deg. | |
| Volume | 880.3(4) Å3 |
| Z, Calculated density | 1, 1.341 g/cm3 |
| Absorption coefficient | 0.087 mm−1 |
| F(000) | 376 |
| Crystal size | 0.15 × 0.05 × 0.05 mm |
| Theta range for data collection | 2.47 to 27.09° |
| Reflections collected/unique | 4890/4207 [R(int) = 0.0174] |
| Goodness-of-fit on F2 | 0.969 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0726, wR2 = 0.1959 |
| R indices (all data) | R1 = 0.1098, wR2 = 0.2396 |
| 2θ (°) | d Value (Å) | 2θ (°) | d Value (Å) |
|---|---|---|---|
| 6.14 | 14.38 | 24.91 | 3.57 |
| 10.67 | 8.28 | 25.18 | 3.53 |
| 12.33 | 7.17 | 26.49 | 3.36 |
| 16.35 | 5.42 | 26.72 | 3.33 |
| 18.56 | 4.78 | 26.76 | 3.33 |
| 21.46 | 4.14 | 28.14 | 3.17 |
| 22.36 | 3.97 | 28.54 | 3.13 |
| 23.60 | 3.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T. Highly Efficient One-Pot Synthesis of Hexakis(m-phenyleneimine) Macrocyle Cm6 and the Thermostimulated Self-Healing Property through Dynamic Covalent Chemistry. Polymers 2023, 15, 3542. https://doi.org/10.3390/polym15173542
Matsumoto T. Highly Efficient One-Pot Synthesis of Hexakis(m-phenyleneimine) Macrocyle Cm6 and the Thermostimulated Self-Healing Property through Dynamic Covalent Chemistry. Polymers. 2023; 15(17):3542. https://doi.org/10.3390/polym15173542
Chicago/Turabian StyleMatsumoto, Toshihiko. 2023. "Highly Efficient One-Pot Synthesis of Hexakis(m-phenyleneimine) Macrocyle Cm6 and the Thermostimulated Self-Healing Property through Dynamic Covalent Chemistry" Polymers 15, no. 17: 3542. https://doi.org/10.3390/polym15173542
APA StyleMatsumoto, T. (2023). Highly Efficient One-Pot Synthesis of Hexakis(m-phenyleneimine) Macrocyle Cm6 and the Thermostimulated Self-Healing Property through Dynamic Covalent Chemistry. Polymers, 15(17), 3542. https://doi.org/10.3390/polym15173542






