Three-Dimensional Printed Shape Memory Gels Based on a Structured Disperse System with Hydrophobic Cellulose Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. CNF Modification and Ink Preparation
2.2.2. Rheology
2.2.3. Atomic Force Microscopy (AFM)
2.2.4. Three-Dimensional Printing
2.2.5. Fourier Transform Infrared Spectroscopy
2.2.6. Mechanical Properties
2.2.7. Dynamic Mechanical Analysis (DMA)
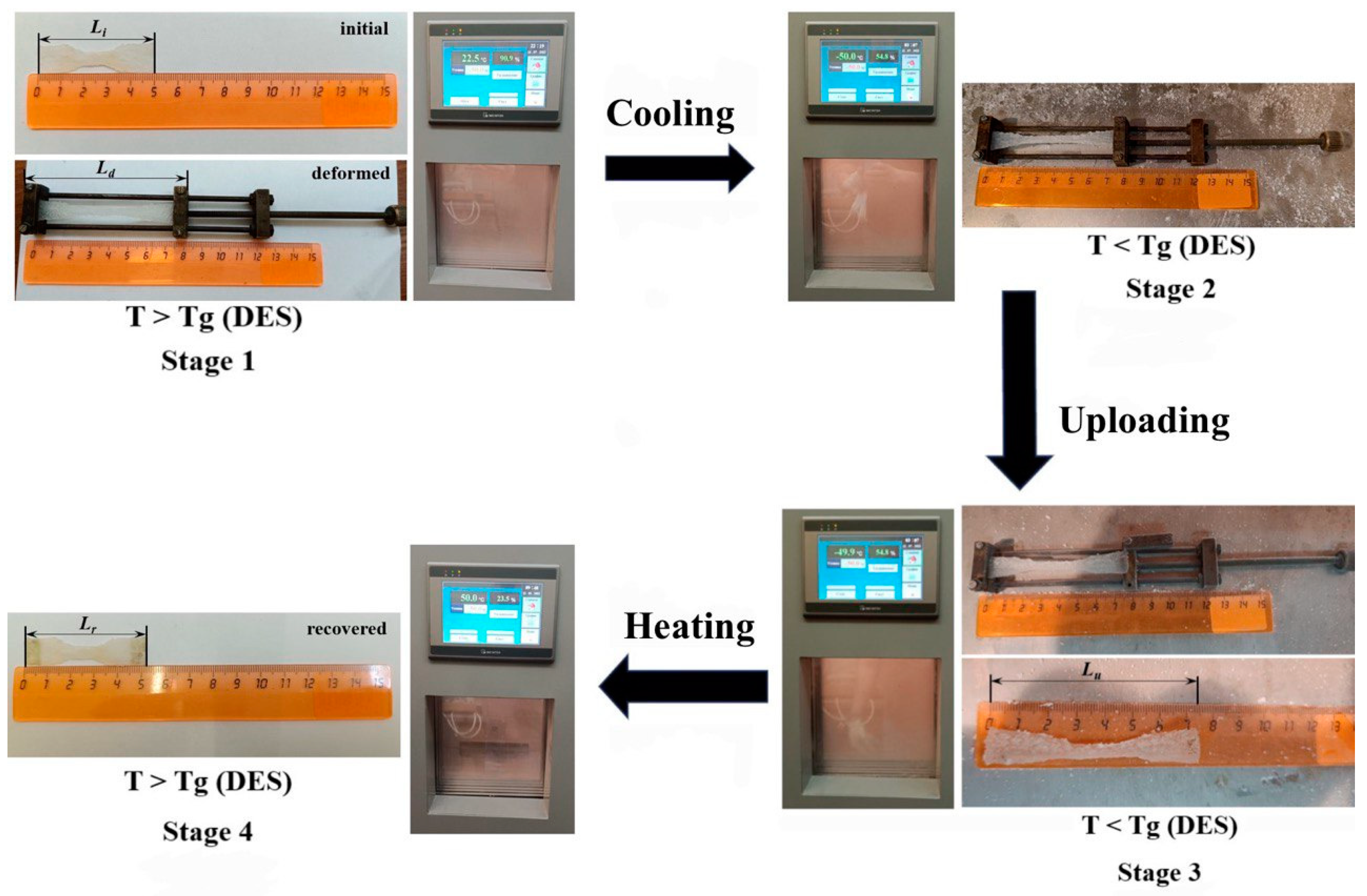
2.2.8. Shape Memory Properties
3. Results and Discussion
3.1. Preparation of Dispersions
3.2. Atomic Force Microscopy
3.3. Rheological Properties of Inks
3.4. Mechanical Properties
3.5. Dynamic Mechanical Analysis
3.6. Shape Memory Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acrylic acid |
| AFM | Atomic force microscopy |
| ChCl | Choline chloride |
| CNF | Cellulose nanofibers |
| DES | Deep eutectic solvent |
| DMA | Dynamic mechanical analysis |
| DN | Double network |
| FTIR | Fourier transform infrared spectroscopy |
| GelMA | Methacrylated gelatin |
| LVE | Linear viscoelastic region |
| M-CNF-0 | Sample with anhydrous 4wt% TMSPM-modified cellulose nanofibers |
| M-CNF-5 | Sample with 4wt% TMSPM-modified cellulose nanofibers and 5 wt% of water |
| M-CNF-12 | Sample with 4wt% TMSPM-modified cellulose nanofibers and 12 wt% of water |
| M-CNF-30 | Sample with 4wt% TMSPM-modified cellulose nanofibers and 30 wt% of water |
| pDES | Polymerized deep eutectic solvent |
| PVA | Polyvinyl alcohol |
| SMP | Shape memory properties |
| TMSPM | 3-(trimethoxysilyl) propyl methacrylate |
References
- Lionetto, F.; Sannino, A.; Mensitieri, G.; Maffezzoli, A. Evaluation of the Degree of Cross-Linking of Cellulose-Based Superabsorbent Hydrogels: A Comparison between Different Techniques. Macromol. Symp. 2003, 200, 199–208. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of Acrylamide/Acrylic Acid Cellulose Hydrogels for the Adsorption of Heavy Metal Ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef]
- Ganesan, M.; Juvekar, V.A. Reduction Self-Assembly of Three-Dimensional Graphene Hydrogels: Implication as Adsorbents. ACS Appl. Nano Mater. 2020, 3, 10823–10834. [Google Scholar] [CrossRef]
- Oh, M.J.; Yoo, P.J. Graphene-Based 3D Lightweight Cellular Structures: Synthesis and Applications. Korean J. Chem. Eng. 2020, 37, 189–208. [Google Scholar] [CrossRef]
- Areyano, M.; Valois, E.; Sanchez Carvajal, I.; Rajkovic, I.; Wonderly, W.R.; Kossa, A.; McMeeking, R.M.; Waite, J.H. Viscoelastic Analysis of Mussel Threads Reveals Energy Dissipative Mechanisms. J. R. Soc. Interface 2022, 19, 828. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Vaccaro, E.; Sun, C.; Lucas, J.M. Elastomeric Gradients: A Hedge against Stress Concentration in Marine Holdfasts? Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yu, X.; Wu, H.; Yang, L.; Lv, H.; Yang, X. Injectable and Cytocompatible Dual Cross-Linking Hydrogels with Enhanced Mechanical Strength and Stability. ACS Biomater. Sci. Eng. 2020, 6, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion-Contraction of Photoresponsive Artificial Muscle Regulated by Host-Guest Interactions. Nat. Commun. 2012, 3, 1270–1278. [Google Scholar] [CrossRef]
- Xia, L.W.; Xie, R.; Ju, X.J.; Wang, W.; Chen, Q.; Chu, L.Y. Nano-Structured Smart Hydrogels with Rapid Response and High Elasticity. Nat. Commun. 2013, 4, 3226. [Google Scholar] [CrossRef] [PubMed]
- Bierbrauer, K.L.; Alasino, R.V.; Barclay, F.E.; Belotti, E.M.; Ortega, H.H.; Beltramo, D.M. Biocompatible Hydrogel for Intra-Articular Implantation Comprising Cationic and Anionic Polymers of Natural Origin: In Vivo Evaluation in a Rabbit Model. Polymers 2021, 13, 4426. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite Hydrogels for Tissue Engineering Applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, K.; Xiao, W.; Zheng, L.; Xiao, Y.; Fan, H.; Zhang, X. Preparation of Collagen-Chondroitin Sulfate-Hyaluronic Acid Hybrid Hydrogel Scaffolds and Cell Compatibility in Vitro. Carbohydr. Polym. 2011, 84, 118–125. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Saraiva, J.N.; Cavaco, G.; Portela, R.P.; Leal, C.R.; Sobral, R.G.; Almeida, P.L. Crosslinked Bacterial Cellulose Hydrogels for Biomedical Applications. Eur. Polym. J. 2022, 177, 111438. [Google Scholar] [CrossRef]
- Lei, Z.; Wu, P. A Highly Transparent and Ultra-Stretchable Conductor with Stable Conductivity during Large Deformation. Nat. Commun. 2019, 10, 11364. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.Y. Hydrogel Soft Robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Sun, X.; Yao, F.; Li, J. Nanocomposite Hydrogel-Based Strain and Pressure Sensors: A Review. J. Mater. Chem. A 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Naficy, S.; Kawakami, S.; Sadegholvaad, S.; Wakisaka, M.; Spinks, G.M. Mechanical Properties of Interpenetrating Polymer Network Hydrogels Based on Hybrid Ionically and Covalently Crosslinked Networks. J. Appl. Polym. Sci. 2013, 130, 2504–2513. [Google Scholar] [CrossRef]
- Xu, P.; Shang, Z.; Yao, M.; Ke, Z.; Li, X.; Liu, P. Molecular Insights on the Mechanical Properties of Double-Network Hydrogels Reinforced by Covalently Compositing with Silica-Nanoparticles. J. Mol. Liq. 2022, 368, 120611. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, J.; Huang, K.; Lee, Z.; Lee, X. A Comparison of Biomechanical Properties between Human and Porcine Cornea. J. Biomech. 2001, 34, 533–537. [Google Scholar] [CrossRef]
- Guldberg, R.E.; Duty, A.O. Design Parameters for Engineering Bone Regeneration; Springer: New York, NY, USA, 2006; ISBN 0387955534. [Google Scholar]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. Part B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, S.; Zhou, J.; Chen, W.; Liu, T.; Yang, S.; Long, S.; Li, X. Achieving Swollen yet Strengthened Hydrogels by Reorganizing Multiphase Network Structure. Adv. Funct. Mater. 2023, 33, 1–13. [Google Scholar] [CrossRef]
- Li, B.; Wu, C.; Han, Y.; Ma, X.; Luo, Z. Preparation of Poly(Acrylic Acid) Grafted Reduced Graphene Oxide/Polyacrylamide Composite Hydrogels with Good Electronic and Mechanical Properties by in-Situ Polymerization. J. Macromol. Sci. Part B Phys. 2021, 60, 589–602. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Z.; Chen, C.; Shi, K.; Zhang, L.; Ju, X.J.; Wang, W.; Xie, R.; Chu, L.Y. Reduced Graphene Oxide-Containing Smart Hydrogels with Excellent Electro-Response and Mechanical Properties for Soft Actuators. ACS Appl. Mater. Interfaces 2017, 9, 15758–15767. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, M.; Ruan, W. High-Water-Content Graphene Oxide/Polyvinyl Alcohol Hydrogel with Excellent Mechanical Properties. J. Mater. Chem. A 2014, 2, 10508–10515. [Google Scholar] [CrossRef]
- Park, D.; Kim, J.W.; Shin, K.; Kim, J.W. Bacterial Cellulose Nanofibrils-Reinforced Composite Hydrogels for Mechanical Compression-Responsive on-Demand Drug Release. Carbohydr. Polym. 2021, 272, 118459. [Google Scholar] [CrossRef]
- Yuan, N.; Xu, L.; Zhang, L.; Ye, H.; Zhao, J.; Liu, Z.; Rong, J. Superior Hybrid Hydrogels of Polyacrylamide Enhanced by Bacterial Cellulose Nanofiber Clusters. Mater. Sci. Eng. C 2016, 67, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, M.A.; Fedotova, V.S.; Sokolova, M.P.; Nikolaeva, A.L.; Elokhovsky, V.Y.; Karttunen, M. Polymerizable Choline-and Imidazolium-Based Ionic Liquids Reinforced with Bacterial Cellulose for 3D-Printing. Polymers 2021, 13, 3044. [Google Scholar] [CrossRef]
- Vorobiov, V.K.; Sokolova, M.P.; Bobrova, N.V.; Elokhovsky, V.Y.; Smirnov, M.A. Rheological Properties and 3D-Printability of Cellulose Nanocrystals/Deep Eutectic Solvent Electroactive Ion Gels. Carbohydr. Polym. 2022, 290, 119475. [Google Scholar] [CrossRef]
- Hu, D.; Zeng, M.; Sun, Y.; Yuan, J.; Wei, Y. Cellulose—Based Hydrogels Regulated by Supramolecular Chemistry. SusMat 2021, 1, 17. [Google Scholar] [CrossRef]
- Liu, T.; Chen, W.; Li, K.; Long, S.; Li, X.; Huang, Y. Toughening Weak Polyampholyte Hydrogels with Weak Chain Entanglements via a Secondary Equilibrium Approach. Polymers 2023, 15, 2644. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F.; Chen, H.; Zheng, J.; Ma, J.; Chen, J. Alginate/Graphene Double-Network Nanocomposite Hydrogel Beads with Low-Swelling, Enhanced Mechanical Properties, and Enhanced Adsorption Capacity. J. Mater. Chem. A 2016, 4, 10885–10892. [Google Scholar] [CrossRef]
- Jing, Z.; Xu, A.; Liang, Y.Q.; Zhang, Z.; Yu, C.; Hong, P.; Li, Y. Biodegradable Poly(Acrylic Acid-Co-Acrylamide)/ Poly(Vinyl Alcohol) Double Network Hydrogels with Tunable Mechanics and High Self-Healing Performance. Polymers 2019, 11, 952. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Putra, A.; Kakugo, A.; Furukawa, H.; Gong, J.P. Ligament-like Tough Double-Network Hydrogel Based on Bacterial Cellulose. Cellulose 2010, 17, 93–101. [Google Scholar] [CrossRef]
- Buyanov, A.L.; Gofman, I.V.; Saprykina, N.N. High-Strength Cellulose–Polyacrylamide Hydrogels: Mechanical Behavior and Structure Depending on the Type of Cellulose. J. Mech. Behav. Biomed. Mater. 2019, 100, 103385. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; Van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of Hydrogels Using Three-Dimensionally Printed Microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, Z.; Liang, Q.; Li, H.; Peng, L.; Wu, M.; Zhao, X.; Cui, X.; Ruan, C.; Liu, W. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef]
- Prosvirnina, A.P.; Bugrov, A.N.; Dobrodumov, A.V.; Vlasova, E.N.; Fedotova, V.S.; Nikolaeva, A.L.; Vorobiov, V.K.; Sokolova, M.P.; Smirnov, M.A. Bacterial Cellulose Nanofibers Modification with 3-(Trimethoxysilyl)Propyl Methacrylate as a Crosslinking and Reinforcing Agent for 3D Printable UV-Curable Inks. J. Mater. Sci. 2022, 57, 20543–20557. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Zhang, H.; Li, Q.; Ma, N.; Zhang, X. High-Strength Double-Network Conductive Hydrogels Based. Molecules 2023, 28, 1900867. [Google Scholar]
- Rehbinder, P.A. Formation of Structures in Disperse Systems. Pure Appl. Chem. 1965, 10, 337–358. [Google Scholar] [CrossRef]
- Uriev, N.B. Physicochemical Dynamics of Disperse Systems. Usp. Khim. 2004, 73, 39–63. [Google Scholar] [CrossRef]
- Sonntag, H.; Strenge, K. Coagulation Kinetics and Structure Formation; Vincent, B., Ed.; Springer: Boston, MA, USA, 1987; ISBN 978-1-4757-0619-2. [Google Scholar]
- Xu, J.; Song, J. Thermal Responsive Shape Memory Polymers for Biomedical Applications; InTech: Shanghai, China, 2011; ISBN 978-953-307-309-5. [Google Scholar]
- Fedotova, V.S.; Sokolova, M.P.; Vorobiov, V.K.; Sivtsov, E.V.; Lukasheva, N.V.; Smirnov, M.A. Water Influence on the Physico-Chemical Properties and 3D Printability of Choline Acrylate—Bacterial Cellulose Inks. Polymers 2023, 15, 2156. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers, 2nd ed.; Vincentz Network: Hannover, Germany, 2006; ISBN 3-87870-1174-8. [Google Scholar]
- Li, M.; Chen, D.; Sun, X.; Xu, Z.; Yang, Y.; Song, Y.; Jiang, F. An Environmentally Tolerant, Highly Stable, Cellulose Nanofiber-Reinforced, Conductive Hydrogel Multifunctional Sensor. Carbohydr. Polym. 2022, 284, 119199. [Google Scholar] [CrossRef]
- Das, D.; Bhattacharjee, S.; Bhaladhare, S. Preparation of Cellulose Hydrogels and Hydrogel Nanocomposites Reinforced by Crystalline Cellulose Nanofibers (CNFs) as a Water Reservoir for Agriculture Use. ACS Appl. Polym. Mater. 2023, 5, 2895–2904. [Google Scholar] [CrossRef]
- Jeencham, R.; Tawonsawatruk, T.; Numpaisal, P.O.; Ruksakulpiwat, Y. Reinforcement of Injectable Hydrogel for Meniscus Tissue Engineering by Using Cellulose Nanofiber from Cassava Pulp. Polymers 2023, 15, 2092. [Google Scholar] [CrossRef]
- Bednarz, S.; Fluder, M.; Galica, M.; Bogdal, D.; Maciejaszek, I. Synthesis of Hydrogels by Polymerization of Itaconic Acid-Choline Chloride Deep Eutectic Solvent. J. Appl. Polym. Sci. 2014, 131, 40608. [Google Scholar] [CrossRef]
- Didenko, A.L.; Kamalov, A.M.; Smirnova, V.E.; Vaganov, G.V.; Popova, E.N.; Kuznetcov, D.A.; Svetlichnyi, V.M.; Yudin, V.E.; Kudryavtsev, V.V. Multiblock (Segmented) Shape Memory Copolymers Containing Polyurethane and Rigid-Chain Polyimide Blocks. Russ. Chem. Bull. 2022, 71, 766–776. [Google Scholar] [CrossRef]
- Cai, C.; Wei, Z.; Wang, X.; Mei, C.; Fu, Y.; Zhong, W.H. Novel Double-Networked Polyurethane Composites with Multi-Stimuli Responsive Functionalities. J. Mater. Chem. A 2018, 6, 17457–17472. [Google Scholar] [CrossRef]






| Samples | Young’s Modulus, MPa | Tensile Strength, MPa | Elongation at Break, % |
|---|---|---|---|
| M-CNF-0 | 23 ± 2 | 5.5 ± 0.9 | 100 ± 5 |
| M-CNF-5 | 5.3 ± 0.7 | 3.4 ± 0.4 | 202 ± 19 |
| M-CNF-12 | 103 ± 10 | 11.9 ± 0.9 | 300 ± 60 |
| M-CNF-30 | 11.3 ± 3.8 | 4.4 ± 0.9 | 224 ± 41 |
| Sample | E′max, MPa | Tg, °C |
|---|---|---|
| M-CNF-0 | 173 | −11 |
| M-CNF-12 | 829 | 3 |
| Samples | No. Cycle | ε0, μm | εm, μm | ε, μm | εrec, μm | Rf,% | Rr,% |
|---|---|---|---|---|---|---|---|
| M-CNF-0 | 1 | 45 | 1779 | 1759 | 887 | 98.9 | 50.9 |
| 2 | 887 | 1836 | 1815 | 1011 | 98.9 | 86.7 | |
| 3 | 1011 | 1839 | 1817 | 1062 | 98.8 | 93.8 | |
| M-CNF-12 | 1 | 30 | 1511 | 1500 | 1163 | 99.3 | 22.9 |
| 2 | 1163 | 1617 | 1605 | 1366 | 99.3 | 54.1 | |
| 3 | 1366 | 1681 | 1670 | 1478 | 99.3 | 63.2 |
| Samples | Li, mm | Ld, mm | Lu, mm | Lr, mm | Rfs,% | Rrs,% |
|---|---|---|---|---|---|---|
| M-CNF-0 | 50 | 80 | 74 | 51 | 80.0 | 95.8 |
| M-CNF-12 | 50 | 80 | 55 | 52 | 16.7 | 60.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosvirnina, A.P.; Bugrov, A.N.; Bobrova, N.V.; Sivtsov, E.V.; Nikolaeva, A.L.; Kamalov, A.M.; Sokolova, M.P.; Smirnov, M.A. Three-Dimensional Printed Shape Memory Gels Based on a Structured Disperse System with Hydrophobic Cellulose Nanofibers. Polymers 2023, 15, 3547. https://doi.org/10.3390/polym15173547
Prosvirnina AP, Bugrov AN, Bobrova NV, Sivtsov EV, Nikolaeva AL, Kamalov AM, Sokolova MP, Smirnov MA. Three-Dimensional Printed Shape Memory Gels Based on a Structured Disperse System with Hydrophobic Cellulose Nanofibers. Polymers. 2023; 15(17):3547. https://doi.org/10.3390/polym15173547
Chicago/Turabian StyleProsvirnina, Angelina P., Alexander N. Bugrov, Natalya V. Bobrova, Eugene V. Sivtsov, Alexandra L. Nikolaeva, Almaz M. Kamalov, Maria P. Sokolova, and Michael A. Smirnov. 2023. "Three-Dimensional Printed Shape Memory Gels Based on a Structured Disperse System with Hydrophobic Cellulose Nanofibers" Polymers 15, no. 17: 3547. https://doi.org/10.3390/polym15173547






